
Metastasis patterns and prognosis in patients with gastric cancer: a Surveillance, Epidemiology, and End Results-based analysis
Highlight box
Key findings
• Patients with single-site metastasis, particularly to the liver and lungs, had better survival outcomes compared to those with multisite metastasis. Key factors influencing survival included age, N stage, surgery of primary tumor, surgery of the metastases, and chemotherapy.
What is known and what is new?
• In the American Joint Committee on Cancer staging system, the prognosis of patients with gastric cancer is mainly based on tumor stage as classified according to primary tumor, lymph nodes, and distant metastases.
• This study is the largest of its kind to investigate the association between metastatic pattern and prognosis among patients with gastric cancer using data from the Surveillance, Epidemiology, and End Results (SEER) database. The results showed that the liver was the most common metastatic site and was associated with a longer overall survival when compared with bone metastasis. Meanwhile, the multivariate analysis showed that N stage, surgery of the primary tumor and metastases, chemotherapy, and metastatic pattern were independent prognostic factors in patients with metastatic gastric cancer.
What is the implication, and what should change now?
• Patients with gastric cancer with liver-only or lung-only metastasis tend to have better life expectancy compared with patients with bone-only metastasis and multisite metastasis. Further studies are warranted to validate whether these factors can be used as new predictive indicators for guiding decision-making in clinical management.
Introduction
Gastric cancer is the fifth most commonly diagnosed malignancy and the fifth leading cause of cancer-related death worldwide (1). Due to the absence of specific symptoms in the early stages, the majority of patients with gastric cancer are diagnosed at an advanced stage, leading to limited treatment options and poor prognosis (2). Currently, the median survival in late-stage gastric cancer is only approximately 6 months (3). Life expectancy plays a critical role in guiding treatment strategies in daily clinical practice. Intensive therapy is presently being overused, while palliative therapy is not being applied properly (4). An accurate prognosis prediction for patients with late-stage gastric cancer is essential for clinical decision-making and management.
The prognosis of gastric cancer is mainly based on the American Joint Committee on Cancer (AJCC) staging system, which classifies tumor stage according to primary tumor (T), lymph nodes (N), and distant metastases (M) (5). However, some prognosis-related risk factors, such as age at diagnosis, gender, ethnicity, previous history of chemotherapy and radiotherapy, and metastatic pattern, are not included in this classification system, making it difficult to accurately predict the prognosis of patients with advanced gastric cancer (6). Several observational studies have demonstrated the association between the survival time and different metastatic sites in solid tumors (7-9). However, the association between metastatic patterns and survival outcomes in patients with advanced gastric cancer has not been fully explored.
The Surveillance, Epidemiology, and End Results (SEER) database (https://seer.cancer.gov/) is the largest publicly available database, which was developed and maintained by the National Cancer Institute (NCI).
In this study, we used SEER database to evaluate the clinical characteristics of patients with gastric cancer with single or multiple distant metastases. In addition, we analyzed the influence of metastatic pattern on survival prognosis, with the aim of providing a reference for clinicians in the selection of treatment strategies. We present this article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-738/rc).
Methods
Data collection
In this retrospective study, we extracted and reviewed data from the SEER database. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
All medical records of patients who were diagnosed with stage IV gastric cancer between 2010 and 2015 were reviewed. The eligibility criteria were as follows: (I) histopathologically confirmed stage IV gastric cancer; (II) distant metastases, such as bone metastasis, brain metastasis, and liver metastasis; and (III) a record of survival outcomes. Meanwhile, the exclusion criteria were as follows: (I) missing information on metastatic site, TNM status, follow-up time, or surgery (II) and patients with primary malignant tumors other than gastric cancer.
Multiple variables were extracted from the SEER database, including race, age, gender, tumor node metastasis (TNM) stage (AJCC seventh edition), site of distant metastases (bone, brain, liver, lung), treatment modalities (surgery, chemotherapy, radiotherapy), and overall survival (OS).
