
Emerging role of spasmolytic polypeptide-expressing metaplasia in gastric cancer
Introduction
Despite advancements in early screening and treatment strategies, gastric cancer (GC) remains the fifth most common cancer and the third leading cause of cancer-related deaths worldwide (1). Both the genetic susceptibility and environmental influences play crucial roles in the progression of precancerous lesions and the eventual development of gastric adenocarcinoma (2,3). Environmental factors, including Helicobacter pylori infection, dietary habits, and lifestyle choices could significantly increase the risk of developing precancerous lesions and subsequent malignancy (4). Previous attention has predominantly focused on intestinal metaplasia (IM), characterized by the presence of goblet cells and the secretion of mucin-producing cells, which was considered as a well-established precursor to GC (5,6). However, increasing evidence has shed light on an underrecognized progress, spasmolytic polypeptide-expressing metaplasia (SPEM), which represents a regenerative alteration within the gastric mucosa, harboring the potential to precede IM and the development of gastric adenocarcinoma (7). The emergence of SPEM has been extensively documented as a metaplastic response to chronic gastric injury, characterized by the replacement of native gastric epithelial cells with mucous-secreting cells reminiscent of pyloric or intestinal phenotypes. Previous investigations have delineated the induction of a lineage expressing trefoil factor 2 (TFF2), spasmolytic peptide (SP) and other mucus cell proteins, correlating with gastric acid atrophy in both rodent models and the human gastric fundus glands (8). Moreover, studies involving Helicobacter pylori-infected Mongolian gerbils lend support to the early onset of SPEM under chronic infection conditions, subsequently progressing to secondary metaplastic transformation into IM (9). Together, this review supports the concept that SPEM lineages could serve as direct precursor of dysplasia and eventual GC. Herein, this review aims to provide a comprehensive overview of SPEM, focusing on its phenotype, underlying mechanisms, association with GC, diagnostic approaches, and future research directions.
Histopathological features of SPEM
SPEM represents a critical histopathological alteration observed within the gastric mucosa, holding significant implications for understanding gastric pathophysiology, particularly in the context of chronic gastritis, GC, and other related disorders. In response to continuous insults, its epithelial cells undergo migration and proliferation to repair damages. At the histological level, SPEM is characterized by the replacement of normal gastric epithelial cells, predominantly parietal (ATP4A, ATP4B, H+-K+ ATPase) and chief cells, with cells that express specific markers such as TFF2, GSII and MUC6 (10). These metaplastic cells often exhibit a distinct mucus-secreting phenotype, resembling intestinal goblet cells (MUC5AC, MUC6, MUC2), thereby altering the protective and digestive functions of the gastric mucosa (10,11). Architecturally, the transition from native gastric epithelium to SPEM is associated with glandular disarray, reduced glandular complexity, and the emergence of aberrant foveolar hyperplasia, indicative of tissue remodeling and regeneration.
The molecular mechanisms driving SPEM formation are multifaceted, encompassing a spectrum of signaling pathways and transcriptional regulators. Notably, inflammatory cytokines, including interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α), play pivotal roles in initiating and perpetuating the metaplastic transformation (12,13). These cytokines activate downstream signaling cascades, such as the NF-κB pathway, leading to the upregulation of metaplasia-associated genes like Spdef and Mist1 (14). Concurrently, alterations in Wnt/β-catenin signaling, epidermal growth factor (EGF) receptor signaling, and Notch signaling pathways contribute synergistically to the reprogramming of gastric epithelial cells towards a SPEM phenotype (15). Epigenetic modifications, including DNA methylation and histone modifications, further modulate gene expression patterns, orchestrating the intricate molecular landscape of SPEM development. In addition, in the context of acute injury healing, SPEM primarily manifests as transient regeneration and repair of the mucosal epithelial cells. This condition is reversible and benign, typically accompanied by the expression of growth factors and repair proteins associated with acute healing, such as hepatocyte growth factor (HGF) and fibroblast growth factor (FGF). In contrast, SPEM associated with progression to GC exhibits persistent pathological changes and a higher malignant potential, often accompanied by abnormal expression of precancerous markers, such as increased levels of proliferative markers like CD44, MUC1, MUC5AC, and Ki67.
