Beyond HER2: recent advances and future directions in targeted therapies in esophagogastric cancers
Introduction
Esophagogastric cancers (EGCa) are a heterogenous group of malignancies that comprise tumors arising in the esophagus, gastroesophageal junction, and stomach. Taken together these diseases were diagnosed in about 1,407,400 individuals worldwide in 2012, and caused 1,123,300 deaths (1). Historically, and globally, most esophageal cancers are histologically squamous cell carcinomas. However, in the United States and Western Europe, most esophageal malignancies are adenocarcinomas (1,2).
In the era of cytotoxic chemotherapies, the distinction between adenocarcinomas and squamous cell carcinomas did not seem to matter, and the standard therapy for metastatic disease has been a combination of a platinum and a fluoropyrimidine (3). With the advent of molecularly targeted therapies, the differences between these histologies have raised the potential of a divergence in therapy for esophageal squamous cell carcinomas, and gastric, gastroesophageal junction and distal esophageal adenocarcinomas. For example, though it is outside the scope of this article, the first targeted therapy that has been demonstrated to be effective in EGCa, trastuzumab (Herceptin) has been studied primarily in, and appears to be effective mainly in gastroesophageal adenocarcinomas (4,5).
The brief history of the development of targeted therapies of EGCa has been littered with failed studies. A general consensus has been that this is, at least in part, because of a lack of biological markers suggesting optimal target populations for therapy, akin to HER2 amplification in gastroesophageal adenocarcinomas. The growing understanding of the biology of EGCa and the genetic and molecular changes underlying its development suggest a great promise for progress in the treatment of these malignancies (Table 1).

Full table
Recently, studies have been conducted evaluating the genome of gastric and esophageal cancers, attempting to better characterize these malignancies, and identify genetic alterations that may be used to optimize therapy. Most prominently, The Cancer Genome Atlas Research Network classified gastric adenocarcinomas into four subtypes: Epstein-Barr virus-high, with extensive DNA promoter hypermethylation representing about 9% of patients; microsatellite instability (MSI-high), with hypermethylation of MLH1, in about 22% of patients; chromosomal instability (CIN), which included extensive copy number aberrations, including in p53, ERBB3, PIK3CA, k-ras and ARID1A, representing about 50% of patients; and genomically stable (GS) tumors, including about 20% of patients who did not have significant copy number aberrations, though most commonly, p53, k-ras, PIK3CA, ARID1A, ERBB2, and the β-catenin and transforming growth factor-β pathways were mutated (6). As these profiles were derived from a mixed population of patients, that was primarily non-metastatic gastric adenocarcinoma, how well these classifications will reflect patients with metastatic EGCa remains uncertain. However, others have categorized gastric cancers from different study populations, and esophageal adenocarcinomas and squamous cell carcinoma, and have also reported noting a distinct population with MSI-high gastric, again representing about 15–20% of patients, but also noting some differences (7-9). Interestingly, none of these classifications specify that the HER2 amplified gastric cancers represent a distinct group of patients. Nonetheless, taken together, these classifications support the notion that there are new and distinct subgroups of gastric adenocarcinoma that may be amenable to further study, and specific therapy (Table 2).
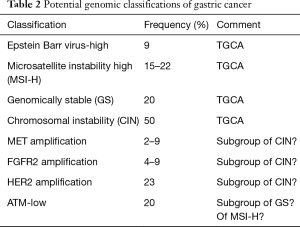
Full table
Epidermal growth factor receptor (EGFR)
It is possible and potentially important, that different pathways have varying import, mechanisms of action and resistance in different tumor types. For example, agents that target the EGFR including monoclonal antibodies cetuximab and panitumumab have demonstrated activity in colorectal cancer, and small molecule inhibitors gefinitib and imatinib in non-small cell lung cancer. Conversely, the monoclonal antibodies do not have definite activity in non-small cell lung cancer, and the small molecule inhibitors do not appear active in colorectal cancer. Mutations in the ras/raf pathway are one mechanism of resistance to these agents.
