
Colorectal metastasis from gastric cancer: insights from a 14-year case series at a tertiary hospital
Highlight box
Key findings
• Gastric cancer with colorectal metastasis showed aggressive histology, with 38.5% of the cases exhibiting concurrent peritoneal metastasis.
• The median overall survival (OS) was 11 months, and chemotherapy after colorectal surgery correlated with improved OS. R0 resection tended to be associated with improved OS.
What is known, and what is new?
• Gastric cancer with colorectal metastasis has poor survival outcomes.
• Chemotherapy after R0 resection may be associated with improved survival outcomes in gastric cancer with colorectal metastasis.
What is the implication, and what should change now?
• Active efforts directed towards achieving R0 resection and administering chemotherapy are imperative in the management of colorectal metastasis from gastric cancer.
Introduction
Gastric cancer, the world’s fifth most commonly diagnosed cancer and fourth leading cause of cancer death (1), poses a significant public health burden, particularly in East Asian countries where its prevalence is notably higher. While the most common sites for gastric cancer metastasis include the liver, peritoneum, lungs, and bones, metastasis to the gastrointestinal tract (including the colorectum) has been reported in only 3.7% of cases (2). Colorectal metastasis from gastric cancer is especially rare, and when it occurs, it can lead to severe complications such as obstruction and perforation. These complications require prompt and appropriate management, which may significantly affect patient outcomes.
Despite its clinical significance, colorectal metastasis from gastric cancer remains poorly understood owing to its rarity. The existing literature on this subject is limited to only a few case reports (3-6), resulting in a significant gap in our understanding of the clinicopathological features and prognosis associated with colorectal metastasis from gastric cancer. This paucity of comprehensive studies hampers the development of effective diagnostic and therapeutic strategies for affected patients.
To address this knowledge gap, we capitalized on the higher incidence of gastric cancer in East Asia to collect a series of relevant cases at our tertiary institution. Through detailed analysis of these cases, our study aimed to investigate the clinicopathological features and prognosis of colorectal metastasis originating from gastric cancer. We present this article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-689/rc).
Methods
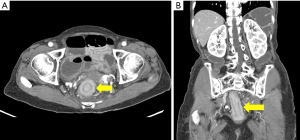
This retrospective study was approved by the Institutional Review Board of Chonnam National University Hwasun Hospital (No. CNUHH-2024-121), with informed consent waived owing to the retrospective nature of the analysis. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Patients undergoing surgery for colorectal metastasis from gastric cancer at our tertiary hospital from January 2010 to June 2023 were included. Colorectal metastasis was diagnosed based on histological confirmation and/or radiological findings. For the radiological diagnosis of colorectal metastasis, abdominopelvic computed tomography (CT) or magnetic resonance imaging (MRI) was performed. The criteria for determining colorectal metastasis through imaging studies were based on a study by Jang et al. (7), which included long segmental colonic wall thickening showing target enhancement and interval progression of wall thickening. Figure 1 shows a representative CT scan of a patient with colorectal metastasis. Preoperative endoscopic biopsies were performed whenever possible. Only cases with histopathological confirmation of gastric cancer colorectal metastasis were included in the analysis. Confirmation was obtained either through resection specimens or colonoscopic biopsy when resection was not performed. Histopathological evaluation was conducted by comparing the morphology of the metastatic colorectal lesions with the pathology of the initial gastric cancer using H&E staining, and, where applicable, immunohistochemistry for markers such as CK7, CK20, and CDX2 was performed to support the diagnosis.
Patient demographic and clinical data, including age, sex, cancer histopathology, surgery type, and metastatic site in the colorectum, were collected. Metachronous cancer was defined as cancer in another organ diagnosed 6 months or more after the initial cancer. For patients with metachronous colorectal metastasis who had previously undergone surgery for gastric cancer, we reviewed the initial surgery type, pathological findings of gastric cancer—including its tumor-node-metastasis (TNM) stage—adjuvant treatment information, and disease-free interval (DFI). The TNM classification was restaged according to the American Joint Committee on Cancer staging manual, eighth edition. The DFI was defined as the period from the initial gastric surgery to the detection of any recurrence.