Murine studies of SPEM
Among the various animal models employed to investigate SPEM, rodent models, particularly murine models, predominate due to their genetic tractability, cost-effectiveness, and physiological similarities to humans. Chemically induced models, including the non-inflammatory DMP-777 model, the L635 model under inflammatory conditions, the high-dose estrogen receptor modulator tamoxifen model, and the Helicobacter pylori chronic infection model, which have been employed to induce acute gastric injury and subsequent SPEM formation, offering insights into the reparative and regenerative processes underlying metaplastic transformation (Table 1). Among these, the DMP-777 and L635 models involve oral administration and can be costly and challenging to obtain. The high-dose tamoxifen model, administered intraperitoneally, exhibits the capability for spontaneous recovery after a brief cessation period. In contrast, the Helicobacter pylori infection model can trigger SPEM and extensive parietal cell loss after 6 months of sustained infection, eventually progressing to dysplasia or tumorigenesis after 10 months of ongoing infection. SPEM represents a precursor lineage of dysplasia in the animal model of GC caused by chronic Helicobacter pylori infection (16). Helicobacter pylori expands its niche from the antrum to the gastric corpus by promoting and exploiting epidermic changes that may lead to tumorigenesis (17). All the four models lead to the depletion of gastric fundic chief cells, replaced by TFF2 immunoreactive mucous cell lineage, displaying cellular morphology akin to the deep cells of Brunner’s glands or pyloric glands.
Table 1
| Features | Mouse model | |||
|---|---|---|---|---|
| Helicobacter pylori infection | DMP-777 treatment | L635 treatment | Tamoxifen treatment | |
| Administration | i.g. | i.g. | i.g. | i.g. or i.p. |
| Time | Months | 14 days | 3 days | 3 days |
| Drug dose | – | 350 mg/kg | 350 mg/kg | 250 mg/kg |
| SPEM | Yes | Yes | Yes | Yes |
| IM | No | No | No | No |
| Inflammatory infiltrate | Yes | No | M2 macrophage | Scant |
| Invasive glands | Yes | No | No | No |
| Reversibility | No | Yes | Yes | Yes |
| Intestinalized SPEM | Yes | No | Yes | No |
SPEM, spasmolytic polypeptide-expressing metaplasia; i.g., oral gavage; i.p., intraperitoneal injection; IM, intestinal metaplasia.
Additionally, genetically engineered mouse models (GEMMs), such as the Mist1-null mouse and the Dmp1-Cre; Rb1 flox/flox mouse, have been instrumental in elucidating the molecular pathways governing SPEM development (18). several other animal models have reported the presence of SPEM lineages, including insulin-gastrin transgenic mice (19), mice subjected to deoxycholic acid gavage (20), and rats treated with MNNG6 after gastric resection (21). AR−/− mice develop SPEM, which causes goblet cell IM and invasive fundus dysplasia. AR−/− mice represent the first mouse model in which spontaneously developed fundus SPEM progresses to IM (19). Together, these models facilitate conditional gene knockout or overexpression strategies, enabling researchers to dissect the roles of specific genes, signaling pathways, and environmental factors implicated in SPEM pathogenesis.
Hypothetical pathways of SPEM
SPEM represents a highly conserved cellular program that emerges in response to glandular injury, subsequently acquiring immunomodulatory functions within a chronic inflammatory environment (22). Experimental animal models have delineated three distinct pathways elucidating the emergence of SPEM following acute parietal cell loss: proliferation of neck mucous cells (8,23), transdifferentiation of chief cells (24-26) and the activation of basal progenitor cells (27) (Figure 1).

In the normal gastric fundic mucosa, progenitor cells situated within the glandular neck differentiate into four distinct epithelial cell types. These include zymogenic chief cells that secrete pepsinogen, parietal cells responsible for gastric acid secretion, as well as surface and neck mucous cells that facilitate mucus secretion (Figure 1). Upon encountering drug-induced acute parietal cell loss, a compensatory proliferation of neck mucous cells ensues to facilitate epithelial repair. Furthermore, gastric chief cells exhibit notable plasticity, capable of transdifferentiating into SPEM cells (28,29). However, this plasticity is contingent upon the maturity and age of chief cells, with only mature and non-senescent chief cells manifesting optimal transdifferentiation potential (30). The precise regulatory mechanisms modulating the plasticity of mature chief cells during their transdifferentiation in response to gastric injury remain an area of active investigation. The physiological context is characterized by intricate redox dynamics, with redox reactions representing foundational chemical processes within the organism (31). While the body produces a plethora of reactive oxygen species (ROS) during metabolic processes, it concurrently deploys robust defense mechanisms to mitigate oxidative damage (32). Disruptions in redox equilibrium can perturb gene transcription, cell signaling cascades, enzymatic activities, and various physiological and pathological phenomena, including cellular proliferation, differentiation, apoptosis, and necrosis (33). Emerging evidence underscores the pivotal role of redox homeostasis in facilitating cellular reprogramming processes. Specifically, following gastric injury, the reprogramming of chief cells into SPEM cells necessitates xCT activity, with xCT-mediated cysteine uptake serving as a critical determinant for chief cell plasticity and ROS detoxification (34,35).
