
Colorectal cancer: local results and significance in Hungary
Highlight box
Key findings
• The morbidity and mortality of colorectal cancer (CRC) in Hungary are among the highest in the world.
• The adenoma detection rate (ADR) in this subsect of Hungarian population is extremely high at 57.1%, similar to a study completed in Csongrad County, representing the paucity of CRC screening.
• To reach maximum cost-effectiveness, the participation rate of the 2-step screening program must be >60% of the population with >80% of fecal immunochemical test (FIT) positive test results being referred to colonoscopy.
• Implementation of a population-based CRC screening program is likely beneficial in every considerable aspect, through population outreach via mailed FIT.
What is known and what is new?
• The burden of CRC is well-characterized worldwide. Hungary has one of the highest healthcare burdens of CRC in the world.
• This manuscript argues in favor of implementation of a mandatory population-based CRC screening program in Hungary. It illustrates some of the eye-opening realities of the CRC disease burden in Hungary. 2-step screening would be preferable in comparison to 1-step screening colonoscopy given the population health illiteracy and limited capacity of the country to complete colonoscopy.
What is the implication, and what should change now?
• This manuscript implies that the implementation of a nation-wide CRC screening program with the 2-step screening program will benefit Hungary in every considerable aspect.
• The government of Hungary should make CRC screening mandatory. Health consciousness should be stressed and participation strongly encouraged for a macroscopic benefit.
Introduction
Background
According to GLOBOCAN 2020 data, colorectal cancer (CRC), among both sexes, was the third leading age-standardized incident cancer in the world. CRC also was the fourth leading cause of cancer-related mortality standardized both to age and sex. Over time, the incidence of CRC has been steadily increasing, particularly in the developing nations adopting a westernized diet and culture (1). Hungary has been shown to have a strikingly high incidence and mortality for CRC, with an incidence of 45.3 per 100,000 and a mortality of 20.2 per 100,000, compared to the international average of 19.5 per 100,000 and 7 per 100,000, respectively.
Rationale and knowledge gap
The high incidence and mortality of CRC in Hungary is a trend that can be readily reversed through both primary and secondary interventions. In most cases, it is known to arise from benign neoplasms which can transform into malignant cancer over the period of many years. This slow cancer progression provides an opportunity to both detect and remove these precursor lesions through screening before undergoing malignant transformation. For these reasons, among others, CRC is one of the most preventable cancers through regular screening. In 2019, Hungary implemented a nationwide 2-step screening program voluntary for both general practitioners and patients to reduce the high mortality rate and raise awareness for CRC.
Objective
I decided to summarize results of the colonoscopies from the 2-step screening program. Additionally, I provide a brief literature review of CRC etiopathogenesis, management, and screening guidelines. I compared the results with that of established benchmark criteria in anticipation that the detection rate and types/localizations of adenomas will be significantly different than expected, due to the population being under screened. This article was presented in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-318/rc).
Gross anatomy of the colon
The colon is a hollow muscular tube that is on average 150 centimeters long (2). It is composed of 6 main parts: the appendix, cecum, ascending colon, transverse colon, descending colon, and the sigmoid colon (Figure 1). In that order, the diameter of the lumen decreases from as much as 7.5 cm in the cecum to 2.5 cm in the sigmoid. The exterior is typified by 3 bands of muscle named the taenia coli (anterior, posteromedial, posterolateral) formed from the fusion of the inner circular and outer longitudinal layers of the colon and the haustra, which are outpouchings of the colonic wall between the taeniae (2). Each haustrum is separated by the so-called plicae semilunaris, which are semicircular folds of the colon protruding into the lumen. The posteromedial taenia, also known as the taenia mesocolica, connects the colon to the mesocolon; the posterolateral taenia, known as the taenia omentalis, connects the colon to the omentum. The anterior taenia has no attachments; therefore, it is known as the taenia libra.

Gross anatomy of the rectum
The rectum is bounded superiorly by the rectosigmoid junction and inferiorly by the dentate line which is the anatomical demarcation of the rectum from the anus. The rectum ranges from 12 to 15 cm in length (3). It contains 2 antero-posterior flexures: a concavity at the level of the sacrum called the sacral flexure and a convexity named the anorectal flexure more distally. There are 3 lateral flexures, also known as the valves of Houston. They are created by submucosal folds that protrude into the lumen. The most distal portion of the rectum forms the ampulla, which is a dilated segment that rests on the pelvic diaphragm (Figure 2). The ampulla acts as a temporary storage for feces before defecation.

General layers of the colon
The colon possesses all of the rudimentary layers of the gastrointestinal (GI) tract. The layers are mostly uniform throughout its length. The layers from the colonic lumen to the outer surface include the mucosa, the submucosa, the muscularis propria, and the serosa (Figure 3). The mucosa is subdivided into the epithelium, composed of enterocytes and goblet cells, the lamina propria, which contains loose connective tissue and vessels, and the muscularis mucosae, which divides the epithelium from the submucosa (4). The submucosa that contains part of the enteric nervous system is called the submucosal plexus, also known as “Meissner’s plexus”. The muscularis propria—consisting of inner circular and outer longitudinal muscle layers—contains Auerbach’s plexus, also known as the myenteric plexus (4). The outermost layer contains the serosa and/or subserosa or adventitia, depending on the location of the colon.

Arterial supply of the colon and rectum
The arterial supply of the colon arises from the superior mesenteric artery and the inferior mesenteric artery. As a general rule, the superior mesenteric artery supplies the colon up to the proximal 2/3 of the transverse colon (Figure 4A). The inferior mesenteric artery supplies the distal third of the transverse colon up to the proximal third of the rectum and anastomoses with the superior mesenteric artery forming the arc of Riolan (Figure 4B). The rectum is supplied by the inferior mesenteric artery for the proximal third, and the middle and inferior rectal arteries originating from the internal iliac artery and internal pudendal artery, respectively (Picture: “Die Arteria mesenterica superior und ihre Äste” by Henry Gray is licensed under Public Domain).

Venous and lymphatic drainage of the colon and rectum
The venous drainage of the colon parallels the arteries that supplied the embryological derivatives of the foregut, midgut, and hindgut (the celiac, superior mesenteric, and inferior mesenteric arteries) (5). The veins are named accordingly (Figure 5). The superior mesenteric vein drains the third part of the duodenum up to the proximal 2/3 of the transverse colon. The inferior mesenteric vein drains the remaining part of the transverse colon up to the proximal part of the rectum. These veins eventually merge with the splenic, gastric, and cystic veins to form the portal vein (5). The portal vein then enters the liver. Therefore, most of the metastases of colon cancer involve the liver. The distal 2/3 of the rectum drain into the systemic venous system, so metastases are more likely to occur in the lungs than in the liver.

The colon has a “4-tier system” of lymphatic drainage (Figure 6A,6B). It consists of the epicolic nodes on the colonic wall, the paracolic and intermediate nodes on the arcade of Drummond and arterial trunks, and the central nodes located on the root of the arterial trunk (6). The lymph drains in that order, into the cisterna chyli and up into the thoracic duct. The lymph ultimately drains into the venous system at the confluence of the subclavian and internal jugular veins.

CRC
CRC is a malignant neoplasm that is derived from the epithelial cells of the large intestine or rectum (1). Although both types of cancer can be defined as separate entities, they are combined frequently because of the similarities in clinical and biological features (7). CRC was a relatively rare cancer in the 1950s, but it has become an increasingly common malignancy in Western countries; now attributable to about 10% of the cases of cancer-related mortality (8). Some of the factors that sustained this increase include decreased levels of physical activity, excess of nutrition, poor diet, and smoking. Projections for the year of 2040 according to CANCER TOMORROW on the Global Cancer Observatory estimate an increase in number of new cases of about 67%, with an increase in number of deaths of about 76%. The situation in Hungary is dismal, where the incidence and mortality of CRC are among the highest in the world. During the past 30 years, the mortality of CRC has increased in Hungary compared to the average in the European Union (EU) (9).
Epidemiology of CRC
CRC is the second most common cancer in men and the third most common cancer in women. In both sexes together, CRC is the third most common malignancy and accounts for around 10% of all malignancies that do not include non-melanoma skin cancer (10). However, CRC is not distributed equally around the world (11). The incidence of CRC varies with geography, and it is correlated positively with the Human Development Index (HDI), age-specific incidence rate (ASIR), and age-specific mortality rate (ASMR) (12). The HDI is defined as the mean of the 3 indices of a long and healthy life, knowledge, and the standard of living. It was created to underline the principle that the population and their human capabilities should be the foremost criteria for assessing the development of a country. Developed countries are at the highest risk for CRC. Colon cancer incidence is highest in Northern Europe, Australia/New Zealand, and Southern Europe. Rectal cancer incidence is highest in the regions of Eastern Europe, Eastern Asia, and also in Australia/New Zealand (1). Additionally, North America is among the continents with the highest incidences for both forms of cancer. Not only does the incidence of CRC vary as much as 8-fold between different countries; within a nation, the variation can be as much as 3-fold, as seen in the United States (US) between Alaska relative to the Southwest region (1). The age-standardized incidence of CRC per 100,000 population was the highest in Hungary among all countries for both 2018 and 2020. According to GLOBOCAN 2018 data, CRC held the second top spot for mortality worldwide. Colon cancer was in the top 5 most deadly cancers, whereas rectal cancer ranked within the top 10. The mortality from CRC differs with the developmental status of a nation, as it does for incidence, albeit to a lower degree (1); it still maintains a positive correlation. The country with the highest mortality rate among nations in 2018 was Hungary, with a rate of about 31.2 per 100,000 among males and 14.8 per 100,000 among females.
Pathogenesis of CRC
The hallmark feature of CRC pathogenesis is the presence of epigenetic and genetic alterations that transform glandular epithelial cells into invasive adenocarcinomas (13). The sequence of transformation from polyps to carcinomas was first proposed in a model by Fearson and Vogelstein, termed the “Seminal and Classic Tumor Progression Model” in September of 1988. It consists of 3 steps that progress chronologically. The first step is the formation of a benign neoplasm (adenomas and sessile serrated polyps), followed by the second step that forms a more histologically advanced neoplasm, and concluding with the third and final step that transforms the tumor into an invasive adenocarcinoma (Figure 7) (14). The latency period for the formation of invasive cancer from an adenomatous polyp is around 7–10 years (15). Many studies have shown that the polyp to CRC progression is highly heterogenous (16-20). Therefore, throughout the years, many modifications have been made to the model as a result of the availability of new information regarding the molecular pathogenesis of CRC. For example, it was once thought that the sole precursor lesions to CRC were tubular and tubulovillous adenomas. Now, it is generally accepted that sessile serrated adenomas and traditional serrated adenomas also have potential to undergo carcinogenesis (21).