Surgical intervention was primarily aimed at resection whenever feasible, with R0 resection pursued as the primary goal when complete removal was deemed achievable, even in the presence of peritoneal seeding or distant metastasis. When R0 resection was not possible, surgical intervention was still considered to alleviate symptoms such as obstruction. In such cases, palliative resection was generally preferred over diverting stoma formation. Diverting stoma was performed only when resection was deemed unfeasible or without meaningful clinical benefit. All decisions regarding surgical approach and extent were made at the discretion of the surgeon. According to the residual tumor (R) classification, resections were categorized as radical resection (R0), microscopic presence of residual tumor (R1), or macroscopic presence of residual tumor (R2).
Postoperative chemotherapy was recommended for all patients, with the decision as to whether to administer it made by medical oncologists depending on each patient’s general condition, such as their Eastern Cooperative Oncology Group (ECOG) performance status (PS). Patients with an ECOG PS of 2 or lower were generally considered for chemotherapy, while those with higher scores or poor general condition were not. Postoperative chemotherapy included platinum/fluoropyrimidine-based regimens, paclitaxel, and targeted agents such as ramucirumab. To check for disease re-recurrence or progression, the treating team conducted chest and abdominopelvic CT scans every 3 to 6 months, taking into account each patient’s disease status. Re-recurrence was defined as radiologically or histologically confirmed recurrence of cancer in patients who had undergone R0 resection for colorectal metastasis. Overall survival (OS) was calculated from the date of colorectal metastasis diagnosis to the date of death or last follow-up.
Statistical analysis
The Kaplan-Meier method and log-rank test were employed for survival analysis, with P-values smaller than 0.05 considered statistically significant. Missing data were excluded from the analysis. Statistical analyses were conducted using SPSS version 27.0 (IBM Inc., Armonk, NY, USA).
Results
A total of 13 patients with colorectal metastasis from gastric cancer were identified, whose demographic and clinicopathological data are presented in Table 1. They included 7 males (53.8%) and had a median age of 60 years (range, 39–81 years). The vast majority (n=12, 92.3%) experienced metachronous metastasis, with a median DFI of 34.5 months (range, 16–92 months), while only 1 patient (7.7%) exhibited synchronous colonic metastasis. The initial TNM staging of the gastric cancer showed that 33.3% of the patients were in stage II, 33.3% in stage III, and 25.0% in stage IV. The predominant histological type of these initial tumors was poorly differentiated adenocarcinoma (58.3%), followed by signet ring cell carcinoma (16.7%). The sites of colorectal metastasis were the right colon in 2 patients (15.4%), the left colon in 7 patients (53.8%), and the rectum in 4 (30.8%). Distant metastases other than to the colorectum were also observed, including peritoneal seeding in 5 patients (38.5%), and liver, lung, and ovarian metastases in 1 patient each.
Table 1
| Characteristics | Value |
|---|---|
| Sex | |
| Male | 7 (53.8) |
| Female | 6 (46.2) |
| Age (years) | 60 (39–81) |
| Timing of colonic metastasis | |
| Metachronous | 12 (92.3) |
| Synchronous | 1 (7.7) |
| Disease-free interval (months) | 34.5 (16–92) |
| Initial TNM stage of gastric cancer | |
| II | 4 (33.3) |
| III | 4 (33.3) |
| IV | 3 (25.0) |
| Unknown | 1 (8.3) |
| Initial pathology | |
| Adenocarcinoma, MD | 1 (8.3) |
| Adenocarcinoma, PD | 7 (58.3) |
| Signet ring cell carcinoma | 2 (16.7) |
| Unknown | 2 (16.7) |
| Chemotherapy after gastric surgery | |
| Yes | 9 (75.0) |
| No | 2 (16.7) |
| Unknown | 1 (8.3) |
| Location of colorectal metastasis | |
| Right-sided colon | 2 (15.4) |
| Left-sided colon | 7 (53.8) |
| Rectum | 4 (30.8) |
| Other site of distant metastasis | |
| Peritoneum | 5 (38.5) |
| Liver | 1 (7.7) |
| Lung | 1 (7.7) |
| Ovary | 1 (7.7) |
| Pathology of colorectal mass | |
| Adenocarcinoma, PD | 10 (76.9) |
| Signet ring cell carcinoma | 3 (23.1) |
| Radicality | |
| R0 | 5 (38.5) |
| R2 (including diverting ostomy) | 8 (61.5) |
Data are presented as number (percent) or median (range). TNM, tumor-node-metastasis; MD, moderately differentiated; PD, poorly differentiated.
