
Comparison of various liver cancer staging systems in predicting prognosis after initial transcatheter arterial chemoembolization: a retrospective study from China
Highlight box
Key findings
• Portal vein tumor thrombus (PVT), alpha fetoprotein levels, and ineffective response to the initial transcatheter arterial chemoembolization (TACE) are independent risk factors for progression-free survival (PFS), while PVT and ineffective response to the initial TACE predict poor overall survival (OS). Most of the classical primary liver cancer staging systems effectively predict post-TACE PFS and OS, whereas the tumor-node-metastasis (TNM) staging system demonstrated inferior predictive ability.
What is known and what is new?
• Classical hepatocellular carcinoma (HCC) staging systems are still the mainstream tool for stratification and prognosis prediction of patients with hepatocellular carcinoma. However, the variable characteristics and emphases between different classical HCC staging systems cause the clinical selection to be inconsistent.
• Our results demonstrate that the majority of HCC staging systems can reliably predict the prognosis of patients after initial TACE, while TNM staging system shows inferior predictive ability due to its exclusion of the hepatic function.
What is the implication, and what should change now?
• The majority of HCC staging systems are useful and comparable in clinical practice, but more relevant and practical clinical features should be incorporated to enhance the efficacy of these systems.
Introduction
Primary liver cancer (PLC) is the sixth most commonly diagnosed cancer and the third leading cause of cancer-related death worldwide, while in China, it is the fourth most commonly diagnosed cancer and second leading cause of cancer-related death (1-3). Hepatocellular carcinoma (HCC) constitutes approximately 75–85% of PLC cases and is a significant health burden to Chinese society (4). As a consequence of the high prevalence of hepatitis B virus in China, patients with HCC are frequently asymptomatic during initial diagnosis, progress rapidly, and are less response to specific treatment such as transcatheter arterial chemoembolization (TACE) and systemic treatment (2,5). A majority of patients with HCC do not have the opportunity to undergo radical therapies at the time of initial diagnosis, such as transplantation, liver resection (LR), and ablation (6,7). Moreover, HCC is a highly vascular tumor that originates from hepatocytes, making it vulnerable to an ischemic environment (8). Consequently, TACE is commonly used to control the growth of tumors as a form of palliative therapy or used to downstage selected patients whose tumor is beyond acceptable criteria for liver transplant (9). TACE targets the tumor’s blood vessels to deliver chemo-embolic drugs, causing cell death and tissue necrosis, thus halting tumor growth (10).
There are many staging systems for HCC, including but not limited to the Chinese liver cancer (CNLC) staging system, Barcelona Clinic Liver Cancer (BCLC) staging system, Hong Kong Liver Cancer (HKLC) staging system, modified Japanese Integrated Staging (mJIS) system, modified Cancer of the Liver Italian Program (mCLIP) staging system, and Tumor-Node-Metastasis (TNM) staging system (2,11-15). Although, some innovative predicting systems integrate radiomics, artificial intelligence, pathological information, or even genetic information for more precise prognosis and treatment response prediction, these systems still lack practical and clinical application (16-20). Classical HCC staging systems are still the mainstream tool for the stratification of patients with HCC and prediction of treatment efficacy. Although classical primary liver cancer staging systems mainly estimate intrahepatic tumor burden, extrahepatic metastasis and liver function, etc. These staging systems differ mainly in each scoring or grading sub-item, so in clinical practice, it remains unclear which one is the most appropriate, especially for HCC patients undergoing TACE (21,22). Thus far, no study has compared different liver cancer staging systems in predicting the efficacy of TACE in patients with HCC, especially in the Chinese mainland. Therefore, this retrospective study enrolled patients with HCC who had received TACE as initial treatment in Hunan Provincial People’s Hospital in recent years. Six liver cancer staging systems, including CNLC, BCLC, HKLC, mJIS, mCLIP, and TNM, were compared in terms of their ability to predict the efficacy of TACE in patients with HCC. We present this article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-2024-850/rc).
Methods
Study population
This single-center, retrospective study was approved by the medical ethics committee of Hunan Provincial People’s Hospital (No. [2024]-168) and was performed following the Declaration of Helsinki (as revised in 2013) (23). The requirement for written informed consent was waived by the institutional review boards due to the retrospective nature of the analysis.
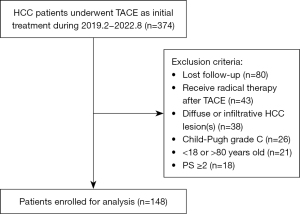
This study included 374 consecutive patients with HCC who underwent TACE as initial treatment at the Department of Interventional Vascular Surgery of Hunan Provincial People’s Hospital from February 2019 to August 2022. The inclusion criteria were (I) pathologically confirmed HCC or Liver Imaging Reporting and Data System (LI-RADS) 5 (LR-5) HCC and (II) TACE was carried out as the initial treatment. The exclusion criteria were as follows: (I) loss to follow-up after initial treatment; (II) administration of radical treatment after initial TACE treatment, such as liver transplantation, surgical resection, and ablation; (III) diffuse or infiltrative HCC lesions; (IV) Child-Pugh grade C; (V) age younger than 18 years or older than 80 years; and (VI) a performance status (PS) score of 2 or above. Finally, 148 eligible patients were enrolled for analysis. The flowchart of participants’ inclusion in this study is shown in Figure 1.