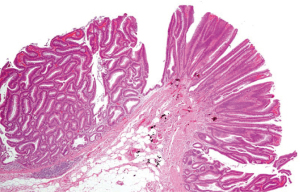
The several types of genetic/epigenetic aberrations that have been implicated in CRC pathogenesis, including: (I) microsatellite instability (MSI); (II) chromosome instability; (III) DNA methylation abnormalities; (IV) non-MSI hypermutability; and (V) global DNA hypomethylation (13). Certain classifications of precursor lesions have also been shown to arise from specific molecular phenotypes; CpG island methylator phenotype (CIMP) is correlated with serrated polyps, whereas conventional tubular adenomas are more frequently associated with inactivation of the adenomatous polyposis coli (APC) tumor suppressor gene and chromosome instability (22). Histological subtypes of adenomas include tubular (Figure 8), villous (Figure 9), and tubulovillous (Figure 10) subtypes (villous adenomas harbor the most malignant potential, followed by tubulovillous adenomas and then tubular adenomas). They gradually show dysplasia, which makes them distinct from hyperplastic polyps (23). However, serrated polyps are potentially an alternative path to malignancy whereby a classification of hyperplastic polyps called microvesicular hyperplastic polyps continue to become serrated neoplasms (24). Chromosomal instability, defined as a numerical alteration in the chromosomes or structural alterations in the chromosomes, can be found in as many as 85% of polyps (13). MSI is found almost always in serrated polyps, with the exception of tubular adenomas in Lynch syndrome (25). MSI is understood well and is due to inactivation of genes in the mismatch repair pathway (26). Indirect data also suggests that MSI can accelerate the adenoma to carcinoma sequence: CRC can arise in as little as 2–3 years from a polyp with MSI (14). The epigenetic instability in CRC is manifested through DNA hypermethylation or global DNA hypomethylation. The methylation of DNA takes place at loci designated as CpG islands, areas of DNA where the base sequence contains repeats of cytosine and guanine (27). The mechanisms that can give rise to CIMP are under investigation, though there is a high probability that they are very heterogenous. The clinical utility of CIMP is burdened by the differing criteria for determining CIMP; however, retrospective studies implicate that CIMP could be a prognostic and predictive marker for CRC (28).


Modifiable risk factors for CRC
Numerous modifiable risk factors have been implicated in the pathogenesis of CRC. These risk factors are predominantly lifestyle-related, and proper educational steps and lifestyle interventions can be taken to reduce the risk of development of CRC.
- Obesity: obesity and a sedentary lifestyle are correlated positively with risk for development of CRC. Obesity is thought to increase the risk of CRC due to the effects on metabolic processes and the disruption of the normal microbial flora of the GI tract. Adipose tissue, which is in higher excess in obesity, stores excess energy and is capable of synthesis of a number of metabolically active compounds that can impact homeostasis (29). Adipose tissue releases inflammatory cytokines that can predispose to carcinogenesis. Excess bodyweight can predispose to malignancy through metabolic derangement and generation of mutagenic free radicals (30). A pooled study of 13 cohort studies demonstrated that a 5 kg gain of weight was associated with a 3% increase in CRC risk (31).
- Smoking: cigarette smoke contains around 60 different carcinogens (32). Smoking is known to increase the risk of various different cancers, including CRC. A meta-analysis of smoking and the risk of CRC published in the American Journal of Gastroenterology analyzed 188 studies found that the relative risk (RR) for CRC of smokers compared to nonsmokers was 1.14 [95% confidence interval (CI): 1.10–1.18], whereas the RR for former smokers compared to nonsmokers was 1.17 [95% CI: 1.15–1.20] (33).
- Diet: diet is known to impact the intestinal microbiome. In particular, bile acids and protein-laden foods may promote the formation of carcinogenic metabolites and pro-inflammatory molecules (34). A large meta-analysis of 60 studies has shown that red meats (RR =1.12; 95% CI: 1.03–1.21) and processed meats (RR =1.15; 95% CI: 1.07–1.24) increase the risk of CRC (35). Fiber found in different foods (vegetables, fruits, etc.) has been shown to decrease the transit times for stool, and subsequently, exposure to carcinogens (36).
- Alcohol: alcohol consumption has been shown to increase the risk of developing CRC, if taken in moderate or severe amounts (37). The RR for drinking alcohol in moderate amounts was 1.12 (95% CI: 1.13–1.28) and 1.52 for severe amounts (95% CI: 1.27–1.81) (37).
- Medications: prolonged periods of use of non-steroidal anti-inflammatory drugs (NSAIDs) such as aspirin has been associated with a decrease in risk for CRC. The risk decrease has not been quantified, however the recommendations from the US Preventive Service Task Force include low-dose aspirin for individuals with a heightened risk of cardiovascular disease or CRC (38).
- v Diabetes or insulin resistance: the incidence of type II diabetes and obesity continue to increase in the developed world. This has led to extensive speculation that this could be one of the causes of the steady increase in incidence of CRC throughout the years. A meta-analysis of 29 prospective cohort studies conducted in China showed a 27% increase in risk of CRC in those with diabetes (39).
Nonmodifiable risk factors for CRC
Nonmodifiable risk factors for CRC cannot be changed through lifestyle interventions, although knowledge of these risk factors may identify the so-called “at risk population” and impact screening intervals or medical care.
- Race and ethnicity: racial and ethnic differences in the incidence, mortality, and stage of CRC diagnosis were presented in the Surveillance, Epidemiology, and End Results (SEER) program data (40). Incidence and mortality adjusted for age were highest for African-Americans (41). According to the SEER program, since the 1980s, the rates of CRC for African Americans and Whites have diverged (42). These racial differences are hypothesized to be a result of differences in access to healthcare and other socioeconomic factors (43). As a result, the US Preventative Services Task Force (USPSTF) guidelines for screening for CRC differ in African Americans and Whites.
- Age: SEER data shows that in the US, those over the age of 65 years have an increased risk of about 3 times for CRC compared to those aged 50–64 years, and about 10 times more than this risk than for those aged 25–49 years.
- Sex: GLOBOCAN 2018 data shows that individuals of the male gender have a 1.5 times higher risk of CRC than females when adjusting for confounders.
- Inflammatory bowel disease: according to a met-analysis of 10 studies, CRC accounts for 10–15% of the mortality in ulcerative colitis (UC) and Crohn’s disease (44). The chronic inflammation of the intestinal mucosa is a clear etiologic factor. A cohort study of about 9,400 patients with childhood-onset inflammatory bowel disease (IBD) showed that patients with UC were at higher risk [hazard ratio (HR) =33.3; 95% CI: 23.1–49.1] than those with Crohn’s disease (HR =5.8; 95% CI: 3.2–10.4) (45).
- Hereditary causes: approximately 1 in 20 CRC cases can be attributed to inherited genetic aberrations (46). The inherited diseases that predispose to CRC are widely categorized among the presence of adenomatous polyps, hamartomatous polyps, or the absence of polyps (47). The most common cause of CRC associated with an inherited cancer syndrome is Lynch syndrome [hereditary nonpolyposis colorectal cancer syndrome (HNPCC)]. It is inherited in an autosomal dominant fashion and accounts for around 2–4% of CRC (47). Consistent with the name, CRC arises in the absence of polyposis. In contrast, familial adenomatous polyposis (FAP), also inherited in an autosomal dominant fashion, arises from marked polyposis. It has a higher penetrance (100% if left untreated) than HNPCC, and its incidence is around 1 in 8,000 (48). It accounts for less than 1% of CRC cases (47), and is the second most common cause of inherited CRC. The hamartomatous polyp syndromes (Peutz-Jegher’s, juvenile polyposis, Cowden syndrome, etc.) listed occur at a very low incidence rate of 1 in 100,000 (48).
- Other nonmodifiable risk factors for CRC include family history, radiation to the abdomen, cystic fibrosis, androgen deprivation therapy, and cholecystectomy (49).
Staging and prognosis of CRC
The most widely used method for staging CRC is the tumor, node, metastasis (TNM) method, primarily used for solid tumors. In general, the T for “tumor” reflects the size of the cancer and its invasion into the abutting tissue, the N for “node” is used to reflect the lymph node involvement in and around the tumor, whereas the M for “metastasis” reflects the spread beyond the lymph nodes (50). In CRC, T ranges from T1 to T4; T1 indicating invasion into the submucosa, T2 the invasion of the muscularis layer, T3 subserosal invasion, and T4 the invasion through all of the colonic layers into nearby structures. N ranges from N1 to N3, differing by the degree and sheer number of lymph node involvement (N1 is 1–3 lymph nodes, N2 4–6 lymph nodes, and N3 7+ lymph nodes). M is subclassified M1a, M1b, and M1c according to the distinct areas involved: M1a representing 1 area, M2b representing 2 areas, and M1c representing involvement of the peritoneal surface (50). The TNM stage can be stratified into stage groups which largely reflect the prognosis of CRC and influences its treatment. The Union for International Cancer Control (UICC) defined stage groups based upon the TNM staging of CRC, with higher stages reflecting a decreased 5-year survival rate. The UICC stages include stage I, stage II, stage III, and stage IV. Stages may be subdivided based upon the combination of TNM, which will not be elaborated upon to maintain simplicity. The American Join Committee on Cancer (AJCC) updates the stage groups and TNM staging periodically.
Management of CRC
The management of CRC depends upon the stage of the disease upon diagnosis. Distinguishing sigmoid from rectal cancer can be challenging. An accepted approach for distinguishing between the two would be to analyze the aggregate data on local recurrences—cancers located 12 cm proximal to the anal verge should be classified as colon cancers because their recurrence rates are consistent with those of more proximal colon cancers (51). Meanwhile, cancers within 12 cm of the anal verge should be classified as rectal cancers due to a similar logic (51). Therefore, it is commonplace to define the length of the colon by proctoscopy in that the colon length begins 13 cm from the anal verge and the rectal length extends 12 cm from the anal verge (52).