Despite initially promising results, anti-EGFR agents have not been demonstrated to have definitive activity in gastroesophageal adenocarcinomas, despite the fact that ras mutations have been relatively uncommon. In esophageal adenocarcinomas, phase II studies of gefitinib (10) and erlotinib (11) reported response rates of 11% and 0%, respectively, while erlotinib induced responses in 15% of patients with esophageal squamous cell carcinomas (12). A SWOG study in gastroesophageal junction and gastric adenocarcinomas resulted in responses in 9% of patients (11). Cetuximab in a similar population produced a response in 3% of patients (13). However, the only randomized phase III study of a single agent EGFR inhibitor, gefitinib in the second line setting in esophageal cancers (75% adenocarcinoma) in this case, did not demonstrate a survival benefit compared to placebo (3.73 months compared to 3.67 months) (14). Similarly, in combinations with chemotherapy in the first line setting, the anti-EGFR monoclonal antibodies also did not demonstrate any survival benefit for cetuximab or panitumumab, in combination with cisplatin-capecitabine (15) and epirubicin-oxaliplatin-capecitabine (16) respectively. These failures to improve survival in EGCa highlight the limitations in our understanding of the EGFR pathway.
Other elements that have been hypothesized to influence the growth factor receptor pathways, including PI3KCA/akt/mTOR, have also been explored. Despite initial suggestions of possible activity of everolimus (Affintor), manifested as stable disease in a single arm phase II study (17), this treatment did not significantly improve survival in the 2nd/3rd line setting, compared to placebo in a phase III study, thought it did double the disease control rate from 22.0% to 43.3%, and increase the likelihood of being free from progressive disease at 6 months from 4.3% to 12.0% (18). The failure of this study was a reflection of our nascent understanding of the biology of gastric cancers, as well as the drug development process.
Vascular endothelial growth factor (VEGF)
With the demonstration of the anticancer potential of anti-angiogenic therapy, many studies have subsequently been undertaken with agents that target the VEGF pathway. The initial study was undertaken with bevacizumab, a monoclonal antibody that binds the ligand VEGF-A, in combination with chemotherapy as first line therapy in gastroesophageal adenocarcinomas However, while there was an increase in response and progression-free survival, the study failed to meet its primary endpoint of survival improvement. This was potentially ascribed to differences in treatment patterns and outcomes in Asia compared to Europe, and in particular the Americas (19). Nonetheless, these results, concerns about bleeding with squamous cell carcinomas, and the modest single agent activity for sunitinib (response rates of 3.9–12%, but median progression free survival was less than 2 months, as second line therapy) (20,21) and sorafenib (response rate of 3%, but stable disease in and 56%, median progression free survival of 3.6% in refractory disease) (22) conspired to dampen the enthusiasm for anti-VEGF therapy in gastroesophageal cancers.
In contrast to these disappointing early results, in the early part of this decade, novel anti-VEGF therapies including ramucirumab, a monoclonal antibody inhibiting the VEGF receptor (VEGFR), and apatinib, an oral tyrosine kinase inhibitor of the VEGFR have been demonstrated to produce modest single agent response rates, but significantly increased survival in the second-line setting of patients with gastroesophageal junction adenocarcinoma. Fuchs et al evaluated single agent ramucirumab administered intravenously every 2 weeks, compared to placebo in the REGARD study. The ramucirumab demonstrated a 3% response rate, but induced stable disease in 45% of patents, and increased the likelihood of progression free survival at 12 weeks from 15.8% with placebo to 40.1%, and overall survival at 6 months from 31.6% to 41.8% (23). In combination with weekly paclitaxel as well, ramucirumab also further improved outcomes compared to paclitaxel, including increasing the response rate from 16% with paclitaxel to 28% with the combination, and the disease control rate from 64% to 80%, respectively. Similarly, the addition of ramucirumab to paclitaxel improved survival by 20%, including a 6 months survival from 57% to 72%, and survival at 1 year from 30% to 40% (24). In China, apatinib has been studied in a randomized phase III study of 273 chemotherapy-refractory patients with gastric and gastroesophageal adenocarcinoma, compared to placebo. The oral VEGFR TKI only induced responses in 2.84% of patients, and disease control in 42.05%. Nonetheless, survival was increased by about 30% in the patients treated with apatinib, with a mean overall survival of 6.5 months compared to 4.7 months (25). The well-known class toxicities of anti-VEGF therapies were noted with both agents, most notably grade 3–4 hand foot syndrome in 8.5% of patients with apatinib, as well as severe hypertension in 4.5% of patient and proteinuria in 2.3%, while the most common severe toxicities with ramucirumab were grade 3 hypertension in 8% of patients, and fatigue and abdominal pain in 6% each (23-25).