Detailed information about the 13 patients is provided in Table 2. Surgical resection was performed in 10 cases (76.9%), with the remaining 23.1% undergoing diverting enterostomy. Among those with surgical resection, 50% (5/10) achieved R0 resection, while the other 50% (5/10) had R2 resection. Nine (69.2%) patients received postoperative chemotherapy (ECOG PS 1 in 5 patients, ECOG PS 2 in 4 patients). Of the 4 patients who did not receive chemotherapy, 3 had an ECOG PS of 3 or higher, and 1 died of sepsis postoperatively. The metastatic colorectal tumors were histologically identified as either poorly differentiated adenocarcinoma (76.9%) or signet ring cell carcinoma (23.1%). Table 3 presents the results of immunohistochemical staining for colorectal tumors. CK7 was positive in 81.8% (9/11), while CK20 was mostly negative, with only three cases showing focal positivity. CDX2 and c-erbB2 were positive in two cases each.
Table 2
| No. | Sex/age (year) | Gastric surgery | Initial TNM stage | Initial radicality | Histology of gastric cancer | CTx1 | DFI (mo) | Colorectal surgery | Colorectal metastatic site | Radicality | Other metastasis | Histology of colorectal metastasis | CTx2 | Regimen of CTx2 | Time to re-recurrence (mo) | Status | OS (mo) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F/81 | TG RYEJ | T4aN3bM0 | R0 | ADC, PD | Yes | 22 | D colon segmental resection | D | R0 | None | ADC, PD | No | N/A | 5 | Death | 7 |
| 2 | F/52 | TG RYEJ | T4aN0M0 | R0 | ADC, PD | Yes | 21 | Laparoscopic low anterior resection | R | R0 | Peritoneum | SRC | Yes | XELOX | 7 | Death | 21 |
| 3 | F/39 | TG RYEJ | T4aN0M0 | R0 | ADC, PD | Yes | 50 | Laparoscopic Hartmann’s op. | R | R0 | Ovary | ADC, PD | Yes | XELOX | 4 | Alive | 12 |
| 4 | F/43 | TG RYEJ | T4aN0M0 | R0 | ADC, PD | No | 34 | Laparoscopic ultra-low anterior resection | R | R0 | None | ADC, PD | Yes | FOLFOX | 70 | Alive | 158 |
| 5 | F/73 | TG RYEJ | T4aN3bM1 (cytology) | R1 | ADC, PD | Yes | 38 | Hartmann’s op. | R | R0 | None | ADC, PD | No | N/A | N/A | Death | 0 |
| 6 | M/66 | DG BII | T4aN3aM0 | R0 | ADC, MD | Yes | 35 | Hartmann’s op. | D | R2 | Liver | ADC, PD | Yes | Paclitaxel | N/A | Death | 6 |
| 7 | M/41 | TG RYEJ | T3N3aM0 | R0 | ADC, PD | Yes | 16 | CRSc with HIPEC | SF | R2 | Peritoneum | ADC, PD | Yes | Paclitaxel | N/A | Death | 4 |
| 8 | M/60 | DG BI | T4aN3aM1 (peritoneuma) | R0 | Unknown | Yes | 25 | D colon segmental resection | D | R2 | Peritoneum | ADC, PD | Yes | FOLFOX + ramucirumab | N/A | Alive | 27 |
| 9 | M/53 | Unknown | Unknown | R0 | Unknown | Unknown | 71 | Anterior resection | S | R2 | Peritoneum | ADC, PD | No | N/A | N/A | Death | 3 |
| 10 | M/72 | Completion TG | T4aN0M0 | R0 | ADC, PD | Yes | 92 | Cecostomy | SF | R2 | None | SRC | Yes | FOLFOX | N/A | Death | 19 |
| 11 | F/67 | TG RYEJ | T4aN2M0 | R0 | SRC | No | 17 | Double-barrel colostomy | HF | R2 | Peritoneum | ADC, PD | No | N/A | N/A | Death | 4 |