Additionally, comprehensive investigations have elucidated the indispensable role of parietal cells in orchestrating the differentiation and maturation of diverse gastric mucosal cell lineages. Notably, the loss of mature parietal cells can precipitate disruptions in the differentiation trajectory of zymogenic lineages, culminating in heterogeneous mucosal cell remodeling patterns (36). Although extensive data suggest a potential association between parietal cell loss and IM, it is imperative to acknowledge that the induction of metaplasia subsequent to parietal cell depletion can transpire independently of acute drug-induced interventions (37). Therefore, parietal cell loss alone is insufficient to induce metaplasia, and metaplasia reprogramming requires mechanisms beyond parietal cell injury or death to collectively induce metaplasia.
Contrary to prevailing theories positing the aberrant differentiation of normal progenitor cells as the primary origin of SPEM cells, accumulating evidence suggests that basal progenitor cells within the gastric body can be activated in response to routine injuries. Furthermore, select studies have postulated that mature gastric chief cells retain latent progenitor-like characteristics, reacquiring proliferative potential within the context of mucosal injury and inflammation (38). The necessary condition for the occurrence of SPEM is the loss of parietal cells, which was previously reported to occur only in the stomach body and gastric antrum. However, recent investigations have extended our understanding by demonstrating the presence of parietal cells within the cardia. Consequently, the potential of parietal cell depletion to instigate SPEM within the cardia necessitates further empirical validation (39).
Underlying mechanisms of IM
IM denotes a physiological process wherein one differentiated tissue type is substituted by another, serving as an adaptive response within biological systems. Notably, IM emerges as a recognized precursor lesion for various gastrointestinal malignancies (40,41). In the gastrointestinal tract, metaplastic lesions predominantly comprise mucous-secreting cells, instrumental in safeguarding and facilitating repair within areas subjected to significant injury. Importantly, metaplasia often precedes the progression to low-grade dysplasia, ultimately escalating to high-grade dysplasia and malignant transformation (42). Chronic inflammation remains a pivotal etiological determinant predisposing to gastric mucosal dysplasia and subsequent tumorigenesis. Specifically, both SPEM and IM intricately associate with the development of inflammation-induced gastric tumors. The development of gastric inflammation is mediated through multifaceted signal transduction cascades, modulating the release of cytokines and chemokines. Historically, the identification of SPEM was pioneered in C57BL/6 murine models infected with Helicobacter pylori, establishing a representative paradigm for human Helicobacter pylori-induced gastric pathologies. Mechanistically, Helicobacter pylori proficiently infiltrates SPEM glands via interactions with its adhesin SabA and sLex, subsequently undergoing expansive proliferation within these domains (17). However, after SPEM recovery, a notable regression in Helicobacter pylori colonization proximally transpires (43) (Figure 2).
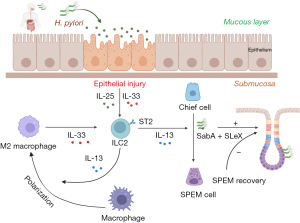
Emerging evidence underscores the pivotal roles of endocrine signaling pathways, inflammatory mediators, and epidermal growth factor receptor (EGFR) ligands in IM modulation (44). Specifically, parietal cell loss precipitates metaplasia and instigates M2 macrophage polarization. In this context, inflammatory M2 macrophages exacerbate SPEM progression amidst an inflammatory milieu. The mechanism underlying metaplasia and macrophage polarization involves the regulation of the IL-33/IL-13 cytokine signaling pathway, which is a combined response to mucosal damage and infiltrating M2 macrophages in the stomach (44) (Figure 2). Intriguingly, IL-13 exerts direct regulatory effects on chief cells via its cognate receptor, IL13RA1 (45). Subsequent studies have also found that in the context of parietal cell loss, epidermal-related cytokines IL-33 and IL-13 are essential inducers of metaplasia and may represent a key link between chronic gastritis and IM in the stomach. Therapeutic interventions targeting the IL-33/IL-13 axis may hold promise in mitigating gastrointestinal neoplastic precursors and potentially reversing gastric mucosal metaplasia evolution.
A salient contributor within the IL33/IL13 signaling pathway is the type 2 innate lymphoid cells (ILC2), inherently resident within the gastric mucosa (46). Functionally, ILC2 play essential roles in the formation of gastric mucosal immunity, tissue damage repair and remodeling, and are critical in host defense, inflammation regulation, and immune homeostasis maintenance. ILC2 facilitate SPEM induction and amplification via the secretion of IL-13 and additional cytokines and growth factors, including amphiregulin (AR) and IL-4 (47) (Figure 2). Notably, ILC2 depletion of ILC2 attenuates metaplasia induction following acute parietal cell loss and inhibit the proliferation of central lobules and zymogenic cells as well as the infiltration or activation of macrophages after injury (46). Thus, ILC2 are crucial intrinsic regulators of early responses to severe gastric injury.