- Colon cancer management: generally, for early-stage tumors defined as UICC stage I, a hemicolectomy with lymph node dissection and no additional treatment is advised (53). Those with low-risk tumors (T1 and low grade) can be treated locally such as with endoscopic mucosal resection or laparoscopic segmental resection. Those with nodal involvement or involvement of the serosa are recommended to undergo adjuvant treatment. With UICC stage II tumors, a study has shown that there is a small gain in 5-year survival (2% to 3%) with adjuvant chemotherapy of 5-fluorouracil (5-FU) or capecitabine (54). Tumors with defective mismatch repair are not recommended for adjuvant therapy (55). Patients with involvement of lymph nodes, or UICC stage III, benefit from adjuvant treatment with 5-FU, which increases 5-year survival by about 10–15%. The standard regiments for UICC stage III are capecitabine plus oxaliplatin (56) or FOLFOX (folinic acid, 5-FU, oxaliplatin) (57). For UICC stage IV (unresectable, metastasis), the FOLFOX protocol was shown to increase overall survival (OS) to a median of 20 months (58). The addition of biologicals such as bevacizumab [anti-vascular endothelial growth factor (VEGF) monoclonal antibody] or cetuximab/panitumumab [anti-epidermal growth factor receptor (EGFR) monoclonal antibodies] to those regimens increased median OS by about 4 months (59-61). Second-line treatment and later-line treatment involving pharmacotherapy with the addition of aflibercept, or new compounds interfering with thymidylate synthesis have shown promising results (53).
- Rectal cancer management: rectal cancer accounts for about a third of all CRC (53). Due to the more complex anatomy and potential for recurrence, management decisions for rectal cancer are more complex (62). The stage and the location of the cancer determine the subsequent management. Rectal cancer can be classified into distinct groups based on identifiable features that include early or advanced stage, low or high location (62). Generally, stage I is the only stage where surgery alone is indicated (T1 or T2 and N0). The rest of the stages are deemed as advanced stages, where treatment with multiple different modalities is considered standard of care.
- Early-stage low rectal cancer (T1 or T2 and N0): surgery alone is standard therapy. The circumferential resection margin (CRM) should be negative for tumor growth for any disease confined to the rectal wall (62). Total mesorectal excision (TME) and low anterior resection with anastomosis with or without temporary diversion yields excellent outcomes. Unfortunately, if at the low sphincter level, complete resection requires resection of the perineum and consequently an end colostomy to ensure a negative margin.
- Early-stage high rectal cancer: for this category, a 1 cm resection margin length plus mesorectal clearance hold equivalent importance. Complete TME should be performed with a clear CRM. If the pathology of the specimen reveals a more advanced disease, the surgery must ensure the minimization of local recurrence followed by adjuvant therapy (62).
- Late-stage low rectal cancer: late-stage low rectal cancer is treated with neoadjuvant radiochemotherapy followed by radical resection. Diverting colostomy is a better option than resection when faced with positive CRM, lymph node, or sphincter involvement (62). There is ongoing research in those that have complete response to neoadjuvant therapy where “watchful waiting” is taking precedence (62).
- Late-stage high rectal cancer: for late-stage high rectal cancer with positive CRM or distal resection margin (DRM), surgery is delayed for radiochemotherapy or chemotherapy. There is potential research that should be undertaken upon the use of the different induction therapies (radiochemotherapy, chemotherapy, or radiation therapy) before surgical resection. As of now, the decision rests on the surgeon and the assessment of the patient and the risks/benefits of the different modalities (62).
- Surgical management of colon cancer: the surgical management of right-sided lesions differs from that of left-sided lesions. Although debatable, it is generally recommended that a minimum of 5 or 10 cm on either side of the colon be removed (63). This is because the anastomoses between the colon parts are more susceptible to leakage (63). Generally, obstructing lesions of the ascending colon are managed by resection and anastomosis, with additional removal of the proximal transverse colon (64). Complete mesocolic excision (CME) with central vein ligation with regard to right-sided colon cancer is analogous to TME in rectal cancer (65). The proper management of left-sided lesions remains controversial. In the past, left-sided tumor management consisted of 3 separate operations: the first surgery was a decompressing surgery, the second involved resection of the lesion, and the third restored continuity in the bowel by internal anastomosis (63). However, this resulted in an unacceptably high mortality (66). The Hartmann’s procedure is a “2-stage procedure” involving segmental resection of the bowel and subsequent closure of the distal stump. Alternatively, a stoma can be created followed by a restoration of intestinal integrity. The Hartman procedure has acceptable morbidity and mortality for those unfit to undergo primary anastomosis (63). A subtotal colectomy with ileosigmoid or ileorectal anastomosis is championed by a sizeable number of practitioners due to no stoma formation, shorter postoperative stay, and definitive treatment of unnoticed preexisting lesions (63). The addition of on-table lavage has been shown to remedy the previously feared complications of anatomic dehiscence as a result of the dilated, edematous, unprepared bowel in segmental resection with primary anastomosis (67). Depending on several factors, metastasectomy can potentially be performed for liver or lung metastases (65).
Screening of CRC
Most cases of CRC arise from adenomatous or serrated polyps. Worldwide, CRC is a significant cause of morbidity and mortality. This is unfortunate because CRC is a disease that can be screened relatively easily, as the precursor lesion is readily detectable by various different methods, and prompt intervention can significantly improve prognosis. According to many sources, the latency for transformation from a polyp into CRC is about 10 years. The screening for CRC for a population can take place in 2 different ways: opportunistic or organized ways. The opportunistic screening for CRC is when a health professional is asked by the patient to screen for the disease. The organized way is when a screening program is instituted nationally or regionally. The most widely used screening modalities include, but are not limited to: (I) stool-based tests such as guaiac-based fecal occult blood test (gFOBT), fecal immunochemical test (FIT), and multitarget stool DNA testing (Cologard©); (II) direct visualization of the colon through invasive tests such as colonoscopy and sigmoidoscopy; (III) radiologic examinations such as computed tomographic colonography, double-contrast barium enema, and capsule endoscopy (68).
Stool-based tests
The 3 noninvasive stool-based tests for CRC screening are gFOBT, FIT, and multitarget stool DNA testing. These tests detect blood that is shed by vascularized lesions, which could include cancer, polyps, or adenomas (69). The gFOBT detects blood based upon the peroxidase enzymatic activity of heme. It requires a moderate amount of heme; it is generally considered not sensitive to the presence of blood. A study estimated that the sensitivity of gFOBT for CRC is 50% (70). This is due to the fact that it can cross-react with dietary substances and/or medications. Despite this, a follow up after 30 years in the Minnesota Colon Cancer Control Study for patients assigned to either annual/biennial gFOBT or usual care revealed a 32% decrease in mortality (71). The FIT is based upon an antibody reaction to the globin chains of hemoglobin, and it does not cross-react with dietary meats. It is simple and requires less fecal samples than gFOBT; a recent meta-analysis of 19 studies showed that FIT had a sensitivity of 79% and specificity of 94% for the detection of CRC (72). A disadvantage of this method is the low sensitivity for detection of colon polyps (73). The multitarget stool DNA test detects abnormally structured DNA in the stool. A study from multiple centers showed that the multitarget stool DNA test had a higher sensitivity (92%) but a lower specificity (87–90%) for detecting CRC (74). Unfortunately, it was unable to detect more than half of all advanced adenomas.
Colonoscopy and sigmoidoscopy
Colonoscopy is widely considered the gold standard for diagnosis and prevention of CRC due to its high sensitivity and specificity as well as the opportunity to detect and resect precursor lesions. It is a procedure in which an endoscope is inserted through the rectum and the entire colon is visualized in real-time. The rate of perforation is around 1/1,000, and it is most often due to polypectomy rather than colonoscopy itself (68). It requires sedation and bowel preparation. A meta-analysis of many observational studies showed that colonoscopy has a 68% lower mortality, however it differs in its ability to detect cancers in different locations of the colon, such as the proximal colon (75). Sigmoidoscopy requires less bowel preparation than colonoscopy, and its beneficial effects are limited to the distal colon. Large, randomized controlled trials have shown that periodic sigmoidoscopy (every 3–5 years) reduced mortality by as much as 31% compared to the population that did not undergo screening (75).
Radiologic screening methods
The radiologic screening methods for CRC involve radiographic visualization of polyps or cancers. They consist of computed tomography (CT) colonography, capsule endoscopy, and double-contrast barium enema. CT colonography is a radiologic imaging test that does not require sedation. It can also evaluate extra-colonic tissues and organs. CT colonography and colonoscopy have similar sensitivity for detection of large polyps; however, CT colonography is less sensitive for polyps less than 8 mm in size (76). CT colonography appears to not differ significantly compared to colonoscopy for the detection of CRC. Double-contrast barium enema involves X-rays of the colon after liquid barium is instilled into the rectum. Double-contrast barium enema can unmask most malignant and premalignant lesions (77). It has been approved as a screening option for high- or medium-risk individuals by the current Medicare guidelines in the US. A study showed that the overall sensitivity for polyp detection for barium enema was 71%, and it increased to 80% with polyps greater than or equal to 10 mm (78). Another study examined the sensitivity of double-contrast barium enema for detection CRC. It revealed that the sensitivity for detection for CRC was 96.5%; in other words, it had a false negative rate of 3.5% (79). This is consistent with previous studies showing approximately the same false negative rate. Capsule endoscopy is a wireless, disposable capsule that is swallowed and takes pictures of the GI tract during transit. Data on the performance of capsule endoscopy for screening is scarce, the first systematic review was published on 13 January 2021. This systematic review combined results of 13 studies; for capsule endoscopy, the polyp detection rate was 24–74%, the sensitivity for polyps >6 mm was 79–96%, and the specificity 66–97% (80). For polyps greater than or equal to 1 cm, the sensitivity was superior to that of CT colonography. More studies should be undertaken, but it seems to be a good alternative to screening colonoscopy (80).
CRC screening programs worldwide
In 2003, the Council of the EU recommended that all EU member states conduct CRC screening for the average risk population (ages 50–74 years) with FIT followed by colonoscopy if positive. By 2015, countries such as Spain, the Netherlands, Ireland, Italy, Croatia, Lithuania, Slovenia, England, France, and the Czech Republic had established screening programs based on this premise (81). Other countries did not have an organized screening program; for example: Bulgaria, Slovakia, Albania, and Bosnia (82). Poland implemented a population-based colonoscopy screening program in 2012 that required just 1 step: a colonoscopy every 10 years (83). In the region of East Asia, the Asian Pacific Colorectal Cancer Screening Group proposed screening in the average risk population for those with the highest CRC incidence. The screening also consists of an FIT (annual or biennial) followed by a colonoscopy if positive. Some of the countries/regions that have screening programs based upon these principles are Japan, South Korea, Thailand, and Taiwan (84). In North America, Canada has a similar screening program to those aforementioned. Screening in the US makes use of almost all of the available modalities on an opportunistic basis: (I) annual FIT; (II) colonoscopy every 10 years; (III) flexible sigmoidoscopy every 5 years; (IV) double-contrast barium enema every 5 years; (V) multi-target stool DNA test every 3 years; or (VI) CT colonography every 5 years (85). In the US, colonoscopy is the most widely used screening modality, followed by FIT and multitarget stool DNA tests.