| 12 | M/61 | TG RYEJ with PALND | T3N1M1 (peritoneumb) | R0 | SRC | Yes | 41 | T-loop colostomy | T | R2 | Lung | ADC, PD | Yes | Paclitaxel + ramucirumab | N/A | Death | 13 |
| 13 | M/56 | N/A | N/A | N/A | N/A | N/A | N/A | Hartmann’s op. | SF | R2 | None | SRC | Yes | XELOX | N/A | Death | 11 |
a, localized peritoneal seeding at posterior wall of stomach; b, diagnosed via laparoscopic biopsy and disappeared after 7 cycles of preoperative XELOX chemotherapy; c, subtotal colectomy, distal pancreatectomy, and peritonectomy. TNM, tumor-node-metastasis; CTx1, chemotherapy after gastric surgery; DFI, disease-free interval; CTx2, chemotherapy after colorectal surgery; OS, overall survival; F, female; M, male; TG, total gastrectomy; RYEJ, Roux-en-Y esophagojejunostomy; ADC, adenocarcinoma; PD, poorly differentiated; D, descending colon; R, rectum; S, sigmoid colon; T, transverse colon; N/A, not applicable; SRC, signet ring cell carcinoma; XELOX, capecitabine + oxaliplatin; FOLFOX, FU + leucovorin + oxaliplatin; DG, distal gastrectomy; BII, Billroth II reconstruction; MD, moderately differentiated; op., operation; CRS, cytoreductive surgery; HIPEC, hyperthermic intraperitoneal chemotherapy; SF, splenic flexure colon; BI, Billroth I reconstruction; HF, hepatic flexure colon; PALND, paraaortic lymph node dissection.
Table 3
| No. | Sex/age (years) | CK7 | CK20 | CDX2 | c-erbB2 |
|---|---|---|---|---|---|
| 1 | F/81 | Not done | Not done | Not done | Not done |
| 2 | F/52 | (+) | (–) | (–) | Not done |
| 3 | F/39 | (+) | (–) | (–) | (–) |
| 4 | F/43 | (–) | (–) | (–) | (–) |
| 5 | F/73 | (+) | (–) | Not done | (–) |
| 6 | M/66 | (+) | (+): focal | (+) | (+) |
| 7 | M/41 | (+) | (–) | (–) | (+) |
| 8 | M/60 | (–) | (–) | (+) | (–) |
| 9 | M/53 | (+) | (–) | (–) | Not done |
| 10 | M/72 | (+) | (–) | (–) | (–) |
| 11 | F/67 | Not done | Not done | Not done | Not done |
| 12 | M/61 | (+) | (+): focal | (–) | Not done |
| 13 | M/56 | (+) | (+): focal | (–) | (–) |
F, female; M, male.
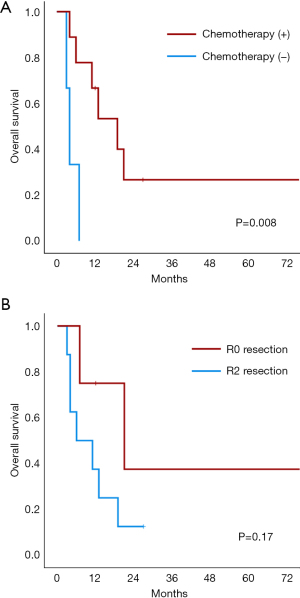
The median follow-up period was 11 months (range, 0–158 months). Of those with R0 resection (n=5), 4 (80.0%) experienced recurrence, and 1 (20%) died during postoperative hospitalization. Overall, 10 patients (76.9%) died, resulting in a 2-year OS rate of 18.5%. Survival analysis was performed on 12 patients, excluding the patient who died during postoperative hospitalization. Table 4 shows the results of the univariable analysis of factors affecting OS. The 2-year OS of the 9 patients who received chemotherapy after colorectal surgery was 26.7%, which was significantly higher than that of those who did not receive chemotherapy (0.0%, P=0.008) (Figure 2A). Although R0 resection tended to be related to OS (2-year OS, R0 37.5% vs. R2 12.5%; P=0.17), this association was not statistically significant (Figure 2B). Notably, one patient who achieved R0 resection and received postoperative chemotherapy has survived for 158 months (case 4, Table 2).