The loss of gastric mucosal parietal cells is a critical step in the pathogenesis of chronic gastritis and gastric adenocarcinoma (48,49). The loss of parietal cells results in the emergence of metaplastic lineages that are prone to tumor transformation. It is known that gastric parietal cells secrete ligands for EGFR, a key regulator of gastric mucosal differentiation. Previous studies have shown that the loss of EGFR signaling in waved-2 mice accelerates the development of metaplasia following acute hypochlorhydria induction (50). Subsequently, researchers attempted to determine the role of ligands in regulating metaplasia in response to hypochlorhydria. It is known that all actions of EGFR ligands are mediated through a common EGFR protein, but individual ligands may produce different physiological responses within the body in response to various internal and external environmental stimuli. Gastric parietal cells secrete at least three EGFR ligands: These cells secrete critical EGFR ligands, including transforming growth factor-α (TGF-α), AR, and heparin-binding EGF-like growth factor (HB-EGF), governing ordered differentiation and epithelial functionality within the gastric mucosa (51). Previous studies have determined that the absence of AR accelerates the induction and amplification of SPEM following acute hypochlorhydria, and AR−/− mice spontaneously develop SPEM in the gastric fundus, subsequently leading to the production of mucous neck cell (MNC)-expressing IM and invasive fundic gland dysplasia lesions. In conclusion, these findings suggest that the emergence of SPEM is coordinated by various complex factors, and any single factor may influence the occurrence and development of SPEM.
SPEM canonical markers and diagnostic approaches
Given the intricate association between metaplasia and gastric carcinogenesis, the delineation of specific markers for metaplasia assumes paramount importance for the efficacious identification of precancerous lesions and the facilitation of early tumor detection and therapeutic interventions. Predominantly, the canonical characterization of SPEM is its distinctive morphological features resembling deep gland cells or Brunner’s glands and TFF2 expression. Recent research has initiated the identification of potential specific markers that differentiate the metaplastic process from the normal gastric lineage, including CD44v, Cftr, GKN3, among others (52-54). Specifically, CD44 emerges as a cell surface adhesion molecule ubiquitously expressed within gastric epithelial cells. Its potential implication in carcinogenesis, particularly in synergy with Helicobacter pylori, has garnered attention, with recent identifications designating CD44 as a marker for GC stem cells. The standard CD44 isoforms is mainly expressed in hematopoietic cells and normal epithelial cell subgroups, variant CD44 isoforms exhibit heightened expression within epithelial carcinomas. Notably, CD44v6 delineates a marker indicative of invasive intramucosal carcinoma and precancerous manifestations. Concurrently, CD44v9 surfaces during gastric epithelial repair post-injury, co-expressing alongside other SPEM-associated markers (55,56). Cumulative evidence underscores the affirmative correlation between CD44 and its variant isoforms with GC onset and progression, pivotal for diagnostic, therapeutic, and prognostic stratifications (57).
Furthermore, the secreted whey acidic protein (WAP) domain protein HE4 has been identified as an additional biomarker for SPEM (58). HE4 expression is absent in the normal gastric lineage but is upregulated in murine gastric lineages under conditions of acute drug induction or chronic Helicobacter pylori infection and is also expressed in human metaplastic lineage (59). Additionally, OLFM4, LYZ, and DPCR1, have surfaced as emergent markers delineating SPEM (60). The latest research was revealed that AQP5 is a novel lineage-specific marker for SPEM cells that are localized at the base of metaplastic glands initially and expand to dominate glands after chronic H felis infection. In addition, AQP5 expression was up-regulated early in chief cell reprogramming and was promoted by IL-13 (61). Thus, by combining biomarkers such as TFF2, MUC6, Ki67, CD44, MUC1, MUC5AC, p53, IL-6, VEGF, and novel SPEM lineages, a comprehensive assessment of the malignant potential of SPEM can be achieved. The abnormal expression of proliferative, precancerous markers, and tumor suppressor genes, along with the evaluation of inflammatory and microenvironmental markers, can help identify whether SPEM has the potential to progress toward GC. This multi-marker detection strategy facilitates early detection and prevention of GC. While these SPEM-specific markers have been identified, a broader range of biomarkers is still needed to enhance the specificity of metaplasia diagnosis. As the scientific landscape evolves, the establishment of an expansive biomarker panel holds promise for fostering personalized diagnostic and therapeutic modalities predicated upon individual patient biomarker profiles. Concurrently, the ongoing discovery of novel biomarkers promises to elucidate the intricate trajectory spanning the transition from a normative gastric lineage to epithelial metaplasia (encompassing both SPEM and IM) and culminating in neoplastic transformations.
SPEM therapeutic implications
Given the profound relationship between chronic inflammation, particularly Helicobacter pylori infection, and the induction of SPEM, targeted eradication of Helicobacter pylori represents a cornerstone therapeutic strategy (62). Standard eradication regimens comprising proton pump inhibitors (PPIs) and antibiotic combinations have demonstrated efficacy in mitigating Helicobacter pylori-associated gastritis (63). Both TFF2/SPEM and IM arise in the fundic units by dysregulated trans-differentiation of the zymogenic cell lineage, and some may regress after Helicobacter pylori eradication. Thus, Helicobacter pylori eradication is recommended for the improvement of these premalignant conditions of intestinal-type GC, thereby potentially attenuating SPEM progression. However, the emergence of antibiotic resistance underscores the imperative for innovative therapeutic modalities.