Data collection
We reviewed the electronic medical record system and collected basic clinical information, biochemical tests, and imaging examinations of patients within 1 week before the first TACE; the specific information included age, gender, number of tumors, maximum tumor diameter, intrahepatic vascular invasion, extrahepatic metastasis, the background of viral hepatitis and cirrhosis, Child-Pugh score, alpha-fetoprotein (AFP) level, Tumor staging was performed according to the imaging examinations and liver function before the initial TACE according to the CNLC, BCLC, HKLC, mJIS, mCLIP, and TNM staging systems (2,11-15).
The liver function was re-examined 3–5 days after TACE and compared with the baseline data before treatment. An increase of 2 points or more in the Child-Pugh score was considered to indicate a deterioration in liver function; otherwise, stable liver function was indicated (24). One month after the initial TACE treatment, contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) of the liver was performed, and the treatment response was evaluated according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST), with complete response (CR) and partial response (PR) being defined as effective treatment and stable disease (SD) and progressive disease (PD) being defined as ineffective treatment (25).
TACE procedure
Each patient was discussed at a multispecialty tumor board. TACE was recommended if the patient was not a suitable candidate for any curative treatment option. All TACE procedures were performed by several board-certified senior interventional radiologists. The femoral artery or radial artery was used as a routine access route, with 5-Fr Yashiro or Rosch hepatic) catheters used via the femoral artery and vertebra or multipurpose catheters employed through the radial artery for arteriographic selection. The celiac artery and superior mesenteric artery were routinely selected for angiography to identify all intrahepatic tumors and their supplying arteries.
The microcatheter was used for the tumor-feeding artery, with superselection at a 2.7-F or 2.8-F size for chemotherapy embolization based on angiographic results. For conventional TACE (cTACE), the iodized oil-idarubicin/doxorubicin emulsion was a mixture of up to 15 mL of lipiodol and distilled water, with 10 mg of idarubicin or 40–80 mg of doxorubicin being dissolved at a ratio of 3:1 or 2:1, respectively. Gelfoam slurry/particles, microspheres (Embozene Microspheres, Varian Medical Systems, Palo Alto, CA, USA), or polyvinyl alcohol (PVA) embolization particles (Cook Medical, Bloomington, IN, USA) were injected through the microcatheter to embolize the proximal tumor feeders. If a hepatic arteriovenous fistula was present within the embolization target lesion, PVA particles or microspheres were used before iodized oil embolization. In the drug-eluting bead-TACE (DEB-TACE) procedure, according to angiographic results, appropriately sized DEBs (Biocompatibles, Farnham, UK; Jiangsu Hengrui Medical, Lianyungang, China) were selected and loaded with either idarubicin (10 mg) or doxorubicin (40–80 mg) for 2,030 minutes before being administered into the tumor’s blood supply artery. All the procedures were performed with technical success according to the Society of Interventional Radiology (SIR) guidelines (26).
Follow-up
After the initial TACE, the imaging follow-up was conducted every 1 to 2 months, and TACE treatment was performed on demand according to the patient’s tumor response and liver function. Progression-free survival (PFS) was defined as the time from the patient’s first TACE treatment to disease progression or death. Overall survival (OS) was defined as the time from the patient’s first TACE treatment to death. The last telephone follow-up time was August 21, 2023. If the endpoint event was not reached at the last follow-up, the data were censored.
Statistical analysis
Categorical variables were compared using the χ2 test or Fisher exact test, as appropriate, while continuous variables were compared using the Mann-Whitney test or t-test, as appropriate. The Cox regression model was used to determine the independent risk factors of PFS and OS after TACE, and factors with P<0.1 in the univariate Cox analysis were included in the multivariate analysis. The Kaplan-Meier method was used to evaluate PFS and OS for different tumor stages in each staging system, and the log-rank method was used for testing. To evaluate the predicting efficacy of every staging system, the concordance index (C-index) was calculated using the function concordance.index in the R “survcomp” package (The R Foundation for Statistical Computing; http://www.R-project.org). 95% confidence interval (95% CI) of the C-index were calculated by bootstrap resampling in 1,000 times. To determine whether the C-index of each staging system was statistically significant, the 95% CIs were compared. If these intervals overlapped, the difference was not statistically significant; if there was no overlap, the difference was substantial. A two-sided P value less than 0.05 indicated statistical significance. Statistical analysis was carried out using SPSS 24 statistical software (IBM Corp., Armonk, NY, USA) or R software v. 4.0.2.