Table 4
| Characteristics | Subgroups | No. | 2-year OS (%) | P |
|---|---|---|---|---|
| Sex | Male | 7 | 14.3 | 0.39 |
| Female | 5 | 30.0 | ||
| Age (years) | <65 | 8 | 31.3 | 0.15 |
| ≥65 | 4 | 0.0 | ||
| Initial TNM stage | II, III | 8 | 16.7 | 0.43 |
| IV | 2 | 50.0 | ||
| Initial histology of gastric cancer | ADC, MD | 1 | 0.0 | 0.21 |
| ADC, PD | 6 | 22.2 | ||
| SRC | 2 | 0.0 | ||
| Chemotherapy after gastric surgery | Yes | 8 | 15.6 | 0.67 |
| No | 2 | 50.0 | ||
| DFI (months) | <36 | 7 | 28.6 | 0.38 |
| ≥36 | 5 | 0.0 | ||
| Metastatic pattern | Colon only | 5 | 30.0 | 0.37 |
| With other metastasis | 7 | 14.3 | ||
| Radicality | R0 | 4 | 37.5 | 0.17 |
| R2 | 8 | 12.5 | ||
| Chemotherapy after colorectal surgery | Yes | 9 | 26.7 | 0.008 |
| No | 3 | 0.0 |
OS, overall survival; TNM, tumor-node-metastasis; ADC, adenocarcinoma; MD, moderately differentiated; PD, poorly differentiated; SRC, signet ring cell carcinoma; DFI, disease-free interval.
Discussion
We investigated the characteristics and prognosis of gastric cancer cases with colorectal metastases. Histopathological examination predominantly revealed poorly differentiated adenocarcinoma and signet ring cell carcinoma, with 38.5% of the cases exhibiting concurrent peritoneal metastasis. The median OS was 11 months, and chemotherapy after colorectal surgery correlated with improved OS. Furthermore, R0 resection tended to be associated with improved OS.
In the present study, a total of 13 patients underwent surgical intervention for colorectal metastasis originating from gastric cancer—a relatively high number given that a recent systematic review identified only 26 patients across 24 papers (5). This discrepancy likely reflects both the higher incidence of gastric cancer in East Asia and the tendency for complex cases to be concentrated at our specialized cancer center. Over the 14-year study period, our institution has performed approximately 600 gastric cancer surgeries annually, suggesting that the incidence rate of colorectal metastasis among surgically treated gastric cancer patients could be estimated at around 0.1–0.2%. However, it should be noted that the estimated incidence rate is only an approximation, as some included patients initially underwent gastric cancer surgery at other hospitals, and this study was not designed as a prospective cohort.
Metastatic colorectal tumors present with symptoms similar to those of primary colorectal cancer, such as bowel obstruction, lower gastrointestinal bleeding, anemia, and weight loss (8). However, colonoscopy, imaging studies, and pathological examination in these metastatic cases reveal findings distinct from those typically seen in primary colon cancer. While colorectal metastases cannot be fully distinguished from primary colorectal cancer through imaging modalities such as CT and MRI alone, previous studies have identified a common characteristic: metastatic colorectal lesions from other organs typically grow beneath the gastrointestinal tract mucosa, exhibiting polypoid or linitis plastica features (6,7). These metastases appear as three-layered wall thickening (target sign) on CT scans and can also be identified on T2-weighted and diffusion-weighted imaging of the rectum (9). The presence of linitis plastica in the colorectum is particularly suggestive of a secondary malignancy rather than a primary colorectal cancer (7,9). In such cases, possible primary sources to consider include the stomach, bladder, breast, ovary, and prostate (8).