The elucidation of specific markers, notably CD44 and its variants, within the SPEM trajectory offers promising avenues for targeted therapeutic interventions (64). Leveraging these biomarkers facilitates the development of personalized therapeutic strategies predicated upon individual patient profiles. For instance, antagonizing IL-33/IL-13 signaling pathways, integral to SPEM pathogenesis, emerges as a prospective therapeutic paradigm, with potential implications for attenuating metaplasia progression and subsequent neoplastic transformation (44,65). Emerging pharmacological agents targeting key signaling pathways modulating SPEM progression are currently under rigorous investigation. Notably, agents inhibiting M2 macrophage polarization, pivotal within the inflammatory milieu fostering SPEM, represent a promising therapeutic avenue. Concurrently, agents targeting EGFR ligands, instrumental in parietal cell loss-induced metaplasia, hold therapeutic potential in modulating SPEM progression (65). Therefore, a combination of various strategies, including early screening, lifestyle modifications, pharmacological interventions, and raising public awareness, is necessary to effectively reduce the risk of malignant progression of SPEM.
This article provides a detailed summary of the current research progress on SPEM, including its phenotype, molecular mechanisms, role in GC development, diagnostic approaches, and future research directions, offering valuable insights for early diagnosis and intervention in GC. However, as it relies on published studies, it may not cover the latest experimental findings, potentially leading to information lag. Additionally, while acute injury models for inducing SPEM suggest that this is a reversible process, how does reversion to homeostasis occur? Is there a point at which metaplasia is irreversible?
Conclusions
Our review elucidates current understandings regarding the origin and regulatory pathways of SPEM. However, a comprehensive grasp of the intricate cellular signaling cascades and mechanisms governing SPEM remains elusive. Accumulating evidence posits that SPEM serves predominantly as a benign regenerative response to gastric injury. Insights from acute injury models underscore the reversibility of SPEM, suggesting that upon transient insults, the gastric mucosa typically reverts to its native state post-injury resolution. Most metaplasia during chronic injury processes has an adaptive mucosal response, and these metaplastic lineages can establish protective lineages in the mucosa to mitigate further damage and alleviate harmful symptoms. However, they may also evolve into more proliferative and self-renewing precancerous or dysplastic lineages, leading to cancer development. The current best diagnostic methods for SPEM in clinical practice include endoscopy, tissue biopsy, and biomarker detection. Recommended management strategies involve regular monitoring, Helicobacter pylori eradication, lifestyle modifications, and pharmacological interventions to reduce the risk of malignant transformation and improve patients’ quality of life. Therefore, future research should focus on identifying and treating gastric metaplasia with a cancerous tendency. This imperative will not only refine our understanding of SPEM pathogenesis but also inform targeted therapeutic strategies aimed at mitigating GC progression.
Acknowledgments
Funding: This work was supported in part by
Footnote
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-508/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-508/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Smyth EC, Nilsson M, Grabsch HI, et al. Gastric cancer. Lancet 2020;396:635-48. [Crossref] [PubMed]
- Jin G, Lv J, Yang M, et al. Genetic risk, incident gastric cancer, and healthy lifestyle: a meta-analysis of genome-wide association studies and prospective cohort study. Lancet Oncol 2020;21:1378-86. [Crossref] [PubMed]
- Wang R, Song S, Qin J, et al. Evolution of immune and stromal cell states and ecotypes during gastric adenocarcinoma progression. Cancer Cell 2023;41:1407-1426.e9. [Crossref] [PubMed]
- Malfertheiner P, Camargo MC, El-Omar E, et al. Helicobacter pylori infection. Nat Rev Dis Primers 2023;9:19. [Crossref] [PubMed]
- Goud HK, Mehkari Z, Mohammed L, et al. Significance of E-cadherin Gene Mutations in Patients With Hereditary Diffuse Gastric Cancer Syndrome: A Systematic Review. Cureus 2020;12:e10406. [Crossref] [PubMed]