MET
The MET pathway, which is involved in cell proliferation, invasion and survival, has been suggested to be an important in the development and potentially the treatment of gastric cancer. The mechanism by which this pathway promotes carcinogenesis and survival is unclear, whether it is an oncogenic driver, as measured by MET amplification, or protein expression or overexpression, potentially measuring an escape mechanism for resistance to the EGFR pathway, or chemosensitization (26). Initial reports suggested that high protein expression or amplification each had prognostic import. After much evaluation and conflicting results from retrospective study and case series, results from clinical trials suggest that MET amplification appears to be the most likely biomarker to attempt to select for patients whose disease is most likely to be sensitive to therapy targeting MET, at least with initial chemotherapy (27).
Randomized clinical trials evaluating monoclonal antibodies targeting MET in patient populations that were selected as being MET positive by immunohistochemistry failed to demonstrate an improvement in outcomes in combination with fluoropyrimidine/platinum chemotherapy in the first line setting. In part, this reflects an evolving understanding of the biology of gastroesophageal cancers. Rilotumumab is a monoclonal antibody that binds to hepatocyte growth factor, the ligand for MET. In a randomized phase II study, epirubicin, cisplatin and capecitabine (ECX) at the doses evaluated in the REAL2 study was combined with rilotumumab at 7.5 mg/kg, 15 mg/kg or placebo in the first line setting in 121 unselected patients (39–42 patients per arm) with gastroesophageal junction and gastric adenocarcinoma. There was an improvement in response, progression free survival and overall survival with the 7.5 mg/kg of rilotumumab with ECX, but not the 15 mg/kg dose, compared to placebo. The investigators then evaluated outcomes in the MET positive population, as indicated by 25% membrane staining by IHC, which was not a stratification factor and is therefore subject to possible imbalances. They reported that in the ECX with rilotumumab (both dose levels combined) compared to placebo, the response rate (50% compared to 12%) and median survival (10.6 months compared to 5.7 months) (28). Based on these results, a phase III study was undertaken in patients with gastroesophageal junction and gastric cancer, whose tumors were MET positive by IHC, evaluating ECX with rilotumumab 15 mg/kg or placebo. Unfortunately, even in this population, which was thought to be enriched for responders based on the phase II study, there was no improvement, and indeed there appeared to be a poorer survival in the patients receiving rilotumumab, with median survival of 9.6 months with rilotumumab compared to 11.5 months with placebo, and 12 months overall survival of 38.4% compared to 49.7%. While there was no difference in median progression free survival, the overall survival curves also demonstrated an inferior survival with the addition of rilotumumab, and similarly, the overall response rate was inferior with rilotumumab (30% compared to 39.2%) (29).
Similarly, Shah et al. reported a randomized phase II study in of oxaliplatin, 5-fluorouracil and leucovorin on a FOLFOX schedule, with or without onartuzumab, a humanized anti-MET antibody, in the first line setting. One hundred and twenty three patients with gastroesophageal adenocarcinoma were enrolled regardless of MET status, but with a planned analysis of the MET positive subgroup, as identified by IHC. They, too, failed to identify any benefit with the addition of onartuzumab, though there was no significant negative difference in the primary endpoint of progression free survival in the MET positive group (5.95 months with the addition of onartuzumab, compared to 6.8 months with placebo) (30).