CRC screening in Hungary
Since the 1990s, there have been several pilot CRC screening programs in Hungary. These consisted of an FIT followed by a colonoscopy if positive, termed the so-called “2-step” screening program. Screening is recommended for those over the age of 50 years in the average risk population. The first pilot screening program in Hungary was conducted in 1997 in Budapest, the next was in the city of Ajka in 2003. There were numerous other screening programs, most notably one conducted in Csongrad County in 2015, which was financed by the EU (86). Currently, the Hungarian laws do not mention CRC screening as it relates to a nationwide, organized screening program. The only historical CRC screening programs in Hungary were opportunistic and pilot programs. In fact, in 2002, the government of Hungary sought “to develop an organized CRC screening program based on detection of human-specific fecal occult blood, and in this way, to reduce the CRC mortality by 20% by the year 2010”. However, this was soon shown to be unsuccessful by the National Audit Office in 2008 (87). The Budapest Declaration in 2011 declared the need for high-quality CRC screening programs in accordance with the United European Gastroenterology Federation (UEGF) (88). Only years later, the first attempt at a nationalized screening program was started in December of 2018, based on the 2-step FIT and subsequent colonoscopy if positive.
Methods
In accordance with the regulatory bodies, Semmelweis University, Department of Internal Medicine and Hematology started a 2-step CRC screening program in 2019–2020. It was based upon voluntary participation of both the general practitioners and the patients. Asymptomatic patients over the age of 50 years were invited to undergo an FIT; if positive, they were referred to colonoscopy. I retrospectively analyzed the results of the 168 colonoscopies referred to Semmelweis University Department of Internal Medicine and Hematology during a 1-year period of time from August 2019 to August 2020. I recorded the results of each colonoscopy, including: (I) the number and histological characteristics of the polypoid lesions resected; (II) their locations; (III) the CRC incidence and their locations; (IV) quality indicators for an efficacious colonoscopy; and (V) the experience and performance of the endoscopists in detecting polyps. This data was analyzed with descriptive statistics, and I have provided that absolute and relative data from the aforementioned categories. The endoscopists were ranked from least experience to most experience numerically. Additionally, I gathered information from literature comparing the cost-effectiveness, benefits, and pitfalls of screening colonoscopy. These results gathered were compared to national and international standard criteria. Through this information, I attempted to show that implementing a 2-step nationalized CRC screening process is cost-effective and will benefit the Hungarian population. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Research and Ethics Committees (RECs) in Hungary grant ethical approval for clinical trials, biomedical research, and research conducted on human participants. The country of Hungary does not have REC/ethics committee for non-biomedical and non-clinical trial research. Therefore, ethical approval and informed consent was not obtained due to country-specific policies.
Quality indicators for colonoscopy
In 2006, the American Society for Gastrointestinal Endoscopy (ASGE) released the first version of quality indicators for colonoscopies (89). In 2015, the ASGE published an update to the quality indicators based upon new evidence from various studies. The indicators that were prioritized included those that were supported by evidence-based trials or studies. The indicators that lacked such evidence were chosen by expert opinion. The quality indicators were divided into preprocedural, intraprocedural, and postprocedural time periods with regard to the colonoscopy. Notably, the targets for performance for the quality indicators do not mirror the standard of care; rather, they guide quality improvement (90). In my study, I chose 4 quality indicators from the colonoscopy reports. All of them were intraprocedural due to the study’s retrospective nature and the presence of recorded values for each. The quality indicators chosen included the following: (I) the adenoma detection rate (ADR); (II) the cecal intubation rate; (III) the withdrawal time; and (IV) frequency of bowel preparation adequacy. I characterized the quality indicators based upon the strength of evidence as noted from the ASGE (90).
ADR
ADR is the best evidence-based parameter for screening colonoscopy (91). The grade of recommendation is listed at 1C (clear benefit, methodological strength supporting evidence from observational studies) (90). It is defined as the fraction of colonoscopies with at least 1 adenoma detected. The ASGE proposed guidelines for the ADR and various quality indicators for colonoscopy. An ADR of greater than or equal to 25% in the screening population is considered high-quality colonoscopy. Specifically, screened males should have a greater than 30% ADR, and screened females should have a greater than 25% ADR. Most post-colonoscopy cancers are attributable to missed lesions (92). A study recently showed that there is a 3% reduction in CRC incidence and 5% decrease in CRC mortality with a 1% increase in ADR by the endoscopist (93). The ADR in the population may be more skewed as the population was under screened.
Cecal intubation rate
The cecal intubation rate is the fraction of colonoscopies that visualize the cecum. It is the passage of the colonoscope to a point adjacent to the ileocecal valve, allowing the visualization of the appendiceal orifice and the medial wall of the cecum (90). The grade of recommendation is listed as 1C (clear benefit, methodological strength supporting evidence from observational studies) (90). For screening colonoscopies, the cecal intubation rate should be greater than 95% (90). This is because of the recurrent finding that a high percentage of CRC are located in the proximal colon, which includes the cecum. Low cecal intubation rates have been correlated with higher rates of interval proximal colon cancer (90).
Withdrawal time
In negative-result screening colonoscopies, the withdrawal time should be greater than or equal to 6 minutes on average, according to ASGE guidelines. The grade of recommendation is listed as 2C (unclear, methodological strength supporting evidence from observational studies) (90). This measure of efficacy is not as important as the ADR. In fact, the use of withdrawal time may be to correct the endoscopists to improve their substandard ADRs (94). Several studies have demonstrated a correlation between longer withdrawal time and higher ADRs (95-99). The main premise that the withdrawal time attempts to illustrate is that a careful examination of the colon requires time and effort. A caveat might be that since colon length can differ, individualized withdrawal time is not applicable, since a well-prepared colon that is shorter than average length can be examined thoroughly in less than 6 minutes (89).
Frequency of bowel preparation adequacy
The grade of recommendation for the frequency of bowel preparation adequacy is 3 (unclear, expert opinion only) (90). The ASGE recommends greater than or equal to 85% of outpatient examinations have adequate bowel preparation (100). Endoscopists who do not meet this criterion are recommended to check and revise their protocol for bowel preparation. By far, the most important determinant for preparation adequacy is the time period between the end of preparation and the colonoscopy (101). Quite logically, quality diminishes as this interval increases. The ASGE recommends split-dosed bowel preparations, meaning that half of the dosage is given on the day of the examination and the other half is given the day before (101). The Boston Bowel Preparation Score (BBPS) has been shown to be valid in various clinical studies (102-106) It consists of a score from 0 to 9; each segment (descending, transverse, ascending) of the colon is scored from 0 to 3 based on the colon preparation quality, and the sum determines the end score (102). The latest validation study for the BPPS showed that a total score of ≥6 and all segment scores ≥2 is a standard for adequacy for a 10-year follow-up (105). Currently, this study has been reaffirmed by an additional study that performed 438 colonoscopies in men. This study showed that a BPPS segment of 2 or 3 had adequate bowel preparation for those adenomas >5 mm and is sufficient for repeat colonoscopy at standard, guideline-recommended intervals (107).
Results
There were 7 different endoscopists that performed colonoscopy. Colonoscopy was performed in 168 patients. There were 100 males and 68 females. The average age for the patient population was 63.4 years. A total of 270 polypoid lesions were resected, consisting of 270 polypoid lesions (185 adenomas, 73 hyperplastic polyps, 1 juvenile polyp, 3 sessile serrated adenomas, and 8 CRC, each of which was an adenocarcinoma). The average age of CRC diagnosis was 63.9 years, and the ages ranged from 56 to 68 years. There was a slight female predominance for CRC, with 5 out of 8 patients being female.
Polypoid lesions
Polypoid lesions are thought to consist of syndromic, mesenchymal, or epithelial categories (108). The syndromic polyps consist of the polyps that arise secondarily to inherited syndromes such as FAP, Cowden syndrome, or Cronkhite-Canada syndrome. The mesenchymal polyps can arise from tissues such as vascular tissue, smooth muscle tissue, neural tissue, and fat tissue, or a combination thereof. The epithelial polyps are the most commonly encountered; they consist of colonic adenomas, sessile serrated adenomas, and hyperplastic polyps. Each one of these epithelial polyps harbor neoplastic potential. The colonic adenomas can be subdivided based upon histological subtype into villous, tubulovillous, and tubular adenomas (108). In the patient population, 270 polypoid lesions were resected (185 adenomas, 73 hyperplastic polyps, 1 juvenile polyp, 3 sessile serrated adenomas, and 8 CRC, each of which was an adenocarcinoma). The polypoid lesion localizations were 16 (5.93%) in the cecum, 39 (14.4%) in the ascending colon, 19 (7%) in the transverse colon, 26 (9.6%) in the descending colon, 98 (36.3%) in the sigmoid colon, and 72 (26.7%) in the rectum. The majority (n=170, 63%) were located in the rectosigmoid region, one of the most common places for development of CRC.
Adenomas
Of the 270 polypoid lesions resected, 185 (68.5%) were colonic adenomas, subdivided into the tubulovillous, villous, or tubular histological characteristics (Table 1) (109). It is important to note that even though “sessile serrated adenoma” has adenoma in its name, it is not classified as an adenoma (89). Therefore, the ADR does not apply to the serrated lesions (110). The current discussion revolves around if serrated lesions should have a separate detection rate of 5% in the proximal colon (111). If proximal colon serrated lesion detection and ADR are correlated, there might need to be a new target established (112). Unfortunately, the histological distinction between sessile serrated polyps and hyperplastic polyps varies highly with the observer, making it an undesirable detection target (113). The ADR in the population was 57.1%; the adenoma per patient ratio was 1.13. Of the adenomas, 16 (8.6%) were in the cecum, 33 (17.8%) were in the ascending colon, 16 (8.6%) were in the transverse colon, 22 (11.9%) were in the descending colon, 71 (38.4%) were in the sigmoid colon, and 27 (14.6%) were in the rectum (Table 2). Altogether, 98 (53%) of the adenomas were located in the rectosigmoid region. The colonic adenomas were also characterized based upon their histological subtypes and the degree of their dysplasia [high-grade dysplasia (HGD) or low-grade dysplasia (LGD)]. A total of 135 (73%) of adenomas were tubular LGD, 7 (~4%) were villous LGD, 25 (~13.5%) were tubulovillous LGD, 6 (~3%) were tubular HGD, 3 (~2%) were villous HGD, and 9 (~4%) were tubulovillous HGD.