Statistical analysis
The primary outcome was OS, defined as the time from diagnosis to death of any cause. Categorical data are described as percentages. The Kaplan-Meier method was used to generate survival curves, and the differences among the curves were estimated using the log-rank test. Univariate and multivariate analysis were assessed with hazard ratios (HRs) and 95% confidence intervals (CIs) via the Cox regression model. Variables with a statistically significant level of less than 0.05 in the univariate analysis were included in multivariate analysis to identify the independent risk factors. A two-sided P value of less than 0.05 was considered to be statistically significant. All statistical analyses were performed using SPSS 26.0 software (IBM Corporation, Armonk, NY, USA).
Results
Patient characteristics and treatment
A total of 10,262 patients were enrolled in this study. Among them, 7,506 (73.14%) were white, 1,324 (12.90%) were black, 64.71% were male, 35.29% were female, and 52.2% were above 65 years of age. In total, 1,131 (11.02%) patients underwent surgical resection of the primary tumor, with gastrectomy being the most common surgery type (496, 43.85%), followed by gastrectomy with additional organ resection (217, 19.19%) and gastrectomy with partial resection of the esophagus (130, 11.49%). Additionally, 818 (7.97%) of the patients underwent surgery of the metastases. Radiotherapy was performed in 1,687 (16.44%) patients, while chemotherapy was administered to 6,022 (58.68%) patients (Table 1). The detailed patient information is summarized in Table 1.
Table 1
| Variables | N (%)† |
|---|---|
| Race | |
| White | 7,506 (73.14) |
| Black | 1,324 (12.90) |
| Other‡ | 1,432 (13.95) |
| Age | |
| <65 years | 4,905 (47.80) |
| ≥65 years | 5,357 (52.20) |
| Sex | |
| Male | 6,641 (64.71) |
| Female | 3,621 (35.29) |
| T stage | |
| T0 | 50 (0.49) |
| T1 | 1,811 (17.65) |
| T2 | 513 (5.00) |
| T3 | 1,485 (14.47) |
| T4 | 2,192 (21.36) |
| TX | 4,211 (41.03) |
| N stage | |
| N0 | 3,823 (37.25) |
| N1 | 3,562 (34.71) |
| N2 | 582 (5.67) |
| N3 | 631 (6.15) |
| NX | 1,664 (16.22) |
| Surgery of the primary tumor§ | |
| No | 9,131 (88.98) |
| Yes | 1,131 (11.02) |
| Radiotherapy¶ | |
| No/unknown | 8,575 (83.56) |
| Yes | 1,687 (16.44) |
| Chemotherapy | |
| No/unknown | 4,240 (41.32) |
| Yes | 6,022 (58.68) |
| Surgery of the metastases# | |
| No | 9,444 (92.03) |
| Yes | 818 (7.97) |
†, percentages may not total 100% due to rounding; ‡, other races include American Indian/Alaskan Native and Asian/Pacific Islander; §, surgery of primary tumor includes local tumor destruction, local tumor excision, and gastrectomy; ¶, radiotherapy includes external beam radiation, radioactive implants only, radioisotopes only, combination of beam with implants or isotopes, and radiation not otherwise specified; #, surgery of the metastases includes the surgical resection of distant lymph node(s) or other tissue(s) or organ(s) beyond the primary site.
Patterns of distant metastasis
Among the 10,262 patients, 4,699 (45.79%) had single-site metastasis, 3,358 (32.72%) of whom had liver metastasis, 699 (6.81%) bone metastasis, 560 (5.46%) lung metastasis, and 82 (0.80%) brain metastasis. Meanwhile, 1,308 (12.75%) patients had multisite metastasis. The liver was the most commonly reported metastatic site (4,482, 43.68%), followed by the lung (1,540, 15.01%), bone (1,319, 12.85%), and brain (207, 2.02%) (Table 2).