In the present study, endoscopic findings of colorectal metastasis included mucosal swelling and circumferential stenosis in 5 patients and a bulky mass in 2 patients. Although, consistently with previous case reports, mucosal ulceration was uncommon and not observed in most of our cases, ulceration and polyps can occur in some rare cases (5,6). Diagnosing submucosal lesions through endoscopic biopsy is challenging. In our study, of the 9 patients who underwent colonoscopy, only 5 were confirmed to have malignancy through colonoscopic biopsy.
Histopathological examination of colorectal metastases typically reveals poorly differentiated adenocarcinoma and signet ring cell carcinoma, both associated with a poor prognosis. We performed immunohistochemical staining to distinguish primary colorectal cancer from metastatic gastric cancer to the colorectum, and the results showed a predominance of CK7 positivity and CK20 negativity. Gastric cancer, especially the diffuse type, generally tests positive for CK7, negative for CK20, and positive for CDX2. In contrast, primary colorectal cancer typically exhibits a CK7-negative, CK20-positive, and CDX2-positive staining pattern (8,10,11). However, these staining patterns represent general tendencies rather than absolute rules, and definitive diagnosis hinges on direct comparison with the initial gastric cancer tissue.
There are several hypotheses regarding the pathways through which gastric cancer metastasizes to the colorectum. These potential routes include direct spread through blood vessels, regional lymph nodes (LNs), or peritoneal seeding. The hematogenous route involves a process wherein cancer cells undergo an epithelial-mesenchymal transition, intravasate into blood vessels, and circulate through the portal system. The frequent metastasis of gastric cancer to the liver and lungs supports the theory of hematogenous spread (2). It is believed that interactions with the host microenvironment determine which organs circulating tumor cells will extravasate into and colonize, although the specific properties causing colorectal metastasis still remain unknown (12). Similar to metastasis from other organs to the gastrointestinal tract, gastric cancer cells are theorized to metastasize to the colorectum through the bloodstream and subsequently infiltrate submucosal lymphatics (6).
Peritoneal metastasis is typical in gastric cancer, especially in signet ring cell carcinoma and diffuse-type gastric cancer (8,12-14). Considering the characteristics of concomitant metastatic lesions, gastric cancer tends to metastasize either within the peritoneum or through the bloodstream, but rarely via both routes simultaneously (2,14). Peritoneal metastasis is commonly explained as the process where gastric cancer cells penetrate the gastric serosa, survive in the peritoneal cavity, attach to peritoneal mesothelial cells, and invade the submesothelial space (13,15). The same mechanism may also apply to the colorectum, where cancer cells from the peritoneal cavity could penetrate and colonize the colorectal serosa. In the present study, 38.5% of the patients had peritoneal metastasis as well, making it challenging to clearly distinguish between hematogenous colorectal metastasis and colorectal infiltration from peritoneal seeding. Molecular markers such as E-cadherin, which is known to be associated with peritoneal dissemination, may help identify peritoneal metastasis (16); however, owing to the retrospective design of this study, such molecular analysis was not feasible.
The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) 2024, Japanese Gastric Cancer Treatment Guidelines 2021, and Korean Practice Guidelines for Gastric Cancer 2022 consistently recommend medical care over surgical resection for distant metastasis from gastric cancer, classifying it as unresectable advanced gastric cancer (17-19). These recommendations are based on clinical trials demonstrating that reduction surgery offers no survival benefit (20). However, there is still no established management protocol for colorectal metastasis from gastric cancer. Notably, achieving R0 resection during surgery might offer a survival benefit. Previous case reports have shown long-term survival after resection and chemotherapy in patients with colorectal metastasis from gastric cancer (6,21,22). In the present study, a patient (case 4) with metachronous rectal metastasis from gastric cancer achieved long-term survival of 158 months following R0 resection and chemotherapy, suggesting that R0 resection can have a meaningful impact on survival. In addition, our study found a trend toward improved OS with R0 resection, although this finding was not statistically significant.