Table 1
| Adenoma subtype | Frequency in the population (%) | Estimated frequency in literature (109) (%) |
|---|---|---|
| Tubular | 76 | 80–86 |
| Villous | 5 | 3–16 |
| Tubulovillous | 19 | 8–16 |
Table 2
| Location | Adenoma subtype and dysplasia, n (%) |
|---|---|
| Cecum | 16 (8.6) |
| Tubular LGD | 14 (7.6) |
| Tubulovillous LGD | 1 (0.5) |
| Tubular HGD | 1 (0.5) |
| Ascending colon | 33 (17.8) |
| Tubular LGD | 27 (14.6) |
| Tubulovillous LGD | 6 (3.2) |
| Transverse colon | 16 (8.6) |
| Tubular LGD | 14 (7.6) |
| Tubulovillous LGD | 1 (0.5) |
| Villous HGD | 1 (0.5) |
| Descending colon | 22 (11.9) |
| Tubular LGD | 19 (10.3) |
| Tubular HGD | 1 (0.5) |
| Tubulovillous LGD | 1 (0.5) |
| Villous HGD | 1 (0.5) |
| Sigmoid colon | 71 (38.4) |
| Tubular LGD | 46 (24.9) |
| Tubulovillous HGD | 6 (3.2) |
| Tubulovillous LGD | 12 (6.5) |
| Villous LGD | 6 (3.2) |
| Tubular HGD | 1 (0.5) |
| Rectum | 27 (14.6) |
| Tubular LGD | 15 (8.1) |
| Villous HGD | 1 (0.5) |
| Tubulovillous HGD | 3 (1.6) |
| Tubulovillous LGD | 4 (2.2) |
| Tubular HGD | 3 (1.6) |
| Villous LGD | 1 (0.5) |
LGD, low-grade dysplasia; HGD, high-grade dysplasia.
CRCs
The average age of CRC patients in the population was 63.9 years. The age ranged from 56 to 68 years. The incidence of CRC was of 8 out of 168 colonoscopies or 4.76%. Of the 8 CRC detected, each of them was an adenocarcinoma. Of these 8 adenocarcinomas, 6 were located in the rectosigmoid region and 2 were located proximally in the splenic and hepatic flexures. The lowest age for diagnosis of CRC was 56 years (male patient). The oldest age for diagnosis of CRC was 68 years (female patient). The CRCs showed a slight female predominance: 5 of 8 were females; the remaining 3 were males.
Quality indicators for colonoscopy
I observed 4 intraprocedural colonoscopy quality indicators selected from the ASGE guidelines. For all 4 of the indicators, the colonoscopies performed in the Semmelweis clinic superseded the selected benchmark criteria (Table 3). The “Frequency of Bowel Preparation Adequacy” indicator was determined by using studies on the BBPS, one of the most widely recognized and validated tools to assess bowel preparation. The latest research states that a BPPS of 6 in total and all segment scores ≥2 is adequate preparation for follow-up at regular screening intervals (105). The average bowel preparation score of 6.6 and average of the segments greater than 2 signify that bowel preparation reached these defined standards. Regarding the cecal intubation rate: 84 of the colonoscopies reached the terminal ileum, 80 reached the cecum, 1 reached the sigmoid, 2 reached the hepatic flexure, and 1 did not go appreciably far. Therefore, 164 out of the 168 reached the cecum, making around a 98% cecal intubation rate.
Table 3
| Quality indicator | Our results | Proposed benchmarks (ASGE) | Strength of recommendation |
|---|---|---|---|
| ADR (in patients) | Males: 58%; females: 55.2%; total ADR: 57.1% |
Males: >30%; females: >20%; total: >25% |
1C (ASGE)—clear benefit (90) |
| Cecal intubation rate | ~98% | >95% | 1C (ASGE)—clear benefit (90) |
| Withdrawal time | ~10.8 minutes | ≥6 minutes | 2C (ASGE)—unclear, alternative approaches may be better (90) |
| Frequency of bowel preparation adequacy | ~87% [average bowel prep score of 6.6 (2+2.3+2.3)] | ≥85% | 3 (ASGE)—expert opinion, unclear benefit (90) |
ASGE, American Society for Gastrointestinal Endoscopy; ADR, adenoma detection rate.
Examiner performance
There were 7 different endoscopists of varying experience who performed colonoscopy (Tables 4-9). It is important to note that in order to be qualified to perform the screening colonoscopy, a total of 500 colonoscopies for any indication must have been performed. The examiners were ranked and given a numerical value from 1 to 7, with 1 being the least experienced and 7 being the most experienced (30+ years of experience). The ADR for the colonoscopists ranged from 40% to 72.2% and did not seem to correlate with experience. However, all of the colonoscopists reached the benchmark ADR of >25% set by the ASGE.
Table 4
| Examiner | Number of colonoscopies performed |
|---|---|
| 1 | 15 |
| 2 | 30 |
| 3 | 37 |
| 4 | 17 |
| 5 | 12 |
| 6 | 27 |
| 7 | 30 |
The examiners were ranked given a numerical value from 1–7, ranging from 1 being the least experienced to 7 being the most experienced (30+ years of experience).
Table 5
| Examiner | Total number of polypectomies |
|---|---|
| 1 | 13 |
| 2 | 38 |
| 3 | 66 |
| 4 | 29 |
| 5 | 30 |
| 6 | 52 |
| 7 | 42 |
The examiners were ranked given a numerical value from 1–7, ranging from 1 being the least experienced to 7 being the most experienced (30+ years of experience).
Table 6
| Examiner | Adenomas resected |
|---|---|
| 1 | 10 |
| 2 | 20 |
| 3 | 48 |
| 4 | 25 |
| 5 | 21 |
| 6 | 35 |
| 7 | 26 |
The examiners were ranked given a numerical value from 1–7, ranging from 1 being the least experienced to 7 being the most experienced (30+ years of experience).
Table 7
| Examiner | Adenoma detection rate (%) |
|---|---|
| 1 | 40 |
| 2 | 46.6 |
| 3 | 72.2 |
| 4 | 52.9 |
| 5 | 66.6 |
| 6 | 66.6 |
| 7 | 46.6 |
The examiners were ranked given a numerical value from 1–7, ranging from 1 being the least experienced to 7 being the most experienced (30+ years of experience).
Table 8
| Examiner | Number of high-grade lesions detected |
|---|---|
| 1 | 2 |
| 2 | 1 |
| 3 | 7 |
| 4 | 0 |
| 5 | 2 |
| 6 | 1 |
| 7 | 2 |
The examiners were ranked given a numerical value from 1–7, ranging from 1 being the least experienced to 7 being the most experienced (30+ years of experience).
Table 9
| Examiner | Number of carcinomas detected |
|---|---|
| 1 | 0 |
| 2 | 0 |
| 3 | 4 |
| 4 | 1 |
| 5 | 0 |
| 6 | 2 |
| 7 | 1 |
The examiners were ranked given a numerical value from 1–7, ranging from 1 being the least experienced to 7 being the most experienced (30+ years of experience).
Discussion
CRC is a major cause of morbidity and mortality worldwide. This is exemplified in the country of Hungary, where in 2018 the incidence and mortality of CRC according to GLOBOCAN data was among the highest in the world. This is disheartening because CRC is a neoplasm that can be screened: there are various screening modalities that exist and have been proposed as viable alternatives for each other by organizations such as the USPSTF. According to a publication in 1968 by Wilson and Junger called “The Principles and Practice of Screening for Disease”, 10 principles should be taken into account when making a decision to screen for a disease: (I) the disease should be an important health problem; (II) the natural history of the condition must be understood, specifically, from latent to symptomatic phase; (III) there should be a recognizable latent and symptomatic phase; (IV) there should be a suitable test or examination; (V) the test should be acceptable to the population; (VI) there should be an agreed policy on whom to treat as patients; (VII) there should be an accepted treatment for patients with recognized disease; (VIII) facilities for diagnosis and treatment should be available; (IX) the cost of case-finding should be economically feasible compared to expenditure on the medical field as a whole; (X) case-finding should be a continuing process and not a “once and for all” project (114). CRC meets most of the criteria due to the burden of the disease globally, the long asymptomatic phase required for transformation, the recognizable precursor lesion of the adenoma, and the treatment options available; all of which were outlined previously. Screening for CRC has many different modalities, but the sensitivity of endoscopic methods is unquestionably superior to that of the others. The different screening methods differ also in their specificity, invasiveness, and required screening frequency, making the acceptance to the public different for each procedure (87). Thus, the proper choice for a screening program requires technical as well as practical considerations for its implementation. The hallmark of a good population-based screening program will take into account the well-documented “triple aim” in healthcare: improving the health of the population, reducing the per-capita cost of the screening, and optimizing the experience of care.
CRC screening test compliance
Participation in screening programs varies markedly in population-based screening programs and randomized controlled trials. Some factors that are implicated with disparity in the participation of CRC screening programs include gender inequalities, socioeconomic inequalities, residence, employment and education, wealth, and ethnicity (115). Compliance with screening programs is also highly dependent on the acceptability to the population (116). The participation in screening for CRC is relatively low compared to other screening tests for other diseases such as prostate or cervical cancer (117). The general consensus with CRC screening is that it is efficacious, and its implementation in an organized way is recommended (87). The early programs for screening in Hungary were implemented in selected counties and the results have since been published (118). The regulatory authorities in Hungary have decided to use the “2-step” process of CRC in accordance with European guidelines. This is in contrast to the alternative guidelines to a “1-step strategy” of just screening colonoscopy, where prevention and therapeutic intervention can be performed simultaneously (119). The conflict between these two standpoints have stagnated the implementation of population-based screening in Hungary. Substantial reductions in mortality can be manifested through organized screening, though the crux of the problem is with the participation of the population with the offered screening program. Due to this, it is likely that in the study, the ADR was markedly increased because of the lack of organized screening in the Hungarian population.