- Correa P, Piazuelo MB, Camargo MC. The future of gastric cancer prevention. Gastric Cancer 2004;7:9-16. [Crossref] [PubMed]
- Goldenring JR. Spasmolytic polypeptide-expressing metaplasia (SPEM) cell lineages can be an origin of gastric cancer. J Pathol 2023;260:109-11. [Crossref] [PubMed]
- Liu X, Ma Z, Deng Z, et al. Role of spasmolytic polypeptide-expressing metaplasia in gastric mucosal diseases. Am J Cancer Res 2023;13:1667-81. [PubMed]
- Xiong M, Chen X, Wang H, et al. Combining transcriptomics and network pharmacology to reveal the mechanism of Zuojin capsule improving spasmolytic polypeptide-expressing metaplasia. J Ethnopharmacol 2024;318:117075. [Crossref] [PubMed]
- Zhang M, Hu S, Min M, et al. Dissecting transcriptional heterogeneity in primary gastric adenocarcinoma by single cell RNA sequencing. Gut 2021;70:464-75. [Crossref] [PubMed]
- Muthupalani S, Ge Z, Joy J, et al. Muc5ac null mice are predisposed to spontaneous gastric antro-pyloric hyperplasia and adenomas coupled with attenuated H. pylori-induced corpus mucous metaplasia. Lab Invest 2019;99:1887-905. [Crossref] [PubMed]
- Oshima M, Oshima H, Matsunaga A, et al. Hyperplastic gastric tumors with spasmolytic polypeptide-expressing metaplasia caused by tumor necrosis factor-alpha-dependent inflammation in cyclooxygenase-2/microsomal prostaglandin E synthase-1 transgenic mice. Cancer Res 2005;65:9147-51. [Crossref] [PubMed]
- Lee C, Lee H, Hwang SY, et al. IL-10 Plays a Pivotal Role in Tamoxifen-Induced Spasmolytic Polypeptide-Expressing Metaplasia in Gastric Mucosa. Gut Liver 2017;11:789-97. [Crossref] [PubMed]
- Zeng X, Yang M, Ye T, et al. Mitochondrial GRIM-19 loss in parietal cells promotes spasmolytic polypeptide-expressing metaplasia through NLR family pyrin domain-containing 3 (NLRP3)-mediated IL-33 activation via a reactive oxygen species (ROS) -NRF2- Heme oxygenase-1(HO-1)-NF-кB axis. Free Radic Biol Med 2023;202:46-61. [Crossref] [PubMed]
- Demitrack ES, Gifford GB, Keeley TM, et al. NOTCH1 and NOTCH2 regulate epithelial cell proliferation in mouse and human gastric corpus. Am J Physiol Gastrointest Liver Physiol 2017;312:G133-44. [Crossref] [PubMed]
- Jeong H, Lee B, Kim KH, et al. WFDC2 Promotes Spasmolytic Polypeptide-Expressing Metaplasia Through the Up-Regulation of IL33 in Response to Injury. Gastroenterology 2021;161:953-967.e15. [Crossref] [PubMed]
- Sáenz JB, Vargas N, Mills JC. Tropism for Spasmolytic Polypeptide-Expressing Metaplasia Allows Helicobacter pylori to Expand Its Intragastric Niche. Gastroenterology 2019;156:160-174.e7. [Crossref] [PubMed]
- Ding L, El Zaatari M, Merchant JL. Recapitulating Human Gastric Cancer Pathogenesis: Experimental Models of Gastric Cancer. Adv Exp Med Biol 2016;908:441-78. [Crossref] [PubMed]
- Nam KT, Lee HJ, Mok H, et al. Amphiregulin-deficient mice develop spasmolytic polypeptide expressing metaplasia and intestinal metaplasia. Gastroenterology 2009;136:1288-96. [Crossref] [PubMed]
- Xu X, Cheng J, Luo S, et al. Deoxycholic acid-stimulated macrophage-derived exosomes promote spasmolytic polypeptide-expressing metaplasia in the stomach. Biochem Biophys Res Commun 2020;524:649-55. [Crossref] [PubMed]
- Yamaguchi H, Goldenring JR, Kaminishi M, et al. Association of spasmolytic polypeptide-expressing metaplasia with carcinogen administration and oxyntic atrophy in rats. Lab Invest 2002;82:1045-52. [Crossref] [PubMed]
- Bockerstett KA, Lewis SA, Wolf KJ, et al. Single-cell transcriptional analyses of spasmolytic polypeptide-expressing metaplasia arising from acute drug injury and chronic inflammation in the stomach. Gut 2020;69:1027-38. [Crossref] [PubMed]
- Willet SG, Lewis MA, Miao ZF, et al. Regenerative proliferation of differentiated cells by mTORC1-dependent paligenosis. EMBO J 2018;37:e98311. [Crossref] [PubMed]
- Radyk MD, Burclaff J, Willet SG, et al. Metaplastic Cells in the Stomach Arise, Independently of Stem Cells, via Dedifferentiation or Transdifferentiation of Chief Cells. Gastroenterology 2018;154:839-843.e2. [Crossref] [PubMed]
- Caldwell B, Meyer AR, Weis JA, et al. Chief cell plasticity is the origin of metaplasia following acute injury in the stomach mucosa. Gut 2022;71:1068-77. [Crossref] [PubMed]
- Lee JH, Kim S, Han S, et al. p57(Kip2) imposes the reserve stem cell state of gastric chief cells. Cell Stem Cell 2022;29:826-839.e9. [Crossref] [PubMed]