Results
Baseline patient characteristics
A total of 148 patients were included in this study, with an average age of 58.76±11.83 years; 122 patients were male and 26 female. According to the enhanced CT/MRI before TACE, 103 patients had 3 or more LR-5 lesions, and 45 patients had 1–2 LR-5 lesions. One hundred and six patients had a maximum diameter of the largest HCC lesion more than 5 cm, and 35 patients had bilobar HCC involvement 68 patients presented with portal vein tumor thrombus (PVT), 51 patients had hepatic vein and (or) inferior vena cava tumor thrombus, and 17 patients had extrahepatic metastases. Furthermore, 143 patients had a background of hepatitis, 107 of whom had chronic type-B hepatitis. There were 94 patients with cirrhosis on diagnosis triple phase contrast CT or MR, and 63 patients had a preoperative AFP level exceeding 400 ng/mL. During the initial TACE, 112 patients underwent DEB-TACE, while the other patients received cTACE. Liver function reexamination 3–5 days after TACE showed that 18 patients had deteriorated liver function. One month post-TACE, 99 patients showed a complete or partial treatment response by contrast-enhanced imaging. The baseline characteristics of patients are detailed in Table 1.
Table 1
| Characteristics | Overall (n=148) |
|---|---|
| Age (years) | 58.76±11.83 |
| Gender | |
| Male | 122 (82.4) |
| Female | 26 (17.6) |
| Tumor number | |
| 1–2 | 45 (30.4) |
| ≥3 | 103 (69.6) |
| Maximum diameter of the largest HCC lesion (cm) | |
| <5 | 42 (28.4) |
| ≥5 | 106 (71.6) |
| Scope of involvement | |
| Single lobe | 113 (76.4) |
| Double lobe | 35 (23.6) |
| PVT | |
| Absent | 80 (54.1) |
| Present | 68 (45.9) |
| Hepatic/inferior cava vein tumor thrombus | |
| Absent | 97 (65.5) |
| Present | 51 (34.5) |
| Extrahepatic metastasis | |
| Absent | 131 (88.5) |
| Present | 17 (11.5) |
| Cirrhosis | |
| Absent | 54 (36.5) |
| Present | 94 (63.5) |
| Type of TACE | |
| cTACE | 36 (24.3) |
| DEB-TACE | 112 (75.7) |
| Type of hepatitis | |
| Absent | 5 (3.4) |
| HBV | 107 (72.3) |
| HCV | 12 (8.1) |
| Alcoholic | 24 (16.2) |
| AFP (ng/mL) | |
| <400 | 85 (57.4) |
| ≥400 | 63 (42.6) |
| Alterations in liver function | |
| Stable | 130 (87.8) |
| Deteriorated | 18 (12.2) |
| Efficacy of the initial TACE | |
| Effective (CR + PR) | 99 (66.9) |
| Ineffective (SD + PD) | 49 (33.1) |
Data are presented as mean ± standard deviation or n (%). HCC, hepatocellular carcinoma; PVT, portal vein tumor thrombus; TACE, transcatheter arterial chemoembolization; cTACE, conventional TACE; DEB, drug-eluting bead; HBV, hepatitis B virus; HCV, hepatitis C virus; AFP, alpha fetoprotein; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
Cox regression analysis for PFS and OS
During the 2,760 person-month follow-up period (median 16.3 months, maximum 50.7 months), 104 patients experienced disease progression and 90 patients died. In the Cox univariate regression analysis of PFS, the maximum diameter of the largest HCC lesion, PVT, hepatic/inferior cava vein tumor thrombus, AFP level, and the efficacy of the initial TACE were factors significantly associated with PFS. In the Cox multivariate regression analysis of PFS, the presence of PVT [hazard ratio (HR) =2.178, 95% CI: 1.393–3.406; P=0.001], AFP ≥400 ng/mL (HR =1.655, 95% CI: 1.076–2.545; P=0.02), and ineffective initial TACE treatment (HR =2.185, 95% CI: 1.411–3.385; P<0.001) were independent risk factors for overall disease progression after the initial TACE. Table 2 shows the specific result of Cox univariate and multivariate regression analysis of PFS.