1-step screening versus 2-step screening
Each screening test has its strengths and weaknesses. Colonoscopy is more uncomfortable on average than other CRC screening tests. Almost all of the patients that undergo colonoscopy find the preparation of the bowels to be more uncomfortable than the procedure itself (120). The reason for this is that during the procedure the patient is sedated, and they experience unfavorable side-effects of the medications used for the procedure. Additionally, colonoscopy incurs significant costs due to the equipment used; it requires a skilled operator, and quality colonoscopies have shown to be time-consuming (121). Meanwhile, colonoscopy has to be performed less frequently—every 10 years in average-risk individuals. Some poorer countries could potentially have limited capacity for colonoscopy. In Hungary, this situation is likely the case, as well as the population’s participation being influenced by psychological, behavioral, and cognitive factors (122). The FIT may be a better alternative for mass-screening. It is less complicated to perform than colonoscopy, noninvasive, and only requires biannual examination at most. Despite being of less sensitivity, mass screening using an FIT is an evidence-based alternative, with referral to colonoscopy (the gold standard screening test) if positive. The participation rate for the 2-step screening program according to a pilot screening program conducted in 2015 in Csongrad County, Hungary recorded a participation rate of 47.3%, which was lower than the expected 65% (123). However, the participation for colonoscopy after a subsequent positive test reached 90.1%, achieving the anticipated value (123). This can imply that the greatest difficulty is to elicit the cooperation of the eligible population, but once health-conscious, the motivation to attend may increase. For the population, the 7 different endoscopists were situated in different clinics affiliated with the university; a limitation was that the data for participation rate and compliance with colonoscopy was not recorded as it was a voluntary screening program with no active study recorded at the time. Nevertheless, the “1-step” screening strategy seems less desirable for population-based screening in Hungary because of the low attendance rate and the limited capacity for colonoscopy, among other factors.
Cost-effectiveness of screening
A recent meta-analysis of the cost-effectiveness of various screening methods that were listed in the USPSTF Guidelines showed that FIT, flexible sigmoidoscopy every 5 years with a sensitive FIT, and colonoscopy are reasonably cost-effective strategies for CRC screening (124). In Hungary, the population-based voluntary cancer screening program introduced in December of 2018 underwent cost-benefit analysis projected to the year of 2050 (125). The study showed that under the current screening regimen, the Hungarian population would benefit modestly with a reduction in mortality of about 6.2% compared to no screening at all (125). Increasing the invitation coverage for the population using pharmacies instead of general practitioners or improved computerized systems reduced the morality by up to 16.6%. The scenarios have shown cost-benefit ratios of up to 8,000–8,700 Euros per life-years gained, depending on the adherence of the population to the guidelines (125). A study conducted in Portugal compared FIT to colonoscopy for cost-effectiveness, and it showed that above a participation rate of 55%, the FIT is more acceptable than a colonoscopy price above 100 Euros. Participation rates of over 63% showed that the FIT will provide even more quality life years than that of colonoscopy at a lower price (126). This concurs with the recommendations of the USPSTF of a 60% participation rate (127). The 60% that participate must reach at least 80% participation for the colonoscopy. Long-term statistics from the US have shown that compared to no screening, cost effectiveness with any of the common methods varied between 10,000 and 25,000 dollars per life year saved (128). In Csongrad County in Hungary, despite the participation rate of 47.1% in the 2-step screening program and a colonoscopy participation rate of 57%, the study ruled that the 2-step screening program is still “beneficial in every considerable aspect” (86).
Population outreach
To increase screening rates, many healthcare systems can conduct what is known as population outreach. This refers to the provision of necessary health services and information to communities that might not otherwise have access. An organized, population-based outreach has been shown to increase CRC screening participation rates and adherence in diverse and underserved patient populations (129,130). This entails large-scale cooperation and multicomponent interventions at the level of the government, the health system, the provider, and the patient population. The study in Csongrad County recorded suboptimal participation with its respondents for reasons such as unfamiliarity with CRC screening (53.3%) and the absence of participation in CRC screening previously (68.8%). However, in the case that a physician or reputable health authority suggested screening, the general attitude changed such that the majority (81%) of the respondents were amenable (131). This can imply that two important factors in the Hungarian population that contribute to low screening adherence are the lack of availability of health information and the lack of CRC awareness. These premises are corroborated by similar studies in underserved populations (132,133). By increasing the population coverage for screening invitations in Hungary using a pharmacy or computerized systems, CRC morality is estimated to reduce by up to 16.6% by 2050 using the 2-step screening program. There were favorable cost-benefit ratios of up to 8,000–8,700 Euros per life-years gained (125). To supplement this information, there is well-documented benefit for increasing CRC screening rates with mailed FIT outreach (134-138). An analysis comparing 2 mailed outreach strategies (FIT kits versus colonoscopy invitations) from a randomized clinical trial implied that mailed FIT kits had a lower 10-year average per-person cost ($1,139) compared to colonoscopy invitations ($1,725) (139). In another randomized control trial, FIT outreach had significantly higher screening rates than that of colonoscopy outreach; when the results were stratified, screening rates were higher for any form of outreach compared to usual care (138). Therefore, in the context of resource limitation and limited capacity for colonoscopy such as in Hungary, the mailed FIT may be the preferred strategy as it pertains to the 2-step program. The principle downside is that there may be fewer reported months of screening compliance and advanced neoplasia detected (139). However, the gap of screening compliance may be mitigated through personalized outreach via texts and telephone calls for non-responders, which has been shown to further increase participation rates (129). Although increasing population health literacy may also contribute to increased screening rates, the long-term benefits are not as well-characterized and well-known as population outreach. It is evident that frequent exposure to CRC screening via population outreach is correlated with increased screening participation (140). Undoubtedly, further studies are warranted as it relates to the feasibility and sustainability of the 2-step screening program in the Hungary.
Conclusions
The data has shown a markedly high rate of polyp and CRC detection in this Hungarian subpopulation. Hungary would benefit from continued implementation of the voluntary population-based screening program introduced in December of 2018. It would be beneficial and cost-effective in the long term. Specifically, an organized population-based screening program based upon the principle of the 2-step screening method of annual or biannual FIT followed by colonoscopy if positive is optimal. It would bring about significant reductions in CRC morbidity and mortality, which in Hungary are among the highest in the world. This alternative is proposed in lieu of 1-step screening because of the limited capacity for colonoscopy as well as low health literacy and limited participation rates in CRC screening. The screening program should be coordinated so that more invitees are exposed to screening, and the participation rate must be optimal (>60%) with 80% or greater of non-negative tests participating in colonoscopy to reach the maximum cost-benefit. This could potentially be achieved through population outreach combined with education on the importance of CRC screening, general health, and advertisement. Extensive coordination of members of the health system together with systemic organizational change and multicomponent intervention is needed to address this health concern in Hungary. Consolidation and distribution of the screening program will potentially increase participation rates and subsequently lead to a larger decrease in mortality from CRC in Hungary. Further studies are warranted as it relates to the feasibility and sustainability of the 2-step screening program in the Hungarian population.
Acknowledgments
I would like to thank my supervisor, Dr. Emese Mihalyi, MD, PhD for supervising this project and providing invaluable guidance while I was still studying at Semmelweis University. I would like to thank Dr. David Strelnikov for his help gathering the colonoscopy results from the Semmelweis University database and support with the presentation at the Hungarian National Gastroenterology Conference in June of 2022. I would like to thank Deo Agnila and Dr. Olu Oyesanmi, MD, PhD for helping me edit and prepare the manuscript for submission. The findings of this manuscript were presented in the Hungarian National Gastroenterology Conference (Siofok, Hungary, 10 June, 2022) as well as Semmelweis International Students’ Conference (Budapest, Hungary, 10 February, 2022).
Funding: None.
Footnote
Reporting Checklist: The author has completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-318/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-318/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-318/prf
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-318/coif). S.L. is currently an Internal Medicine Resident at HCA Florida Blake Hospital and an employee of HCA Healthcare. This research was supported in part by HCA Healthcare and/or an HCA Healthcare affiliated entity through proofreading and editing of the manuscript. The views expressed in this publication represent those of the author(s) and do not necessarily represent the official views of HCA Healthcare or any of its affiliated entities. The author has no other conflicts of interest.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Research and Ethics Committees (RECs) in Hungary grant ethical approval for clinical trials, biomedical research, and research conducted on human subjects. The country of Hungary does not have an REC/ethics committee for non-biomedical and non-clinical trial research. Therefore, ethical approval and informed consent was not obtained due to country-specific policies.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol 2019;14:89-103. [Crossref] [PubMed]
- Jorge JMN, Habr-Gama A. Anatomy and Embryology of the Colon, Rectum, and Anus. Available online: https://book.bsmi.uz/web/kitoblar/152370510.pdf
- Wang YHW, Wiseman J. Anatomy, Abdomen and Pelvis, Rectum. Treasure Island, FL, USA: StatPearls Publishing; 2024.
- Tóth DZ. Morphology and histology of the large intestine and the rectum. 2019. Available online: https://www.skypat.no/pathology/wp-content/uploads/2021/06/large-intestineand-rectum.pdf
- Harkins JM, Ahmad B. Anatomy, Abdomen and Pelvis, Portal Venous System (Hepatic Portal System). Treasure Island, FL, USA: StatPearls Publishing; 2024.
- Maurer CA. Colon cancer: resection standards. Tech Coloproctol 2004;8:s29-32. [Crossref] [PubMed]
- American Cancer Society. What is Colorectal Cancer? 2022. Available online: https://www.cancer.org/cancer/types/colon-rectal-cancer/about/what-is-colorectalcancer.html
- Kuipers EJ, Grady WM, Lieberman D, et al. Colorectal cancer. Nat Rev Dis Primers 2015;1:15065. [Crossref] [PubMed]
- Boncz I, Brodszky V, Péntek M, et al. The disease burden of colorectal cancer in Hungary. Eur J Health Econ 2010;10:S35-40. [Crossref] [PubMed]
- World Health Organization. International Agency for Research on Cancer. Cancer Today. Available online: https://gco.iarc.fr
- Boyle P, Langman JS. ABC of colorectal cancer: Epidemiology. BMJ 2000;321:805-8. [Crossref] [PubMed]
- Rafiemanesh H, Mohammadian-Hafshejani A, Ghoncheh M, et al. Incidence and Mortality of Colorectal Cancer and Relationships with the Human Development Index across the World. Asian Pac J Cancer Prev 2016;17:2465-73. [PubMed]
- Grady WM, Carethers JM. Genomic and epigenetic instability in colorectal cancer pathogenesis. Gastroenterology 2008;135:1079-99. [Crossref] [PubMed]
- Grady WM, Markowitz SD. The molecular pathogenesis of colorectal cancer and its potential application to colorectal cancer screening. Dig Dis Sci 2015;60:762-72. [Crossref] [PubMed]
- Harvard Medical School. They found colon polyps: Now what? Available online: https://www.health.harvard.edu/diseases-and-conditions/they-found-colon-polyps-now-what
- Jiang Y, Song F, Hu X, et al. Analysis of dynamic molecular networks: the progression from colorectal adenoma to cancer. J Gastrointest Oncol 2021;12:2823-37. [Crossref] [PubMed]
- Sullivan BA, Noujaim M, Roper J. Cause, Epidemiology, and Histology of Polyps and Pathways to Colorectal Cancer. Gastrointest Endosc Clin N Am 2022;32:177-94. [Crossref] [PubMed]
- Simon K. Colorectal cancer development and advances in screening. Clin Interv Aging 2016;11:967-76. [Crossref] [PubMed]
- Tse BCY, Welham Z, Engel AF, et al. Genomic, Microbial and Immunological Microenvironment of Colorectal Polyps. Cancers (Basel) 2021;13:3382. [Crossref] [PubMed]
- Malki A, ElRuz RA, Gupta I, et al. Molecular Mechanisms of Colon Cancer Progression and Metastasis: Recent Insights and Advancements. Int J Mol Sci 2020;22:130. [Crossref] [PubMed]
- Goldstein NS. Serrated pathway and APC (conventional)-type colorectal polyps: molecular-morphologic correlations, genetic pathways, and implications for classification. Am J Clin Pathol 2006;125:146-53. [Crossref] [PubMed]
- Burnett-Hartman AN, Newcomb PA, Potter JD, et al. Genomic aberrations occurring in subsets of serrated colorectal lesions but not conventional adenomas. Cancer Res 2013;73:2863-72. [Crossref] [PubMed]
- Meseeha M, Attia M. Colon Polyps. Treasure Island, FL, USA: StatPearls Publishing; 2024.