Meanwhile, several small studies have suggested that MET amplification may be the most appropriate too with which to select patients with gastroesophageal cancer for anti-MET therapy. MET amplification has been identified in 2–9% of patients. In an evaluation of patients who were treated at the Massachusetts General Hospital and Dana Farber Cancer Institute, of four patients with MET amplification who were treated with crizotinib, one patient had a partial response, and another had stable disease (31). More provocatively, Kwak et al. reported the results of a phase I study of AMG337, an oral tyrosine kinase inhibitor of MET. Of the 13 patients with MET amplified gastreoesopheal cancers, there was one complete response and six partial responses, producing an overall response rate of 62% (32). Kang et al. have also reported anticancer activity with the anti-c-MET antibody ABT700, with three partial responses in four patients with MET amplifications (33).
Checkpoint inhibitors
Immunotherapy as cancer therapy has been studied, initially with immune effectors such as interferon and interleukin with limited success in most malignancies, for decades. Over the past decade, a greater understanding of the interaction between cancer and the immune system has emerged. In particular, cancers may suppress the immune system by overstimulating and thereby exhausting regulatory cells, so called immune checkpoints, via receptors such as programmed death (PD)-1 and cytotoxic T lymphocyte antigen (CTLA)-4. Therefore, promising therapeutic approaches in solid tumors have been inhibitors of these pathways, for example with inhibitors of PD-1, its ligand PD-L-1, or CTLA-4. The anticancer activity of this approach has been validated in melanoma and renal cell carcinoma, as well as tumors traditionally considered immune resistant, such as non-small cell lung cancer.
Tremelimumab, a monoclonal antibody inhibiting CTLA-4, has been studied in a phase II clinical trial in gastroesophageal adenocarcinoma. While the study did not meet its primary endpoint with regards to objective response, one of the 18 patients enrolled on the study did have a long-standing (more than 32 months, and ongoing at the time of report) partial response. As is often seen in immunotherapy, this response did not develop until nearly 2 years of therapy. Three other patients had stable disease, and the median time to progression was 2.83 months, and the median survival was 4.83 months, but the 12 months survival was 33%, suggesting the promise of this type of approach. However, single agent therapy with anti-CTLA-4 therapy has been surpassed by combination therapies with agents targeting the PD-1 pathway, which has been suggested to be superior in some patients with melanoma (34).
Preclinical and translational studies suggest that PD-1 may be an important and promising therapy in gastric cancers, as gastric cancers and infiltrating T cells have higher levels of PD-1 and PD-L1 than adjacent non-tumorous gastric tissue, and the peripheral blood (35). This buttresses the hypothesis that tumor expression of PD-L-1 may result in the recruitment of T cells, and their subsequent persistent activation and exhaustion, perhaps contributing to carcinogenesis and progression. Therefore, EGCa have also been the subject of evaluation with PD-1 inhibitors.
Muro et al. reported that in the gastric cancer cohort of patients in KEYNOTE 012, a study of pembrolizumab, 22% of 39 patients had an objective response. The 6-month progression free survival was 24%, and the 6-month overall survival was 69%. Correlative studies demonstrated that 40% of patients had at least 1% PD-L1 expression by immunohistochemistry, and that this expression was correlated with prolonged survival. In possible contrast to the experiences with the EGFR inhibitors, immune checkpoint inhibitors appeared to have similar activity in esophageal squamous cell carcinomas (36). In the KEYNOTE 028 study, Doi et al. reported an objective response rate of 30% in 23 patients treated with 10 mg/kg of pembrolizumab every 2 weeks (37). Similarly, Kojima responses in 17% of 65 patients who were treated with nivolumab 3 mg/kg IV every 2 weeks. As a result of these studies, randomized phase III study of nivolumab and pembolizumab are planned in esophageal and gastric cancers, respectively (38).