Table 2
| Characteristics | Cox univariate analysis | Cox multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age | 0.992 (0.976–1.008) | 0.30 | |||
| Gender | 0.17 | ||||
| Male | 1 | ||||
| Female | 0.707 (0.432–1.159) | ||||
| Tumor number | 0.32 | ||||
| 1–2 | 1 | ||||
| ≥3 | 1.263 (0.801–1.993) | ||||
| Maximum diameter of the largest HCC lesion (cm) | 0.002 | 0.95 | |||
| <5 | 1 | 1 | |||
| ≥5 | 2.057 (1.292–3.277) | 1.020 (0.575–1.809) | |||
| Scope of involvement | 0.46 | ||||
| Single lobe | 1 | ||||
| Double lobe | 1.188 (0.755–1.868) | ||||
| PVT | <0.001 | 0.001† | |||
| Absent | 1 | 1 | |||
| Present | 2.749 (1.846–4.092) | 2.178 (1.393–3.406) | |||
| Hepatic/inferior cava vein tumor thrombus | 0.007 | 0.98 | |||
| Absent | 1 | 1 | |||
| Present | 1.733 (1.161–2.588) | 1.006 (0.639–1.583) | |||
| Extrahepatic metastasis | 0.19 | ||||
| Absent | 1 | ||||
| Present | 1.481 (0.826–2.654) | ||||
| Cirrhosis | 0.85 | ||||
| Absent | 1 | ||||
| Present | 0.963 (0.641–1.445) | ||||
| Type of TACE | 0.07 | 0.30 | |||
| cTACE | 1 | 1 | |||
| DEB-TACE | 0.649 (0.404–1.042) | 0.766 (0.462–1.270) | |||
| Type of hepatitis | 0.36 | ||||
| Absent | 1 | ||||
| HBV | 5.395 (0.744–39.119) | ||||
| HCV | 5.119 (0.640–40.942) | ||||
| Alcoholic | 6.222 (0.823–47.054) | ||||
| AFP (ng/mL) | <0.001 | 0.02† | |||
| <400 | 1 | 1 | |||
| ≥400 | 2.389 (1.613–3.540) | 1.655 (1.076–2.545) | |||
| Alterations in liver function | 0.95 | ||||
| Stable | 1 | ||||
| Deteriorated | 0.980 (0.535–1.792) | ||||
| Efficacy of the initial TACE | <0.001 | <0.001† | |||
| Effective (CR + PR) | 1 | 1 | |||
| Ineffective (SD + PD) | 2.709 (1.829–4.013) | 2.185 (1.411–3.385) | |||
†, independent risk factors for PFS. PFS, progression-free survival; HCC, hepatocellular carcinoma; PVT, portal vein tumor thrombus; TACE, transcatheter arterial chemoembolization; cTACE, conventional TACE; DEB, drug-eluting beads; HBV, hepatitis B virus; HCV, hepatitis C virus; AFP, alpha fetoprotein; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; HR, hazard ratio; CI, confidence interval.
In the Cox univariate regression analysis of OS, the tumor number, maximum diameter of the largest HCC lesion, scope of involvement (single or double lobe), PVT, hepatic/inferior cava vein tumor thrombus, AFP level, and the efficacy of the initial TACE were factors significantly associated with OS. In the Cox multivariate regression analysis of OS, the presence of PVT (HR =2.228, 95% CI: 1.367–3.630; P=0.001) and ineffective initial TACE treatment (HR =1.666, 95% CI: 1.054–2.633; P=0.03) were independent risk factors for death after initial TACE. Table 3 shows the specific result of Cox univariate and multivariate regression analysis of OS.
Table 3
| Characteristics | Cox univariate analysis | Cox multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age | 0.987 (0.969–1.004) | 0.14 | |||
| Gender | 0.28 | ||||
| Male | 1 | ||||
| Female | 0.743 (0.431–1.280) | ||||
| Tumor number | 0.01 | 0.25 | |||
| 1–2 | 1 | 1 | |||
| ≥3 | 1.972 (1.175–3.310) | 1.395 (0.793–2.456) | |||
| Maximum diameter of the largest HCC lesion (cm) | <0.001 | 0.23 | |||
| <5 | 1 | 1 | |||
| ≥5 | 2.872 (1.646–5.013) | 1.480 (0.786–2.788) | |||
| Scope of involvement | 0.002 | 0.15 | |||
| Single lobe | 1 | 1 | |||
| Double lobe | 2.014 (1.298–3.126) | 1.451 (0.873–2.410) | |||
| PVT | <0.001 | 0.001† | |||
| Absent | 1 | 1 | |||
| Present | 3.088 (2.009–4.746) | 2.228 (1.367–3.630) | |||
| Hepatic/inferior cava vein tumor thrombus | 0.001 | 0.91 | |||
| Absent | 1 | 1 | |||
| Present | 2.085 (1.370–3.173) | 1.028 (0.639–1.653) | |||
| Extrahepatic metastasis | 0.28 | ||||
| Absent | 1 | ||||
| Present | 1.402 (0.761–2.584) | ||||
| Cirrhosis | 0.80 | ||||
| Absent | 1 | ||||
| Present | 1.058 (0.687–1.631) | ||||
| Type of TACE | 0.15 | ||||
| cTACE | 1 | ||||
| DEB-TACE | 0.685 (0.408–1.150) | ||||
| Type of hepatitis | 0.33 | ||||
| Absent | 1 | ||||
| HBV | 4.434 (0.614–32.001) | ||||
| HCV | 4.572 (0.561–32.275) | ||||
| Alcoholic | 5.894 (0.783–44.346) | ||||
| AFP (ng/mL) | <0.001 | 0.22 | |||
| <400 | 1 | 1 | |||
| ≥400 | 2.104 (1.388–3.189) | 1.339 (0.844–2.124) | |||
| Alterations in liver function | 0.92 | ||||
| Stable | 1 | ||||
| Deteriorated | 1.032 (0.548–1.943) | ||||
| Efficacy of the initial TACE | 0.001 | 0.03† | |||
| Effective (CR + PR) | 1 | 1 | |||
| Ineffective (SD + PD) | 2.065 (1.351–3.158) | 1.666 (1.054–2.633) | |||
†, independent risk factors for OS. OS, overall survival; HCC, hepatocellular carcinoma; PVT, portal vein tumor thrombus; TACE, transcatheter arterial chemoembolization; cTACE, conventional TACE; DEB, drug-eluting beads; HBV, hepatitis B virus; HCV, hepatitis C virus; AFP, alpha fetoprotein; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; HR, hazard ratio; CI, confidence interval.