- Hata M, Kinoshita H, Hayakawa Y, et al. GPR30-Expressing Gastric Chief Cells Do Not Dedifferentiate But Are Eliminated via PDK-Dependent Cell Competition During Development of Metaplasia. Gastroenterology 2020;158:1650-1666.e15. [Crossref] [PubMed]
- Petersen CP, Mills JC, Goldenring JR. Murine Models of Gastric Corpus Preneoplasia. Cell Mol Gastroenterol Hepatol 2016;3:11-26. [Crossref] [PubMed]
- Quante M, Marrache F, Goldenring JR, et al. TFF2 mRNA transcript expression marks a gland progenitor cell of the gastric oxyntic mucosa. Gastroenterology 2010;139:2018-2027.e2. [Crossref] [PubMed]
- Weis VG, Petersen CP, Weis JA, et al. Maturity and age influence chief cell ability to transdifferentiate into metaplasia. Am J Physiol Gastrointest Liver Physiol 2017;312:G67-76. [Crossref] [PubMed]
- Di Gennaro P, Bargna A, Sello G. Microbial enzymes for aromatic compound hydroxylation. Appl Microbiol Biotechnol 2011;90:1817-27. [Crossref] [PubMed]
- Rosa CP, Belo TCA, Santos NCM, et al. Reactive oxygen species trigger inflammasome activation after intracellular microbial interaction. Life Sci 2023;331:122076. [Crossref] [PubMed]
- Seitz R, Tümen D, Kunst C, et al. Exploring the Thioredoxin System as a Therapeutic Target in Cancer: Mechanisms and Implications. Antioxidants (Basel) 2024;13:1078. [Crossref] [PubMed]
- Meyer AR, Engevik AC, Willet SG, et al. Cystine/Glutamate Antiporter (xCT) Is Required for Chief Cell Plasticity After Gastric Injury. Cell Mol Gastroenterol Hepatol 2019;8:379-405. [Crossref] [PubMed]
- Miao ZF, Sun JX, Huang XZ, et al. Metaplastic regeneration in the mouse stomach requires a reactive oxygen species pathway. Dev Cell 2024;59:1175-1191.e7. [Crossref] [PubMed]
- Zhao Y, Deng Z, Ma Z, et al. Expression alteration and dysfunction of ion channels/transporters in the parietal cells induces gastric diffused mucosal injury. Biomed Pharmacother 2022;148:112660. [Crossref] [PubMed]
- Burclaff J, Osaki LH, Liu D, et al. Targeted Apoptosis of Parietal Cells Is Insufficient to Induce Metaplasia in Stomach. Gastroenterology 2017;152:762-766.e7. [Crossref] [PubMed]
- Burclaff J, Willet SG, Sáenz JB, et al. Proliferation and Differentiation of Gastric Mucous Neck and Chief Cells During Homeostasis and Injury-induced Metaplasia. Gastroenterology 2020;158:598-609.e5. [Crossref] [PubMed]
- Wang Z, Wang Q, Chen C, et al. NNMT enriches for AQP5(+) cancer stem cells to drive malignant progression in early gastric cardia adenocarcinoma. Gut 2023;73:63-77. [Crossref] [PubMed]
- Jencks DS, Adam JD, Borum ML, et al. Overview of Current Concepts in Gastric Intestinal Metaplasia and Gastric Cancer. Gastroenterol Hepatol (N Y) 2018;14:92-101. [PubMed]
- Que J, Garman KS, Souza RF, et al. Pathogenesis and Cells of Origin of Barrett's Esophagus. Gastroenterology 2019;157:349-364.e1. [Crossref] [PubMed]
- Giroux V, Rustgi AK. Metaplasia: tissue injury adaptation and a precursor to the dysplasia-cancer sequence. Nat Rev Cancer 2017;17:594-604. [Crossref] [PubMed]
- Goldenring JR. Pyloric metaplasia, pseudopyloric metaplasia, ulcer-associated cell lineage and spasmolytic polypeptide-expressing metaplasia: reparative lineages in the gastrointestinal mucosa. J Pathol 2018;245:132-7. [Crossref] [PubMed]
- Petersen CP, Meyer AR, De Salvo C, et al. A signalling cascade of IL-33 to IL-13 regulates metaplasia in the mouse stomach. Gut 2018;67:805-17. [Crossref] [PubMed]
- Noto CN, Hoft SG, Bockerstett KA, et al. IL13 Acts Directly on Gastric Epithelial Cells to Promote Metaplasia Development During Chronic Gastritis. Cell Mol Gastroenterol Hepatol 2022;13:623-42. [Crossref] [PubMed]
- Meyer AR, Engevik AC, Madorsky T, et al. Group 2 Innate Lymphoid Cells Coordinate Damage Response in the Stomach. Gastroenterology 2020;159:2077-2091.e8. [Crossref] [PubMed]
- Contreras-Panta EW, Lee SH, Won Y, et al. Interleukin 13 Promotes Maturation and Proliferation in Metaplastic Gastroids. Cell Mol Gastroenterol Hepatol 2024;18:101366. [Crossref] [PubMed]
- Goldenring JR, Nam KT. Oxyntic atrophy, metaplasia, and gastric cancer. Prog Mol Biol Transl Sci 2010;96:117-31. [Crossref] [PubMed]
- Goldenring JR, Nam KT, Wang TC, et al. Spasmolytic polypeptide-expressing metaplasia and intestinal metaplasia: time for reevaluation of metaplasias and the origins of gastric cancer. Gastroenterology 2010;138:2207-10, 2210.e1.