Ataxia telangiectasia mutated (ATM)
One proposed mechanism of action for immunotherapy and PD-1 inhibitors in particular is that more frequent mutations, perhaps because of deficiencies in mismatch repair genes, for example, resulting in the generation of more neoantigens, thereby further stimulating the immune system (39). On the other hand, those and other etiologies of impaired mutational repair may also be a potential target for therapeutic intervention via synthetic lethality (40). This has been demonstrated in ovarian and breast cancer with poly ADP ribose polymerase (PARP) inhibitors such as olaparib demonstrating single agent activity in patients with BRCA1 and two mutations (41,42). ATM has been suggested to be a potential target for PARP inhibitors, and low ATM expression has been identified in about 20% of patients. Therefore, Kang et al. performed a randomized phase II study of paclitaxel with or without olaparib, with stratification by patients’ tumor ATM expression by immunohistochemistry. While there was no clear difference in outcomes between the two treatment arms in the primary endpoint, progression free survival or objective response rate, there was a significant difference in overall survival, favoring paclitaxel/olaparib, both in the overall population (HR =0.56), and in particular in the low ATM population (HR =0.35). This has led to the development of a phase III study in patients with low ATM expression gastric cancers (43).
Fibroblast growth factor receptor (FGFR)
The FGFR has been suggested to be an important potential prognostic factor, and therapeutic target in gastric cancers. After expression of FGFR was initially evaluated, amplification of FGFR2 has been suggested to be associated with a poor prognosis (44,45). Studies have demonstrated amplification in 4–9% of gastroesophageal cancers. Preclinical studies suggest that AZD4547, an inhibitor of FGFR 1, 2 and 3 had activity in cell lines (46). AZD4547 was then evaluated in patients. Smyth et al. reported that they identified FGFR2 amplification in 9% of patients with gastroesophageal cancer, and of the nine patients who were treated with AZD4547 in the phase II study, three had objective responses, a promising finding (47).
Conclusions
Contrary to the initial experiences with targeted therapy in colorectal cancer, aside from trastuzumab, the development of targeted therapies in gastroesophageal cancers was faced with significant initial failures with the initial studies of EGFR and VEGF inhibitors. These setbacks further highlight the limitations of extrapolating approaches targeting one malignancy to another, based on histology alone. However, over the past several years, therapies inhibiting the VEGF pathway have demonstrated a survival benefit in the treatment of patients with gastric and gastroesophageal junction adenocarcinomas, with or without chemotherapy, compared to placebo. Moreover, advances in genomic testing have suggested that subpopulations of patients may benefit of promising therapy with MET and FGFR inhibitors. Immunotherapy is another promising approach in the treatment of gastroesophageal cancers, but interestingly and contrary to other novel and promising therapies, it remains unclear if biomarkers will help identify the optimal population for these potentially expensive treatments.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Gupta B, Kumar N. Worldwide incidence, mortality and time trends for cancer of the oesophagus. Eur J Cancer Prev 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Shah MA. Update on metastatic gastric and esophageal cancers. J Clin Oncol 2015;33:1760-9. [Crossref] [PubMed]
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
- Miura JT, Xiu J, Thomas J, et al. Tumor profiling of gastric and esophageal carcinoma reveal different treatment options. Cancer Biol Ther 2015;16:764-9. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202-9. [Crossref] [PubMed]
- Cristescu R, Lee J, Nebozhyn M, et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med 2015;21:449-56. [Crossref] [PubMed]
- Liu YJ, Shen D, Yin X, et al. HER2, MET and FGFR2 oncogenic driver alterations define distinct molecular segments for targeted therapies in gastric carcinoma. Br J Cancer 2014;110:1169-78. [Crossref] [PubMed]