Prediction of PFS and OS by the PLC staging systems
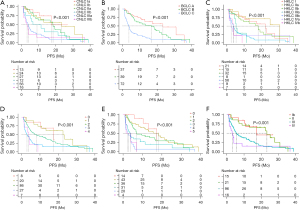
In the survival analysis of PFS, for the CNLC staging system (Figure 2A), the median PFS of stage Ia was not reached, while it was 23.4 months for stage Ib, 18.4 months for stage IIa, 6.7 months for stage IIb, 3.9 months for stage IIIa, and 3.1 months for stage IIIb. For the BCLC staging system (Figure 2B), the median PFS was 23.4 months for stage A, 16.7 months for stage B, and 3.8 months for stage C. For the HKLC staging system (Figure 2C), the median PFS was 22.6 months for stage I, 31.0 months for stage IIa, 18.3 months for stage IIb, 5.4 months for stage IIIa, 3.9 months for stage IIIb, 2.5 months for stage IVa, and 3.8 months for stage IVb. As for the mJIS staging system (Figure 2D), the median PFS for 0, 1, 2, 3, and 4 points was 17.4, 22.6, 8.1, 2.9 and 3.8 months respectively. For the mCLIP (Figure 2E) system, the median PFS was 17.4, 18.3, 7.1, 3.7, 2.3, and 1.6 months for 0 to 5 points respectively. For the TNM staging system (Figure 2F), the median PFS was 17.4, 23.4, 5.5, and 3.1 months for stages I to IV, respectively.
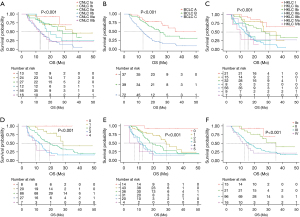
In the survival analysis of OS, for the CNLC staging system (Figure 3A), the median OS was not reached for stage I, and it was 35.5, 28.5, 17.3, 13.2, and 11.5 months for Ib, IIa, IIb, IIIa, and IIIb respectively. For the BCLC staging system (Figure 3B), the median OS was not reached for stage A, and it was 25.0 months for stage B and 13.2 months for stage C. For the HKLC staging system (Figure 3C), the median OS stage I was not reached, and it was 26.7, 27.7, 14.6, 13.2, 15.4, and 9.7 months for stage IIa, IIb, IIIa, IIIb, Iva, and IVb respectively. As for the mJIS staging system (Figure 3D), the median OS was not reached for 0 points, and it was 28.5, 19.3, 12.4, and 9.7 months for 1 to 4 points, respectively. For the mCLIP staging system (Figure 3E), the median OS was not reached for 0 points, and it was 28.5, 19.3, 12.4, 9.7, and 3.9 months for 1 to 5 points, respectively. For the TNM staging system (Figure 3F), the median OS was not reached for stage I, and it was 35.5, 16, and 11.5 months for stages II to IV, respectively.
In the assessment of ability to predict PFS, the HKLC staging system demonstrated the highest C-index of 0.693, while the CNLC and mCLIP staging system achieved a comparable C-index of 0.692 and 0.691, respectively. For the prediction of OS, the mCLIP staging system had the highest C-index of 0.711. Meanwhile, the TNM staging system showed inferior predicting efficacy, with a C-index of 0.633 and 0.632 for PFS and OS, respectively. Table 4 shows the specific C-index values and 95% CI for predicting PFS and OS for each staging system. Figure 4 presents the Error bar plot of C-index and 95% CI for each staging system, and there is no significant difference between each system in predicting PFS and OS performance as the 95% CIs of each system overlapped.