Table 2
| Sites of distant metastasis | N (%)† |
|---|---|
| One site of distant metastasis | 4,699 |
| Bone | 699 (6.81) |
| Brain | 82 (0.80) |
| Liver | 3,358 (32.72) |
| Lung | 560 (5.46) |
| Two sites of distant metastasis | |
| Bone + brain | 19 (0.19) |
| Bone + liver | 270 (2.63) |
| Bone + lung | 135 (1.32) |
| Brain + liver | 26 (0.25) |
| Brain + lung | 18 (0.18) |
| Liver+ lung | 624 (6.08) |
| Three sites of distant metastasis | |
| Bone + brain+ liver | 13 (0.13) |
| Bone + brain+ lung | 12 (0.12) |
| Bone + liver+ lung | 154 (1.50) |
| Brain + liver+ lung | 20 (0.19) |
| Four sites of distant metastasis | |
| Bone + brain+ liver+ lung | 17 (0.17) |
| Other‡ | 4,255 (41.46) |
†, percentages may not total 100% due to rounding; ‡, other includes distant metastases, but none to the bone, brain, liver, or lung.
Association between distant metastatic pattern and prognosis
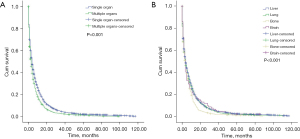
The median OS in patients with single-site metastasis and multisite metastasis was 4 and 3 months, respectively (P<0.001; Figure 1A). The median OS in patients with single-site metastasis to the bone, brain, liver and lung was 4 months (Figure 1B).
For patients with single-site metastasis (n=4,699) and the entire population (n=10,262), univariate analysis showed that age, T stage, N stage, surgery of primary tumor, radiotherapy, chemotherapy, and surgery of the metastases were associated with OS. In patients with single-site metastasis, with bone metastasis serving as a reference, better survival outcomes were achieved in patients with liver metastasis (HR =0.798, 95% CI: 0.735–0.867; P<0.001) or lung metastasis (HR =0.837, 95% CI: 0.748–0.937; P=0.002). For the entire population, univariate analysis showed that the prognosis of patients with single-site metastasis was better than that of patients with multisite metastasis (HR =1.230, 95% CI: 1.158–1.306; P<0.001) (Table 3).
Table 3
| Variables | Subgroups | One site of distant metastasis | Entire cohort | |||||
|---|---|---|---|---|---|---|---|---|
| HR† | 95% CI | P value | HR† | 95% CI | P value | |||
| Race | Other‡ | 1.000 | Reference | 1.000 | Reference | |||
| White | 0.971 | 0.888–1.062 | 0.52 | 1.011 | 0.954–1.071 | 0.72 | ||
| Black | 0.974 | 0.870–1.091 | 0.65 | 1.058 | 0.981–1.142 | 0.15 | ||
| Age | <65 years | 1.000 | Reference | 1.000 | Reference | |||
| ≥65 years | 1.242 | 1.170–1.317 | <0.001 | 1.267 | 1.218–1.318 | <0.001 | ||
| Sex | Female | 1.000 | Reference | 1.000 | Reference | |||
| Male | 1.077 | 0.979–1.186 | 0.13 | 1.014 | 0.973–1.057 | 0.51 | ||
| T stage | T0 | 1.000 | Reference | 1.000 | Reference | |||
| T1 | 0.713 | 0.435–1.168 | 0.18 | 0.996 | 0.743–1.335 | 0.98 | ||
| T2 | 0.561 | 0.336–0.938 | 0.03 | 0.804 | 0.594–1.088 | 0.16 | ||
| T3 | 0.545 | 0.332–0.895 | 0.02 | 0.765 | 0.570–1.026 | 0.07 | ||
| T4 | 0.725 | 0.442–1.190 | 0.20 | 0.972 | 0.725–1.301 | 0.85 | ||
| TX | 0.792 | 0.485–1.296 | 0.35 | 1.158 | 0.866–1.549 | 0.32 | ||