- Bandla S, Pennathur A, Luketich JD, et al. Comparative genomics of esophageal adenocarcinoma and squamous cell carcinoma. Ann Thorac Surg 2012;93:1101-6. [Crossref] [PubMed]
- Ferry DR, Anderson M, Beddard K, et al. A phase II study of gefitinib monotherapy in advanced esophageal adenocarcinoma: evidence of gene expression, cellular, and clinical response. Clin Cancer Res 2007;13:5869-75. [Crossref] [PubMed]
- Dragovich T, McCoy S, Fenoglio-Preiser CM, et al. Phase II trial of erlotinib in gastroesophageal junction and gastric adenocarcinomas: SWOG 0127. J Clin Oncol 2006;24:4922-7. [Crossref] [PubMed]
- Ilson DH, Kelsen D, Shah M, et al. A phase 2 trial of erlotinib in patients with previously treated squamous cell and adenocarcinoma of the esophagus. Cancer 2011;117:1409-14. [Crossref] [PubMed]
- Chan JA, Blaszkowsky LS, Enzinger PC, et al. A multicenter phase II trial of single-agent cetuximab in advanced esophageal and gastric adenocarcinoma. Ann Oncol 2011;22:1367-73. [Crossref] [PubMed]
- Dutton SJ, Ferry DR, Blazeby JM, et al. Gefitinib for oesophageal cancer progressing after chemotherapy (COG): a phase 3, multicentre, double-blind, placebo-controlled randomised trial. Lancet Oncol 2014;15:894-904. [Crossref] [PubMed]
- Lordick F, Kang YK, Chung HC, et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol 2013;14:490-9. [Crossref] [PubMed]
- Waddell T, Chau I, Cunningham D, et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open-label phase 3 trial. Lancet Oncol 2013;14:481-9. [Crossref] [PubMed]
- Doi T, Muro K, Boku N, et al. Multicenter phase II study of everolimus in patients with previously treated metastatic gastric cancer. J Clin Oncol 2010;28:1904-10. [Crossref] [PubMed]
- Ohtsu A, Ajani JA, Bai YX, et al. Everolimus for previously treated advanced gastric cancer: results of the randomized, double-blind, phase III GRANITE-1 study. J Clin Oncol 2013;31:3935-43. [Crossref] [PubMed]
- Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol 2011;29:3968-76. [Crossref] [PubMed]
- Moehler M, Mueller A, Hartmann JT, et al. An open-label, multicentre biomarker-oriented AIO phase II trial of sunitinib for patients with chemo-refractory advanced gastric cancer. Eur J Cancer 2011;47:1511-20. [Crossref] [PubMed]
- Wu C, Mikhail S, Wei L, et al. A phase II and pharmacodynamic study of sunitinib in relapsed/refractory oesophageal and gastro-oesophageal cancers. Br J Cancer 2015;113:220-5. [Crossref] [PubMed]
- Janjigian YY, Vakiani E, Ku GY, et al. Phase II Trial of Sorafenib in Patients with Chemotherapy Refractory Metastatic Esophageal and Gastroesophageal (GE) Junction Cancer. PLoS One 2015;10:e0134731. [Crossref] [PubMed]
- Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014;383:31-9. [Crossref] [PubMed]
- Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 2014;15:1224-35. [Crossref] [PubMed]
- Li J, Qin S, Xu J, et al. Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Apatinib in Patients With Chemotherapy-Refractory Advanced or Metastatic Adenocarcinoma of the Stomach or Gastroesophageal Junction. J Clin Oncol 2016;34:1448-54. [Crossref] [PubMed]
- Blumenschein GR Jr, Mills GB, Gonzalez-Angulo AM. Targeting the hepatocyte growth factor-cMET axis in cancer therapy. J Clin Oncol 2012;30:3287-96. [Crossref] [PubMed]
- An X, Wang F, Shao Q, et al. MET amplification is not rare and predicts unfavorable clinical outcomes in patients with recurrent/metastatic gastric cancer after chemotherapy. Cancer 2014;120:675-82. [Crossref] [PubMed]
- Iveson T, Donehower RC, Davidenko I, et al. Rilotumumab in combination with epirubicin, cisplatin, and capecitabine as first-line treatment for gastric or oesophagogastric junction adenocarcinoma: an open-label, dose de-escalation phase 1b study and a double-blind, randomised phase 2 study. Lancet Oncol 2014;15:1007-18. [Crossref] [PubMed]
- Cunningham D, Tebbutt NC, Davidenko I, et al. Phase III, randomized, double-blind, multicenter, placebo (P)-controlled trial of rilotumumab (R) plus epirubicin, cisplatin and capecitabine (ECX) as first-line therapy in patients (pts) with advanced MET-positive (pos) gastric or gastroesophageal junction (G/GEJ) cancer: RILOMET-1 study. J Clin Oncol 2015;33:abstr 4000.