Nevertheless, it is premature to conclude that R2 resection lacks clinical benefit. Although R2 resection is generally associated with limited survival benefits, it may still provide symptomatic relief and should not be ruled out in select cases. Even in asymptomatic patients, palliative resection may offer benefits, such as maintaining eligibility for chemotherapy and potentially reducing complications from future tumor progression (23). It is of concern that palliative resection may result in slower postoperative recovery compared to diverting stoma formation, potentially delaying the initiation of chemotherapy. However, performing only a diverting stoma has the limitation that it cannot completely prevent complications due to tumor progression. Furthermore, palliative resection can improve quality of life by avoiding stoma formation, as seen in our study, where 3 of 5 patients who underwent R2 resection did not require a stoma. Further large-scale studies are required to establish a basis for the surgical principles of colorectal metastasis from gastric cancer.
Palliative systemic chemotherapy or best supportive care is recommended for distant metastasis and recurrent metastatic disease, based on the patient’s performance score (18). Meanwhile, chemotherapy has been shown to be more beneficial than best supportive care alone for advanced gastric cancer (24,25). Our study corroborated these findings, showing improved survival rates among patients who received chemotherapy compared with those who received best supportive care alone. Chemotherapy was not administered to patients whose general condition did not recover sufficiently to tolerate the treatment, leading to rapid disease progression and death. This suggests that patients unable to receive chemotherapy likely had a poor baseline general condition. Conversely, this implies that patients in good enough condition to undergo chemotherapy could expect a relatively favorable prognosis with chemotherapy.
Our study has several limitations. First and foremost, the number of patients included in the study was very limited, which is attributable to the rarity of colorectal metastasis from gastric cancer. Additionally, patient data collection spanned a 14-year period, which was necessary to achieve even this small sample size but could introduce bias due to potential changes in surgical or medical treatments over time. Given the small sample size, comparing survival differences across chemotherapy regimens was not feasible. Nevertheless, this study holds significant value as it analyzed and reported on one of the largest cohorts of patients with colorectal metastasis from gastric cancer reported to date, despite being conducted at a single institution. Secondly, in some cases, pathological examination could not clearly distinguish between gastric cancer colorectal metastasis and primary colorectal cancer. As mentioned above, although immunohistochemical staining can help distinguish between metastatic and primary colorectal cancer, it is not always possible. However, this study analyzed only patients with gastric cancer colorectal metastasis that was clearly identified both radiologically and clinically. Thirdly, owing to the retrospective study design, information about biomarkers (e.g., human epidermal growth factor receptor 2), microsatellite instability, and Epstein-Barr virus status could not be obtained. Lastly, there was a limitation in elucidating the mechanism of colorectal metastasis, which needs further research.
Conclusions
In conclusion, colorectal metastasis from gastric cancer is a rare occurrence characterized by aggressive histology and often accompanied by metastasis to other organs, particularly peritoneal seeding. Although survival rates are generally poor for patients with this condition, R0 resection and chemotherapy may be associated with improved survival outcomes. Hence, active efforts directed towards achieving R0 resection and administering chemotherapy are imperative in the management of colorectal metastasis from gastric cancer.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-689/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-689/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-689/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-689/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Chonnam National University Hwasun Hospital (No. CNUHH-2024-121) and the requirement of individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Riihimäki M, Hemminki A, Sundquist K, et al. Metastatic spread in patients with gastric cancer. Oncotarget 2016;7:52307-16. [Crossref] [PubMed]