- Bettington M, Walker N, Clouston A, et al. The serrated pathway to colorectal carcinoma: current concepts and challenges. Histopathology 2013;62:367-86. [Crossref] [PubMed]
- O'Brien MJ, Yang S, Mack C, et al. Comparison of microsatellite instability, CpG island methylation phenotype, BRAF and KRAS status in serrated polyps and traditional adenomas indicates separate pathways to distinct colorectal carcinoma end points. Am J Surg Pathol 2006;30:1491-501. [Crossref] [PubMed]
- Grady WM. Genomic instability and colon cancer. Cancer Metastasis Rev 2004;23:11-27. [Crossref] [PubMed]
- Toyota M, Ahuja N, Ohe-Toyota M, et al. CpG island methylator phenotype in colorectal cancer. Proc Natl Acad Sci U S A 1999;96:8681-6. [Crossref] [PubMed]
- Shen L, Toyota M, Kondo Y, et al. Integrated genetic and epigenetic analysis identifies three different subclasses of colon cancer. Proc Natl Acad Sci U S A 2007;104:18654-9. [Crossref] [PubMed]
- Coelho M, Oliveira T, Fernandes R. Biochemistry of adipose tissue: an endocrine organ. Arch Med Sci 2013;9:191-200. [Crossref] [PubMed]
- Lawrence VJ, Kopelman PG. Medical consequences of obesity. Clin Dermatol 2004;22:296-302. [Crossref] [PubMed]
- Karahalios A, Simpson JA, Baglietto L, et al. Change in weight and waist circumference and risk of colorectal cancer: results from the Melbourne Collaborative Cohort Study. BMC Cancer 2016;16:157. [Crossref] [PubMed]
- Hecht SS. Cigarette smoking: cancer risks, carcinogens, and mechanisms. Langenbecks Arch Surg 2006;391:603-13. [Crossref] [PubMed]
- Botteri E, Borroni E, Sloan EK, et al. Smoking and Colorectal Cancer Risk, Overall and by Molecular Subtypes: A Meta-Analysis. Am J Gastroenterol 2020;115:1940-9. [Crossref] [PubMed]
- O'Keefe SJ. Diet, microorganisms and their metabolites, and colon cancer. Nat Rev Gastroenterol Hepatol 2016;13:691-706. [Crossref] [PubMed]
- Zhao Z, Feng Q, Yin Z, et al. Red and processed meat consumption and colorectal cancer risk: a systematic review and meta-analysis. Oncotarget 2017;8:83306-14. [Crossref] [PubMed]
- Song M, Garrett WS, Chan AT. Nutrients, foods, and colorectal cancer prevention. Gastroenterology 2015;148:1244-60.e16. [Crossref] [PubMed]
- Fedirko V, Tramacere I, Bagnardi V, et al. Alcohol drinking and colorectal cancer risk: an overall and dose-response meta-analysis of published studies. Ann Oncol 2011;22:1958-72. [Crossref] [PubMed]
- Rothwell PM, Wilson M, Elwin CE, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet 2010;376:1741-50. [Crossref] [PubMed]
- Jiang Y, Ben Q, Shen H, et al. Diabetes mellitus and incidence and mortality of colorectal cancer: a systematic review and meta-analysis of cohort studies. Eur J Epidemiol 2011;26:863-76. [Crossref] [PubMed]
- Ollberding NJ, Nomura AM, Wilkens LR, et al. Racial/ethnic differences in colorectal cancer risk: the multiethnic cohort study. Int J Cancer 2011;129:1899-906. [Crossref] [PubMed]
- Troisi RJ, Freedman AN, Devesa SS. Incidence of colorectal carcinoma in the U.S.: an update of trends by gender, race, age, subsite, and stage, 1975-1994. Cancer 1999;85:1670-6. [Crossref] [PubMed]
- Irby K, Anderson WF, Henson DE, et al. Emerging and widening colorectal carcinoma disparities between Blacks and Whites in the United States (1975-2002). Cancer Epidemiol Biomarkers Prev 2006;15:792-7. [Crossref] [PubMed]
- Lansdorp-Vogelaar I, Kuntz KM, Knudsen AB, et al. Contribution of screening and survival differences to racial disparities in colorectal cancer rates. Cancer Epidemiol Biomarkers Prev 2012;21:728-36. [Crossref] [PubMed]
- Wanders LK, Dekker E, Pullens B, et al. Cancer risk after resection of polypoid dysplasia in patients with longstanding ulcerative colitis: a meta-analysis. Clin Gastroenterol Hepatol 2014;12:756-64. [Crossref] [PubMed]
- Olén O, Askling J, Sachs MC, et al. Childhood onset inflammatory bowel disease and risk of cancer: a Swedish nationwide cohort study 1964-2014. BMJ 2017;358:j3951. [Crossref] [PubMed]
- Burt R, Neklason DW. Genetic testing for inherited colon cancer. Gastroenterology 2005;128:1696-716. [Crossref] [PubMed]
- Gryfe R. Inherited colorectal cancer syndromes. Clin Colon Rectal Surg 2009;22:198-208. [Crossref] [PubMed]
- de la Chapelle A. Genetic predisposition to colorectal cancer. Nat Rev Cancer 2004;4:769-80. [Crossref] [PubMed]
- Macrae FA. Epidemiology and risk factors for colorectal cancer. Available online: https://www.uptodate.com/contents/epidemiology-and-risk-factors-for-colorectal-cancer
- Rosen RD, Sapra A. TNM Classification. Treasure Island, FL, USA: StatPearls Publishing; 2024.
- Olson RM, Perencevich NP, Malcolm AW, et al. Patterns of recurrence following curative resection of adenocarcinoma of the colon and rectum. Cancer 1980;45:2969-74. [Crossref] [PubMed]
- Nelson H, Petrelli N, Carlin A, et al. Guidelines 2000 for colon and rectal cancer surgery. J Natl Cancer Inst 2001;93:583-96. [Crossref] [PubMed]
- Stintzing S. Management of colorectal cancer. F1000Prime Rep 2014;6:108. [Crossref] [PubMed]
- Quasar Collaborative Group. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet 2007;370:2020-9. [Crossref] [PubMed]
- Sargent DJ, Marsoni S, Monges G, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol 2010;28:3219-26. [Crossref] [PubMed]
- Haller DG, Tabernero J, Maroun J, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol 2011;29:1465-71. [Crossref] [PubMed]
- André T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 2004;350:2343-51. [Crossref] [PubMed]
- Tournigand C, André T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 2004;22:229-37. [Crossref] [PubMed]
- Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 2008;26:2013-9. [Crossref] [PubMed]
- Douillard JY, Siena S, Cassidy J, et al. Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann Oncol 2014;25:1346-55. [Crossref] [PubMed]
- Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol 2014;15:1065-75. [Crossref] [PubMed]
- Wilkinson N. Management of Rectal Cancer. Surg Clin North Am 2020;100:615-28. [Crossref] [PubMed]
- Rossi H, Rothenberger DA. Surgical treatment of colon cancer. Surg Oncol Clin N Am 2006;15:109-27. vii. [Crossref] [PubMed]
- Phillips RK, Hittinger R, Fry JS, et al. Malignant large bowel obstruction. Br J Surg 1985;72:296-302. [Crossref] [PubMed]
- Brown KGM, Koh CE. Surgical management of recurrent colon cancer. J Gastrointest Oncol 2020;11:513-25. [Crossref] [PubMed]
- Adler DG, Baron TH. Endoscopic palliation of colorectal cancer. Hematol Oncol Clin North Am 2002;16:1015-29. [Crossref] [PubMed]
- Dudley HA, Racliffe AG, McGeehan D. Intraoperative irrigation of the colon to permit primary anastomosis. Br J Surg 1980;67:80-1. [Crossref] [PubMed]
- Issa IA, Noureddine M. Colorectal cancer screening: An updated review of the available options. World J Gastroenterol 2017;23:5086-96. [Crossref] [PubMed]
- Carroll MR, Seaman HE, Halloran SP. Tests and investigations for colorectal cancer screening. Clin Biochem 2014;47:921-39. [Crossref] [PubMed]
- Lansdorp-Vogelaar I, van Ballegooijen M, Boer R, et al. A novel hypothesis on the sensitivity of the fecal occult blood test: Results of a joint analysis of 3 randomized controlled trials. Cancer 2009;115:2410-9. [Crossref] [PubMed]
- Shaukat A, Mongin SJ, Geisser MS, et al. Long-term mortality after screening for colorectal cancer. N Engl J Med 2013;369:1106-14. [Crossref] [PubMed]
- Lee JK, Liles EG, Bent S, et al. Accuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta-analysis. Ann Intern Med 2014;160:171. [Crossref] [PubMed]
- Lieberman DA. Clinical practice. Screening for colorectal cancer. N Engl J Med 2009;361:1179-87. [Crossref] [PubMed]
- Imperiale TF, Ransohoff DF, Itzkowitz SH. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med 2014;371:187-8. [Crossref] [PubMed]
- Brenner H, Stock C, Hoffmeister M. Effect of screening sigmoidoscopy and screening colonoscopy on colorectal cancer incidence and mortality: systematic review and meta-analysis of randomised controlled trials and observational studies. BMJ 2014;348:g2467. [Crossref] [PubMed]
- Martín-López JE, Beltrán-Calvo C, Rodríguez-López R, et al. Comparison of the accuracy of CT colonography and colonoscopy in the diagnosis of colorectal cancer. Colorectal Dis 2014;16:O82-9. [Crossref] [PubMed]
- Glick S. Double-contrast barium enema for colorectal cancer screening: a review of the issues and a comparison with other screening alternatives. AJR Am J Roentgenol 2000;174:1529-37. [Crossref] [PubMed]
- Steine S, Stordahl A, Lunde OC, et al. Double-contrast barium enema versus colonoscopy in the diagnosis of neoplastic disorders: aspects of decision-making in general practice. Fam Pract 1993;10:288-91. [Crossref] [PubMed]
- Gillespie JS, Kelly BE. Double contrast barium enema and colorectal carcinoma: sensitivity and potential role in screening. Ulster Med J 2001;70:15-8. [PubMed]
- Vuik FER, Nieuwenburg SAV, Moen S, et al. Colon capsule endoscopy in colorectal cancer screening: a systematic review. Endoscopy 2021;53:815-24. [Crossref] [PubMed]
- Navarro M, Nicolas A, Ferrandez A, et al. Colorectal cancer population screening programs worldwide in 2016: An update. World J Gastroenterol 2017;23:3632-42. [Crossref] [PubMed]
- Schreuders EH, Ruco A, Rabeneck L, et al. Colorectal cancer screening: a global overview of existing programmes. Gut 2015;64:1637-49. [Crossref] [PubMed]
- Kaminski MF, Kraszewska E, Rupinski M, et al. Design of the Polish Colonoscopy Screening Program: a randomized health services study. Endoscopy 2015;47:1144-50. [Crossref] [PubMed]
- Sano Y, Byeon JS, Li XB, et al. Colorectal cancer screening of the general population in East Asia. Dig Endosc 2016;28:243-9. [Crossref] [PubMed]
- Smith RA, Andrews K, Brooks D, et al. Cancer screening in the United States, 2016: A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin 2016;66:96-114. [Crossref] [PubMed]
- Rutka M, Bor R, Molnár T, et al. Efficacy of the population-based pilot colorectal cancer screening, Csongrád county, Hungary, 2015. Turk J Med Sci 2020;50:756-63. [Crossref] [PubMed]
- Döbrőssy DL, Kovács A, Budai A. Conflicts between Clinical and Public Health Viewpoints: Colorectal Screening. Arch Clin Gastroenterol 2016;2:044-9.