- Shah MA, Cho JY, Huat IT, et al. Randomized phase II study of FOLFOX +/- MET inhibitor, onartuzumab (O), in advanced gastroesophageal adenocarcinoma (GEC). J Clin Oncol 2015;33:abstr 2.
- Lennerz JK, Kwak EL, Ackerman A, et al. MET amplification identifies a small and aggressive subgroup of esophagogastric adenocarcinoma with evidence of responsiveness to crizotinib. J Clin Oncol 2011;29:4803-10. [Crossref] [PubMed]
- Kwak EL, LoRusso P, Hamid O, et al. Clinical activity of AMG 337, an oral MET kinase inhibitor, in adult patients (pts) with MET-amplified gastroesophageal junction (GEJ), gastric (G), or esophageal (E) cancer. J Clin Oncol 2015;33:abstr 1.
- Kang YK, LoRusso P, Salgia R, et al. Phase I study of ABT-700, an anti-c-Met antibody, in patients (pts) with advanced gastric or esophageal cancer (GEC). J Clin Oncol 2015;33:abstr 167.
- Ralph C, Elkord E, Burt DJ, et al. Modulation of lymphocyte regulation for cancer therapy: a phase II trial of tremelimumab in advanced gastric and esophageal adenocarcinoma. Clin Cancer Res 2010;16:1662-72. [Crossref] [PubMed]
- Saito H, Kuroda H, Matsunaga T, et al. Increased PD-1 expression on CD4+ and CD8+ T cells is involved in immune evasion in gastric cancer. J Surg Oncol 2013;107:517-22. [Crossref] [PubMed]
- Muro K, Bang YJ, Shankaran V, et al. Relationship between PD-L1 expression and clinical outcomes in patients (Pts) with advanced gastric cancer treated with the anti-PD-1 monoclonal antibody pembrolizumab (Pembro; MK-3475) in KEYNOTE-012. J Clin Oncol 2015;33:abstr 3.
- Doi T, Piha-Paul SA, Jalal SI, et al. Updated results for the advanced esophageal carcinoma cohort of the phase Ib KEYNOTE-028 study of pembrolizumab (MK-3475). J Clin Oncol 2016;34:abstr 7.
- Kojima T, Hara H, Yamaguchi K, et al. Phase II study of nivolumab (ONO-4538/BMS-936558) in patients with esophageal cancer: Preliminary report of overall survival. J Clin Oncol 2016;34:abstr TPS175.
- Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015;372:2509-20. [Crossref] [PubMed]
- Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med 2009;361:123-34. [Crossref] [PubMed]
- Mateo J, Carreira S, Sandhu S, et al. DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. N Engl J Med 2015;373:1697-708. [Crossref] [PubMed]
- Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med 2012;366:1382-92. [Crossref] [PubMed]
- Bang YJ, Im SA, Lee KW, et al. Randomized, Double-Blind Phase II Trial With Prospective Classification by ATM Protein Level to Evaluate the Efficacy and Tolerability of Olaparib Plus Paclitaxel in Patients With Recurrent or Metastatic Gastric Cancer. J Clin Oncol 2015;33:3858-65. [Crossref] [PubMed]
- Ueki T, Koji T, Tamiya S, et al. Expression of basic fibroblast growth factor and fibroblast growth factor receptor in advanced gastric carcinoma. J Pathol 1995;177:353-61. [Crossref] [PubMed]
- Su X, Zhan P, Gavine PR, et al. FGFR2 amplification has prognostic significance in gastric cancer: results from a large international multicentre study. Br J Cancer 2014;110:967-75. [Crossref] [PubMed]
- Xie L, Su X, Zhang L, et al. FGFR2 gene amplification in gastric cancer predicts sensitivity to the selective FGFR inhibitor AZD4547. Clin Cancer Res 2013;19:2572-83. [Crossref] [PubMed]
- Smyth EC, Turner NC, Pearson A, et al. Phase II study of AZD4547 in FGFR amplified tumours: Gastroesophageal cancer (GC) cohort pharmacodynamic and biomarker results. J Clin Oncol 2016;34:abstr 154.