| N stage | N0 | 1.000 | Reference | 1.000 | Reference | |||
| N1 | 0.916 | 0.856–0.980 | 0.01 | 0.908 | 0.867–0.951 | <0.001 | ||
| N2 | 0.794 | 0.691–0.912 | 0.001 | 0.733 | 0.670–0.802 | <0.001 | ||
| N3 | 0.824 | 0.710–0.956 | 0.01 | 0.777 | 0.712–0.846 | <0.001 | ||
| NX | 1.310 | 1.204–1.425 | <0.001 | 1.257 | 1.186–1.333 | <0.001 | ||
| Surgery of the primary tumor§ | No | 1.000 | Reference | 1.000 | Reference | |||
| Yes | 0.633 | 0.567–0.707 | <0.001 | 0.565 | 0.530–0.604 | <0.001 | ||
| Radiotherapy¶ | No/unknown | 1.000 | Reference | 1.000 | Reference | |||
| Yes | 0.894 | 0.829–0.964 | 0.004 | 0.850 | 0.806–0.896 | <0.001 | ||
| Chemotherapy | No/unknown | 1.000 | Reference | 1.000 | Reference | |||
| Yes | 0.347 | 0.326–0.369 | <0.001 | 0.360 | 0.345–0.376 | <0.001 | ||
| Surgery for metastases# | No | 1.000 | Reference | 1.000 | Reference | |||
| Yes | 0.696 | 0.606–0.799 | <0.001 | 0.695 | 0.645–0.748 | <0.001 | ||
| Metastatic sites | Bone | 1.000 | Reference | – | – | – | ||
| Brain | 0.793 | 0.627–1.002 | 0.05 | – | – | – | ||
| Liver | 0.798 | 0.735–0.867 | <0.001 | – | – | – | ||
| Lung | 0.837 | 0.748–0.937 | 0.002 | – | – | – | ||
| Number of metastatic sites | 1 | – | – | – | 1.000 | |||
| >1 | – | – | – | 1.230 | 1.158–1.306 | <0.001 | ||
†, HR based on Cox proportional hazards model; ‡, other races include American Indian/Alaska Native and Asian/Pacific Islander; §, surgery of primary tumor includes local tumor destruction, local tumor excision, and gastrectomy; ¶, radiotherapy includes external beam radiation, radioactive implants only, radioisotopes only, combination of beam with implants or isotopes, and radiation not otherwise specified; #, surgery of the metastases includes the surgical resection of distant lymph node(s) or other tissue(s) or organ(s) beyond the primary site. HR, hazard ratio; CI, confidence interval.
Variables with significant differences in univariate analysis were further analyzed in multivariate Cox regression. The results showed that N stage, surgery of primary tumor, chemotherapy, surgery of the metastases, and metastatic site were independent risk factors associated with OS in patients with single-site metastasis. After other factors were adjusted for, longer OS was achieved in patients with liver metastasis (HR =0.815, 95% CI: 0.748–0.886; P<0.001) and lung metastasis (HR =0.832, 95% CI: 0.743–0.932; P=0.001) with bone metastasis serving as a reference; meanwhile, no significant difference was found in OS between bone metastasis and brain metastasis. For the entire population, the multivariate Cox regression showed that age, N stage, surgery of the primary tumor, chemotherapy, and surgery of the metastases were independent risk factors associated with OS. Moreover, multivariate analysis showed that patients with single-site metastasis had a better prognosis than did those with multisite metastasis (HR =1.290, 95% CI: 1.211–1.375; P<0.001) (Table 4).