Table 4
| PLC staging system | C-index | |
|---|---|---|
| PFS (95% CI) | OS (95% CI) | |
| CNLC | 0.692 (0.6371, 0.7399) | 0.674 (0.6095, 0.7255) |
| BCLC | 0.679 (0.6247, 0.7224) | 0.667 (0.6126, 0.7145) |
| HKLC | 0.693 (0.6273, 0.7388) | 0.683 (0.6251, 0.7353) |
| mJIS | 0.642 (0.5882, 0.6926) | 0.664 (0.6110, 0.7163) |
| mCLIP | 0.691 (0.6323, 0.7361) | 0.711 (0.6555, 0.7612)† |
| TNM | 0.633 (0.5861, 0.6852) | 0.632 (0.5834, 0.6837) |
†, the highest C-index for predicting OS. C-index, concordance index; PFS, progression-free survival; OS, overall survival; PLC, primary liver cancer; CNLC, Chinese liver cancer; BCLC, Barcelona Clinic Liver Cancer; HKLC, Hong Kong Liver Cancer; mJIS, modified Japanese Integrated Staging; mCLIP, modified Cancer of the Liver Italian Program; TNM, tumor-node-metastasis.

Discussion
The efficacy of TACE in patients with HCC is influenced by multiple factors, including tumor burden, tumor vascularity, vascular invasion, extrahepatic metastasis, liver function, and AFP. Recent studies have attempted to incorporate gene-related factors, radiomics, and artificial intelligence, among other auxiliary factors, to strengthen the predictive performance (16-18,27). However, due to these innovative factors being difficult to obtain in clinical practice and lack of universality, the traditional HCC staging system remains the primary tool for predicting TACE outcomes and prognosis.
In our study, several factors, including PVT, AFP ≥400 ng/mL, and ineffective response to initial TACE treatment were identified as independent risk factors of the progression after initial TACE. Meanwhile, the presence of PVT and ineffective response to initial TACE treatment were independent risk factors for poor OS. This finding is consistent with the conclusions of the previous study (4). PVT is the most common form of macrovascular invasion and occurs in 10–60% of patients with HCC (2,12). PVT is an indicator of advanced-stage HCC and is characterized by vulnerability to metastasis, worsening liver function, higher association with portal hypertension, and refractory treatment response compared with PVT-free HCC (28). According to the Liver Cancer Study Group of Japan, the extent of PVT can be classified as Vp1 to Vp4 (29). Extensive PVT involvement, especially Vp3 and Vp4, is strongly associated with a dismal prognosis, and systemic therapy is recommended for these patients (30). For the treatment of patients with HCC with lesser degrees of PVT (i.e., Vp1 or Vp2), there are some discrepancies in the first-line therapy. Systemic therapy is the primary option for most Western guidelines, while more aggressive locoregional anticancer treatments are recommended for select patients in most Asia-Pacific guidelines (2,11,13).
Ineffective response to the initial TACE treatment is also an independent risk factor for poor PFS and OS. A retrospective analysis by Kim et al. revealed that achieving an objective response (CR + PR) after the initial TACE treatment was an independent prognostic factor associated with longer OS compared to those who achieved an objective response after two sessions of TACE or in whom the response was ineffective, with the respective survival times being 53.6, 27.0, and 10.8 months (P<0.001) (31). TACE refractoriness, a more complicated concept for assessing the efficacy of early TACE, is also a significant risk factor for poor prognosis (32). However, the mechanism and subsequent treatment of the ineffective response of TACE refractoriness remains controversial, and more studies in this area are needed to resolve this challenging issue.
Some findings in our study were inconsistent with those of previous studies (17,33). Patients with hepatitis B virus (HBV), hepatitis C virus, or alcoholic hepatitis did not exhibit a significantly higher risk of disease progression or death. This may be attributed to the emphasis on antiviral treatment in clinical practice in China. Chronic hepatitis B is an important pathogenic factor for HCC even in non-cirrhotic patients. Among the patients with HCC included in this study, 96.6% had a viral hepatitis background and 72.3% had HBV infection. HBV is a DNA virus that can integrate into the double-stranded DNA in the cell nucleus for replication (3,33). In addition, we did not find extrahepatic metastasis to be significantly associated with PFS or OS in our study. First, there were fewer patients with HCC and extrahepatic metastasis in this study (n=17, 11.5%). Second, targeted therapy, immunotherapy, and other systemic treatments have been widely applied in HCC. TACE combined with systemic therapy is currently the first-line treatment for many patients with intermediate or advanced HCC (2,34).