- Lim SW, Huh JW, Kim YJ, et al. Laparoscopic low anterior resection for hematogenous rectal metastasis from gastric adenocarcinoma: a case report. World J Surg Oncol 2011;9:148. [Crossref] [PubMed]
- Oh SY, Cunningham J, Saif MW. Colonic metastasis from gastric cancer. Clin Colorectal Cancer 2014;13:255-6. [Crossref] [PubMed]
- Fretwell VL, Kane EG, MacPherson S, et al. Metastases from gastric cancer presenting as colorectal lesions: a report of two cases and systematic review. Ann R Coll Surg Engl 2025;107:76-82. [Crossref] [PubMed]
- Kohno S, Ikegami M, Yamamoto SR, et al. A rare case of colorectal metastasis found 8 years and 10 months after gastrectomy for advanced gastric cancer: A case report and literature review. Oncol Lett 2023;25:203. [Crossref] [PubMed]
- Jang HJ, Lim HK, Kim HS, et al. Intestinal metastases from gastric adenocarcinoma: helical CT findings. J Comput Assist Tomogr 2001;25:61-7. [Crossref] [PubMed]
- Galanopoulos M, Gkeros F, Liatsos C, et al. Secondary metastatic lesions to colon and rectum. Ann Gastroenterol 2018;31:282-7. [Crossref] [PubMed]
- You JH, Song JS, Jang KY, et al. Computed tomography and magnetic resonance imaging findings of metastatic rectal linitis plastica from prostate cancer: A case report and review of literature. World J Clin Cases 2018;6:554-8. [Crossref] [PubMed]
- Park SY, Kim BH, Kim JH, et al. Panels of immunohistochemical markers help determine primary sites of metastatic adenocarcinoma. Arch Pathol Lab Med 2007;131:1561-7. [Crossref] [PubMed]
- Wong HH, Chu P. Immunohistochemical features of the gastrointestinal tract tumors. J Gastrointest Oncol 2012;3:262-84. [PubMed]
- Solomon D, DeNicola N, Feingold D, et al. Signet ring cell features with peritoneal carcinomatosis in patients undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy are associated with poor overall survival. J Surg Oncol 2019;119:758-65. [Crossref] [PubMed]
- Sun F, Feng M, Guan W. Mechanisms of peritoneal dissemination in gastric cancer. Oncol Lett 2017;14:6991-8. [Crossref] [PubMed]
- Koemans WJ, Luijten JCHBM, van der Kaaij RT, et al. The metastatic pattern of intestinal and diffuse type gastric carcinoma - A Dutch national cohort study. Cancer Epidemiol 2020;69:101846. [Crossref] [PubMed]
- Ng D, Ali A, Lee K, et al. Investigating the mechanisms of peritoneal metastasis in gastric adenocarcinoma using a novel ex vivo peritoneal explant model. Sci Rep 2022;12:11499. [Crossref] [PubMed]
- Kanda M, Kodera Y. Molecular mechanisms of peritoneal dissemination in gastric cancer. World J Gastroenterol 2016;22:6829-40. [Crossref] [PubMed]
- Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). Gastric Cancer 2023;26:1-25.
- National Comprehensive Cancer Network (2024) Gastric cancer (version 1.2024). Available online: https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf. Accessed May 20, 2024.
- Kim TH, Kim IH, Kang SJ, et al. Korean Practice Guidelines for Gastric Cancer 2022: An Evidence-based, Multidisciplinary Approach. J Gastric Cancer 2023;23:3-106. [Crossref] [PubMed]
- Fujitani K, Yang HK, Mizusawa J, et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol 2016;17:309-18. [Crossref] [PubMed]
- Noji T, Yamamura Y, Muto J, et al. Surgical resection of colorectal recurrence of gastric cancer more than 5 years after primary resection. Int J Surg Case Rep 2014;5:954-7. [Crossref] [PubMed]
- Wang Y, Ma P, Liu K, et al. Gastric cancer with repeated metastasis in the colonic lumen: a case report and multi-surgical experience. J Int Med Res 2021;49:3000605211018420. [Crossref] [PubMed]
- Kim CW, Baek JH, Choi GS, et al. The role of primary tumor resection in colorectal cancer patients with asymptomatic, synchronous unresectable metastasis: Study protocol for a randomized controlled trial. Trials 2016;17:34. [Crossref] [PubMed]
- Glimelius B, Ekström K, Hoffman K, et al. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol 1997;8:163-8. [Crossref] [PubMed]
- Kang JH, Lee SI, Lim DH, et al. Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol 2012;30:1513-8. [Crossref] [PubMed]