Table 4
| Variables | Subgroups | One site of distant metastasis | Entire cohort | |||||
|---|---|---|---|---|---|---|---|---|
| HR† | 95% CI | P value | HR† | 95% CI | P value | |||
| Age | <65 years | 1.000 | Reference | 1.000 | Reference | |||
| ≥65 years | 1.043 | 0.981–1.109 | 0.18 | 1.085 | 1.041–1.130 | <0.001 | ||
| T stage | T0 | 1.000 | Reference | 1.000 | Reference | |||
| T1 | 0.992 | 0.603–1.632 | 0.98 | 1.040 | 0.776–1.395 | 0.79 | ||
| T2 | 0.809 | 0.482–1.356 | 0.42 | 0.897 | 0.662–1.214 | 0.48 | ||
| T3 | 0.874 | 0.529–1.444 | 0.60 | 0.954 | 0.710–1.282 | 0.76 | ||
| T4 | 1.143 | 0.693–1.885 | 0.60 | 1.180 | 0.880–1.582 | 0.27 | ||
| TX | 1.022 | 0.623–1.676 | 0.93 | 1.116 | 0.834–1.493 | 0.46 | ||
| N stage | N0 | 1.000 | Reference | 1.000 | Reference | |||
| N1 | 1.042 | 0.972–1.118 | 0.24 | 0.985 | 0.939–1.033 | 0.53 | ||
| N2 | 1.083 | 0.937–1.251 | 0.28 | 1.029 | 0.937–1.131 | 0.55 | ||
| N3 | 1.183 | 1.005–1.392 | 0.04 | 1.161 | 1.054–1.277 | 0.002 | ||
| NX | 1.195 | 1.095–1.305 | <0.001 | 1.112 | 1.047–1.181 | 0.001 | ||
| Surgery of the primary tumor‡ | No | 1.000 | Reference | 1.000 | Reference | |||
| Yes | 0.550 | 0.484–0.625 | <0.001 | 0.495 | 0.458–0.535 | <0.001 | ||
| Radiotherapy§ | No/unknown | 1.000 | Reference | 1.000 | Reference | |||
| Yes | 0.997 | 0.921–1.079 | 0.94 | 0.965 | 0.914–1.018 | 0.19 | ||
| Chemotherapy | No/unknown | 1.000 | Reference | 1.000 | Reference | |||
| Yes | 0.336 | 0.315–0.359 | <0.001 | 0.352 | 0.337–0.367 | <0.001 | ||
| Surgery for the metastases¶ | No | 1.000 | Reference | 1.000 | Reference | |||
| Yes | 0.822 | 0.710–0.951 | 0.009 | 0.843 | 0.781–0.910 | <0.001 | ||
| Metastatic sites | Bone | 1.000 | Reference | – | – | – | ||
| Brain | 0.865 | 0.678–1.102 | 0.24 | – | – | – | ||
| Liver | 0.815 | 0.748–0.886 | <0.001 | – | – | – | ||
| Lung | 0.832 | 0.743–0.932 | 0.001 | – | – | – | ||
| Number of metastatic sites | 1 | – | – | – | 1.000 | |||
| >1 | – | – | – | 1.290 | 1.211–1.375 | <0.001 | ||
†, HR based on Cox proportional hazards model; ‡, surgery of primary tumor includes local tumor destruction, local tumor excision, and gastrectomy; §, radiotherapy includes external beam radiation, radioactive implants only, radioisotopes only, combination of beam with implants or isotopes, and radiation not otherwise specified; ¶, surgery of the metastases includes the surgical resection of distant lymph node(s) or other tissue(s) or organ(s) beyond the primary site. HR, hazard ratio; CI, confidence interval.
Discussion
The association between metastatic pattern and prognosis in patients with gastric cancer has not been fully investigated. In our study, through extracting data from the SEER database, we found that the liver was the most common metastatic site and was associated with a longer OS as compared to bone metastasis. Meanwhile, the multivariate analysis showed that N stage, surgery of the primary tumor and metastases, chemotherapy, and metastatic pattern were independent risk factors for prognosis in patients with metastatic gastric cancer. To our knowledge, this is the largest study to examine the association between metastatic pattern and prognosis among patients with gastric cancer and single-site metastasis using data from the SEER database.
For metastatic patterns in gastric cancer, we found that the most frequent metastatic organ in patients with single or multiple metastases was liver. The liver receives all the blood from the stomach via the hepatic portal vein, and the blood carries cancer cells. Furthermore, the immune-suppressive microenvironment in the liver contributes to tumor cell survival and growth (10). In our study, for patients with single-site metastasis, the most common metastatic sites following the liver were the bone, lung, and brain. For patients with multisite metastasis, the most common metastatic sites following the liver were the lung, bone, and brain. Similar results have been reported in previous studies that investigated the metastatic patterns of gastric cancer using data from the SEER database (11,12). Moreover, these results are consistent with those of other studies that examined data from the Swedish Cancer Registry (13) and the National Cancer Database (14). Brain metastases from gastric cancer are rare, but they are associated with a poor prognosis and need to be treated with caution (15,16).