- Ogawa M, Nomura S, Varro A, et al. Altered metaplastic response of waved-2 EGF receptor mutant mice to acute oxyntic atrophy. Am J Physiol Gastrointest Liver Physiol 2006;290:G793-804. [Crossref] [PubMed]
- Nam KT, Varro A, Coffey RJ, et al. Potentiation of oxyntic atrophy-induced gastric metaplasia in amphiregulin-deficient mice. Gastroenterology 2007;132:1804-19. [Crossref] [PubMed]
- Busada JT, Ramamoorthy S, Cain DW, et al. Endogenous glucocorticoids prevent gastric metaplasia by suppressing spontaneous inflammation. J Clin Invest 2019;129:1345-58. [Crossref] [PubMed]
- Bockerstett KA, Lewis SA, Noto CN, et al. Single-Cell Transcriptional Analyses Identify Lineage-Specific Epithelial Responses to Inflammation and Metaplastic Development in the Gastric Corpus. Gastroenterology 2020;159:2116-2129.e4. [Crossref] [PubMed]
- Choi E, Hendley AM, Bailey JM, et al. Expression of Activated Ras in Gastric Chief Cells of Mice Leads to the Full Spectrum of Metaplastic Lineage Transitions. Gastroenterology 2016;150:918-30.e13. [Crossref] [PubMed]
- Teal E, Dua-Awereh M, Hirshorn ST, et al. Role of metaplasia during gastric regeneration. Am J Physiol Cell Physiol 2020;319:C947-54. [Crossref] [PubMed]
- Bertaux-Skeirik N, Wunderlich M, Teal E, et al. CD44 variant isoform 9 emerges in response to injury and contributes to the regeneration of the gastric epithelium. J Pathol 2017;242:463-75. [Crossref] [PubMed]
- Zavros Y. Initiation and Maintenance of Gastric Cancer: A Focus on CD44 Variant Isoforms and Cancer Stem Cells. Cell Mol Gastroenterol Hepatol 2017;4:55-63. [Crossref] [PubMed]
- Nozaki K, Ogawa M, Williams JA, et al. A molecular signature of gastric metaplasia arising in response to acute parietal cell loss. Gastroenterology 2008;134:511-22. [Crossref] [PubMed]
- Weis VG, Goldenring JR. Current understanding of SPEM and its standing in the preneoplastic process. Gastric Cancer 2009;12:189-97. [Crossref] [PubMed]
- Lee HJ, Nam KT, Park HS, et al. Gene expression profiling of metaplastic lineages identifies CDH17 as a prognostic marker in early stage gastric cancer. Gastroenterology 2010;139:213-25.e3. [Crossref] [PubMed]
- Lee SH, Jang B, Min J, et al. Up-regulation of Aquaporin 5 Defines Spasmolytic Polypeptide-Expressing Metaplasia and Progression to Incomplete Intestinal Metaplasia. Cell Mol Gastroenterol Hepatol 2022;13:199-217. [Crossref] [PubMed]
- Kuo HY, Chang WL, Yeh YC, et al. Spasmolytic polypeptide-expressing metaplasia associated with higher expressions of miR-21, 155, and 223 can be regressed by Helicobacter pylori eradication in the gastric cancer familial relatives. Helicobacter 2019;24:e12578. [Crossref] [PubMed]
- Tungtrongchitr N, Bongkotvirawan P, Ratana-Amornpin S, et al. Fourteen-day vonoprazan-based bismuth quadruple therapy for H. pylori eradication in an area with high clarithromycin and levofloxacin resistance: a prospective randomized study (VQ-HP trial). Sci Rep 2024;14:8986. [Crossref] [PubMed]
- Wada T, Ishimoto T, Seishima R, et al. Functional role of CD44v-xCT system in the development of spasmolytic polypeptide-expressing metaplasia. Cancer Sci 2013;104:1323-9. [Crossref] [PubMed]
- Yu S, Yang M, Lim KM, et al. Expression of LRIG1, a Negative Regulator of EGFR, Is Dynamically Altered during Different Stages of Gastric Carcinogenesis. Am J Pathol 2018;188:2912-23. [Crossref] [PubMed]