To the best of our knowledge, this study is the first retrospective analysis to compare the efficacy of multiple classic HCC staging systems in predicting the prognosis of patients with HCC, who received initial TACE. All HCC staging systems were effective in predicting post-TACE PFS and OS (P<0.001). Among these, the HKLC staging system demonstrated the best predictive efficacy for PFS (C-index =0.693) while the mCLIP staging system (C-index =0.711) was the most accurate system in predicting OS. The CNLC, BCLC, HKLC, and mJIS staging systems also showed comparable predictive capabilities for both PFS and OS. Although without statistically significant, the TNM staging system had the lowest prediction ability likely due to its lack of consideration of liver function. The characteristics of patients with HCC who received TACE include repeated TACE sessions, chronic inflammatory stimulation such as hepatitis, and a complex tumor microenvironment, among others (10). These factors tend to cause liver damage, potentially resulting in complications such as hepatic failure, thus hindering the effectiveness of tumor treatment and shortening life expectancy. The outcome of our study supports the important role of hepatic function in HCC.
Some limitations to our study should be mentioned. First, due to the single-center and retrospective nature of our study, potential selection bias could have influenced the accuracy of our conclusions. Second, some stratification analyses were restricted due to the limited number of patients we enrolled. Finally, a short follow-up period led to the inaccurate prediction survival times in patients with early-stage HCC.
Conclusions
In conclusion, PVT, AFP levels, and ineffective response to the initial TACE are independent risk factors for PFS, while PVT and ineffective response to the initial TACE are predictors of poor OS. The CNLC, BCLC, HKLC, mJIS, and mCLIP staging systems provide comparable predictive value for predicting post-TACE PFS and OS outcomes and can thus be used in clinical practice, whereas the TNM staging system, due to its lack of consideration for liver function, demonstrated inferior predictive ability. The future focus of HCC staging systems development should combine more intrinsic features such radiomics and information at the molecular and genetic level.
Acknowledgments
Funding: This project was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-2024-850/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-2024-850/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-2024-850/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-2024-850/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This single-institution retrospective study was approved by the Institutional Medical Ethics Committee of Hunan Provincial People’s Hospital (The First Affiliated Hospital of Hunan Normal University) (No. [2024]-168) and was performed in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was waived by the Institutional Ethics Committee due to the retrospective nature of the analysis.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Xie DY, Zhu K, Ren ZG, et al. A review of 2022 Chinese clinical guidelines on the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg Nutr 2023;12:216-28. [Crossref] [PubMed]
- Vigneron P, Franzè MS, Chalaye J, et al. Selective internal radiation therapy across Barcelona Clinic Liver Cancer (BCLC) stages of hepatocellular carcinoma: literature review. Hepatobiliary Surg Nutr 2024;13:974-90. [Crossref] [PubMed]
- Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2021;7:6. [Crossref] [PubMed]
- Krinsky G, Shanbhogue K. Proliferative versus Nonproliferative Hepatocellular Carcinoma: Clinical and Imaging Implications. Radiology 2021;300:583-5. [Crossref] [PubMed]
- Chiang DY, Villanueva A, Hoshida Y, et al. Focal gains of VEGFA and molecular classification of hepatocellular carcinoma. Cancer Res 2008;68:6779-88. [Crossref] [PubMed]
- Tsai MC, Yong CC, Lin CC, et al. Living donor liver transplantation for Barcelona clinic liver cancer (BCLC) intermediate-stage hepatocellular carcinoma. Hepatobiliary Surg Nutr 2023;12:169-82. [Crossref] [PubMed]
- Hayano K, Desai GS, Kambadakone AR, et al. Quantitative characterization of hepatocellular carcinoma and metastatic liver tumor by CT perfusion. Cancer Imaging 2013;13:512-9. [Crossref] [PubMed]
- Li HZ, Tan J, Tang T, et al. Chemoembolization Plus Microwave Ablation vs Chemoembolization Alone in Unresectable Hepatocellular Carcinoma Beyond the Milan Criteria: A Propensity Scoring Matching Study. J Hepatocell Carcinoma 2021;8:1311-22. [Crossref] [PubMed]
- Kotsifa E, Vergadis C, Vailas M, et al. Transarterial Chemoembolization for Hepatocellular Carcinoma: Why, When, How? J Pers Med 2022;12:436. [Crossref] [PubMed]
- Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol 2022;76:681-93. [Crossref] [PubMed]
- Yau T, Tang VY, Yao TJ, et al. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology 2014;146:1691-700.e3. [Crossref] [PubMed]
- Ikai I, Takayasu K, Omata M, et al. A modified Japan Integrated Stage score for prognostic assessment in patients with hepatocellular carcinoma. J Gastroenterol 2006;41:884-92. [Crossref] [PubMed]
- Takanishi DM Jr, Severino R, Wong LL. The Cancer of the Liver Italian Program (CLIP) score: validation of a new prognostic system for hepatocellular carcinoma. Hawaii Med J 2007;66:209-12. [PubMed]
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471-4.