In terms of the association between metastatic patterns and prognosis, the multivariate results showed that in patients with single-site metastasis, liver and lung metastases were associated with longer OS as compared to bone metastasis. A previous study based on data from the SEER database evaluated the prognosis of patients with metastatic gastric cancer, and the results showed that the median OS in patients with liver metastasis, lung metastasis, brain metastasis, and bone metastasis were 7, 5, 5, and 6 months, respectively (17). Meanwhile, in our study, the median OS in patients with single-site metastasis to the bone, brain, liver, and lung were all 4 months. The longer survival time reported previously might be due to the exclusion of patients with incomplete collaborative stage. In another study that used information from the Swedish Cancer Registry, patients with metastatic gastric cancer had a median OS of 3 months. In addition, patients with bone or liver metastases had a median OS of 2 months. The shorter median OS may be due to the enrollment of information from an earlier time when patients were treated with less mature forms of management (13).
No consensus has been reached regarding the association between metastatic pattern and prognosis in solid tumors (18-20). In patients with metastatic gastric cancer, those with brain metastasis tend to have a worse prognosis (21,22). This might be due to the reduced efficacy of systemic treatment imposed by the blood-brain barrier (23). In contrast, owing to the relatively high incidence rate of liver metastasis in patients with gastric cancer, the treatment strategies for liver metastasis are more mature, resulting in a better prognosis in these patients (24,25).
There are several limitations related to our study. First, we employed a retrospective, observational design, and potential selection bias was inevitable. Second, this study was based on information from the SEER database, and some metastatic sites were not recorded, such as peritoneal metastasis and lymph node metastasis, were not recorded. Therefore, patients classified as having single-site metastases may actually had multiple metastases. Third, the findings derived from our analysis were not externally verified. Fourth, detailed individual information, such as pathological type, physical status, tumor markers, and chemotherapy regimens, are not available from the SEER database.
Conclusions
In conclusion, our showed that liver was the most frequent metastatic site for patients with gastric cancer, and when compared with bone-only or brain-only metastasis, liver-only and lung-only metastasis are associated with a prolonged OS. This study suggests that for patients with metastatic gastric cancer, surgical resection of the primary and metastatic tumors and systemic chemotherapy should be administered as intensely as possible, while radiotherapy is not a required treatment option.
Acknowledgments
The authors would like to thank the staff at SEER for free access to the database.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-738/rc
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-738/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-738/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024;74:229-63. [Crossref] [PubMed]
- Guan WL, He Y, Xu RH. Gastric cancer treatment: recent progress and future perspectives. J Hematol Oncol 2023;16:57. [Crossref] [PubMed]
- Hu HM, Tsai HJ, Ku HY, et al. Survival outcomes of management in metastatic gastric adenocarcinoma patients. Sci Rep 2021;11:23142. [Crossref] [PubMed]
- Gensheimer MF, Henry AS, Wood DJ, et al. Automated Survival Prediction in Metastatic Cancer Patients Using High-Dimensional Electronic Medical Record Data. J Natl Cancer Inst 2019;111:568-74. [Crossref] [PubMed]
- Janczewski LM, Asare EA, Goodman KA. Updates on the Version 9 American Joint Committee on Cancer Staging System for Anal Cancer. Ann Surg Oncol 2024;31:4155-8. [Crossref] [PubMed]
- Alshehri A, Alanezi H, Kim BS. Prognosis factors of advanced gastric cancer according to sex and age. World J Clin Cases 2020;8:1608-19. [Crossref] [PubMed]
- Qin L, Yu X, Xu C, et al. Prognostic impact of metastatic patterns and treatment modalities on overall survival in lung squamous cell carcinoma: A population-based study. Medicine (Baltimore) 2023;102:e34251. [Crossref] [PubMed]
- Wu J, Fang D, Man D, et al. Clinical correlates and prognostic value of different metastatic sites in gastric and colorectal signet ring cell carcinoma. Engineering 2020;6:1028-34. [Crossref]
- Halabi S, Kelly WK, Ma H, et al. Meta-Analysis Evaluating the Impact of Site of Metastasis on Overall Survival in Men With Castration-Resistant Prostate Cancer. J Clin Oncol 2016;34:1652-9. [Crossref] [PubMed]
- Clark AM, Ma B, Taylor DL, et al. Liver metastases: Microenvironments and ex-vivo models. Exp Biol Med (Maywood) 2016;241:1639-52. [Crossref] [PubMed]
- Li W, Tang Y, Wang D, et al. Outcomes in patients with metastatic gastric cancer: a surveillance, epidemiology, and end results program (SEER) database analysis. All Life 2023; [Crossref]
- Zhang H, Cheng X, Guo W, et al. Metastasis patterns and prognosis in young gastric cancer patients: A propensity score-matched SEER database analysis. PLoS One 2024;19:e0301834. [Crossref] [PubMed]
- Riihimäki M, Hemminki A, Sundquist K, et al. Metastatic spread in patients with gastric cancer. Oncotarget 2016;7:52307-16. [Crossref] [PubMed]
- Sirody J, Kaji AH, Hari DM, et al. Patterns of gastric cancer metastasis in the United States. Am J Surg 2022;224:445-8. [Crossref] [PubMed]
- Kraszkiewicz M, Wydmanski J. Brain metastases from stomach cancer - The role of different treatment modalities and efficacy of palliative radiotherapy. Rep Pract Oncol Radiother 2014;20:32-7. [Crossref] [PubMed]
- Arvanitis CD, Ferraro GB, Jain RK. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat Rev Cancer 2020;20:26-41. [Crossref] [PubMed]
- Lin Z, Wang R, Zhou Y, et al. Prediction of distant metastasis and survival prediction of gastric cancer patients with metastasis to the liver, lung, bone, and brain: research based on the SEER database. Ann Transl Med 2022;10:16. [Crossref] [PubMed]
- Wang H, Zhang C, Zhang J, et al. The prognosis analysis of different metastasis pattern in patients with different breast cancer subtypes: a SEER based study. Oncotarget 2017;8:26368-79. [Crossref] [PubMed]
- Piedra-Delgado L, Chambergo-Michilot D, Morante Z, et al. Survival according to the site of metastasis in triple-negative breast cancer patients: The Peruvian experience. PLoS One 2024;19:e0293833. [Crossref] [PubMed]
- Tie X, Wang J, Wang Y, et al. The prognostic effect of metastasis patterns on overall survival in organ metastatic lung adenocarcinoma. Medicine (Baltimore) 2023;102:e33297. [Crossref] [PubMed]
- Li AY, Gaebe K, Zulfiqar A, et al. Association of Brain Metastases With Survival in Patients With Limited or Stable Extracranial Disease: A Systematic Review and Meta-analysis. JAMA Netw Open 2023;6:e230475. [Crossref] [PubMed]
- Rehman MEU, Kulsoom A, Faraz F, et al. Analysis of risk factors and prognostic factors of brain metastasis in gastric cancer: a surveillance, epidemiology and end-results database study. Sci Rep 2023;13:18664. [Crossref] [PubMed]
- Achrol AS, Rennert RC, Anders C, et al. Brain metastases. Nat Rev Dis Primers 2019;5:5. [Crossref] [PubMed]
- Luo Z, Rong Z, Huang C. Surgery Strategies for Gastric Cancer With Liver Metastasis. Front Oncol 2019;9:1353. [Crossref] [PubMed]
- Granieri S, Altomare M, Bruno F, et al. Surgical treatment of gastric cancer liver metastases: Systematic review and meta-analysis of long-term outcomes and prognostic factors. Crit Rev Oncol Hematol 2021;163:103313. [Crossref] [PubMed]