- Xia T, Zhao B, Li B, et al. MRI-Based Radiomics and Deep Learning in Biological Characteristics and Prognosis of Hepatocellular Carcinoma: Opportunities and Challenges. J Magn Reson Imaging 2024;59:767-83. [Crossref] [PubMed]
- Bao Y, Li JX, Zhou P, et al. Identifying Proliferative Hepatocellular Carcinoma at Pretreatment CT: Implications for Therapeutic Outcomes after Transarterial Chemoembolization. Radiology 2023;308:e230457. [Crossref] [PubMed]
- Kim S, Shin J, Kim DY, et al. Radiomics on Gadoxetic Acid-Enhanced Magnetic Resonance Imaging for Prediction of Postoperative Early and Late Recurrence of Single Hepatocellular Carcinoma. Clin Cancer Res 2019;25:3847-55. [Crossref] [PubMed]
- Wu J, Sun M, Li Z, et al. Effect of urokinase-type plasminogen activator combined with clinical stage and Barcelona Clinic Liver Cancer stage on the prognosis of patients with hepatocellular carcinoma. J Gastrointest Oncol 2023;14:1434-50. [Crossref] [PubMed]
- You K, Wang J, Xu J, et al. Development and validation of a predictive scoring system for post-transarterial chemoembolization pain management in liver cancer patients. J Gastrointest Oncol 2024;15:425-34. [Crossref] [PubMed]
- Zhong BY, Jiang JQ, Sun JH, et al. Prognostic Performance of the China Liver Cancer Staging System in Hepatocellular Carcinoma Following Transarterial Chemoembolization. J Clin Transl Hepatol 2023;11:1321-8. [Crossref] [PubMed]
- Zhong BY, Ni CF, Yin GW, et al. Multicentric Assessment of the Hong Kong Liver Cancer Staging System in Chinese Patients Following Transarterial Chemoembolization. Cardiovasc Intervent Radiol 2018;41:1867-76. [Crossref] [PubMed]
- World Medical Association Declaration of Helsinki. ethical principles for medical research involving human subjects. JAMA 2013;310:2191-4. [Crossref] [PubMed]
- Son SH, Jang HS, Jo IY, et al. Significance of an increase in the Child-Pugh score after radiotherapy in patients with unresectable hepatocellular carcinoma. Radiat Oncol 2014;9:101. [Crossref] [PubMed]
- Llovet JM, Lencioni R. mRECIST for HCC: Performance and novel refinements. J Hepatol 2020;72:288-306. [Crossref] [PubMed]
- Gaba RC, Lewandowski RJ, Hickey R, et al. Transcatheter Therapy for Hepatic Malignancy: Standardization of Terminology and Reporting Criteria. J Vasc Interv Radiol 2016;27:457-73. [Crossref] [PubMed]
- Feng L, Chen Q, Huang L, et al. Radiomics features of computed tomography and magnetic resonance imaging for predicting response to transarterial chemoembolization in hepatocellular carcinoma: a meta-analysis. Front Oncol 2023;13:1194200. [Crossref] [PubMed]
- Tao ZW, Cheng BQ, Zhou T, et al. Management of hepatocellular carcinoma patients with portal vein tumor thrombosis: A narrative review. Hepatobiliary Pancreat Dis Int 2022;21:134-44. [Crossref] [PubMed]
- The general rules for the clinical and pathological study of primary liver cancer. Liver Cancer Study Group of Japan. Jpn J Surg 1989;19:98-129. [Crossref] [PubMed]
- Khan AR, Wei X, Xu X. Portal Vein Tumor Thrombosis and Hepatocellular Carcinoma - The Changing Tides. J Hepatocell Carcinoma 2021;8:1089-115. [Crossref] [PubMed]
- Kim BK, Kim SU, Kim KA, et al. Complete response at first chemoembolization is still the most robust predictor for favorable outcome in hepatocellular carcinoma. J Hepatol 2015;62:1304-10. [Crossref] [PubMed]
- Wang TC, An TZ, Li JX, et al. Development and Validation of a Predictive Model for Early Refractoriness of Transarterial Chemoembolization in Patients With Hepatocellular Carcinoma. Front Mol Biosci 2021;8:633590. [Crossref] [PubMed]
- Paterlini-Bréchot P, Saigo K, Murakami Y, et al. Hepatitis B virus-related insertional mutagenesis occurs frequently in human liver cancers and recurrently targets human telomerase gene. Oncogene 2003;22:3911-6. [Crossref] [PubMed]
- Li J, Yang B, Teng Z, et al. Efficacy and safety of first-line treatments for advanced hepatocellular carcinoma patients: a systematic review and network meta-analysis. Front Immunol 2024;15:1430196. [Crossref] [PubMed]
(English Language Editor: J. Gray)