- Wittmann T, Stockbrugger R, Herszényi L, et al. New European initiatives in colorectal cancer screening: Budapest Declaration. Official appeal during the Hungarian Presidency of the Council of the European Union under the Auspices of the United European Gastroenterology Federation, the European Association for Gastroenterology and Endoscopy and the Hungarian Society of Gastroenterology. Dig Dis 2012;30:320-2. [Crossref] [PubMed]
- Rex DK, Petrini JL, Baron TH, et al. Quality indicators for colonoscopy. Gastrointest Endosc 2006;63:S16-28. [Crossref] [PubMed]
- Rizk MK, Sawhney MS, Cohen J, et al. Quality indicators common to all GI endoscopic procedures. Am J Gastroenterol 2015;110:48-59. [Crossref] [PubMed]
- Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Gastrointest Endosc 2015;81:31-53. [Crossref] [PubMed]
- Pohl H, Robertson DJ. Colorectal cancers detected after colonoscopy frequently result from missed lesions. Clin Gastroenterol Hepatol 2010;8:858-64. [Crossref] [PubMed]
- Corley DA, Levin TR, Doubeni CA. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med 2014;370:2541. [Crossref] [PubMed]
- Barclay RL, Vicari JJ, Greenlaw RL. Effect of a time-dependent colonoscopic withdrawal protocol on adenoma detection during screening colonoscopy. Clin Gastroenterol Hepatol 2008;6:1091-8. [Crossref] [PubMed]
- Sanchez W, Harewood GC, Petersen BT. Evaluation of polyp detection in relation to procedure time of screening or surveillance colonoscopy. Am J Gastroenterol 2004;99:1941-5. [Crossref] [PubMed]
- Desai M, Rex DK, Bohm ME, et al. Impact of withdrawal time on adenoma detection rate: results from a prospective multicenter trial. Gastrointest Endosc 2023;97:537-543.e2. [Crossref] [PubMed]
- Zhao S, Yang X, Wang S, et al. Impact of 9-Minute Withdrawal Time on the Adenoma Detection Rate: A Multicenter Randomized Controlled Trial. Clin Gastroenterol Hepatol 2022;20:e168-81. [Crossref] [PubMed]
- Yun GY, Eun HS, Kim JS, et al. Colonoscopic withdrawal time and adenoma detection in the right colon. Medicine (Baltimore) 2018;97:e12113. [Crossref] [PubMed]
- Barclay RL, Vicari JJ, Doughty AS, et al. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med 2006;355:2533-41. [Crossref] [PubMed]
- Froehlich F, Wietlisbach V, Gonvers JJ, et al. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European Panel of Appropriateness of Gastrointestinal Endoscopy European multicenter study. Gastrointest Endosc 2005;61:378-84. [Crossref] [PubMed]
- Kilgore TW, Abdinoor AA, Szary NM, et al. Bowel preparation with split-dose polyethylene glycol before colonoscopy: a meta-analysis of randomized controlled trials. Gastrointest Endosc 2011;73:1240-5. [Crossref] [PubMed]
- Kastenberg D, Bertiger G, Brogadir S. Bowel preparation quality scales for colonoscopy. World J Gastroenterol 2018;24:2833-43. [Crossref] [PubMed]
- Lai EJ, Calderwood AH, Doros G, et al. The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc 2009;69:620-5. [Crossref] [PubMed]
- Calderwood AH, Jacobson BC. Comprehensive validation of the Boston Bowel Preparation Scale. Gastrointest Endosc 2010;72:686-92. [Crossref] [PubMed]
- Kluge MA, Williams JL, Wu CK, et al. Inadequate Boston Bowel Preparation Scale scores predict the risk of missed neoplasia on the next colonoscopy. Gastrointest Endosc 2018;87:744-51. [Crossref] [PubMed]
- Heron V, Parmar R, Ménard C, et al. Validating bowel preparation scales. Endosc Int Open 2017;5:E1179-88. [Crossref] [PubMed]
- Clark BT, Protiva P, Nagar A, et al. Quantification of Adequate Bowel Preparation for Screening or Surveillance Colonoscopy in Men. Gastroenterology 2016;150:396-405; quiz e14-5. [Crossref] [PubMed]
- Mansoor S, Dolkar T, El-Fanek H. Polyps and polypoid lesions of the colon. Int J Surg Pathol 2013;21:215-23. [Crossref] [PubMed]
- Feldman M, Friedman LS, Brandt LJ. Sleisenger and Fordtran's Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, Management. 11th edition. Elsevier; 2020:2215.
- Rex DK, Bond JH, Winawer S, et al. Quality in the technical performance of colonoscopy and the continuous quality improvement process for colonoscopy: recommendations of the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol 2002;97:1296-308. [Crossref] [PubMed]
- Kahi CJ, Li X, Eckert GJ, et al. High colonoscopic prevalence of proximal colon serrated polyps in average-risk men and women. Gastrointest Endosc 2012;75:515-20. [Crossref] [PubMed]
- Kahi CJ, Hewett DG, Norton DL, et al. Prevalence and variable detection of proximal colon serrated polyps during screening colonoscopy. Clin Gastroenterol Hepatol 2011;9:42-6. [Crossref] [PubMed]
- Wong NA, Hunt LP, Novelli MR, et al. Observer agreement in the diagnosis of serrated polyps of the large bowel. Histopathology 2009;55:63-6. [Crossref] [PubMed]
- Dobrow MJ, Hagens V, Chafe R, et al. Consolidated principles for screening based on a systematic review and consensus process. CMAJ 2018;190:E422-9. [Crossref] [PubMed]
- Mosquera I, Mendizabal N, Martín U, et al. Inequalities in participation in colorectal cancer screening programmes: a systematic review. Eur J Public Health 2020;30:416-25. [Crossref] [PubMed]
- Subramanian S, Klosterman M, Amonkar MM, et al. Adherence with colorectal cancer screening guidelines: a review. Prev Med 2004;38:536-50. [Crossref] [PubMed]
- Adler A, Geiger S, Keil A, et al. Improving compliance to colorectal cancer screening using blood and stool based tests in patients refusing screening colonoscopy in Germany. BMC Gastroenterol 2014;14:183. [Crossref] [PubMed]
- Döbrôssy L, Kovács A, Budai A, et al. The state of the colorectal screening in Hungary: lessons of the pilot programs. Orv Hetil 2007;148:1787-93. [Crossref] [PubMed]
- Péter Z, Tulassay Z. Colonoscopy, the primary tool for colonic screening. Orv Hetil 2009;150:299-304. [Crossref] [PubMed]
- Helm J, Choi J, Sutphen R, et al. Current and evolving strategies for colorectal cancer screening. Cancer Control 2003;10:193-204. [Crossref] [PubMed]
- Herszényi L, Lakatos G, Tulassay Z. Quality colonoscopy: assumptions and expectations. Orv Hetil 2010;151:1331-9. [Crossref] [PubMed]
- Döbrőssy L, Kovács A, Cornides A, et al. Factors influencing the participation in colorectal screening. Orv Hetil 2014;155:1051-6. [Crossref] [PubMed]
- Kivés Z, Kovács A, Budai A, et al. Quality and performance indicators of colorectal cancer screening pilot program in Csongrád County, Hungary. Magy Onkol 2019;63:125-32. [PubMed]
- Zauber AG. Cost-effectiveness of colonoscopy. Gastrointest Endosc Clin N Am 2010;20:751-70. [Crossref] [PubMed]
- Csanádi M, Gini A, Koning H, et al. Modeling costs and benefits of the organized colorectal cancer screening programme and its potential future improvements in Hungary. J Med Screen 2021;28:268-76. [Crossref] [PubMed]
- Areia M, Fuccio L, Hassan C, et al. Cost-utility analysis of colonoscopy or faecal immunochemical test for population-based organised colorectal cancer screening. United European Gastroenterol J 2019;7:105-13. [Crossref] [PubMed]
- Robertson DJ, Lee JK, Boland CR, et al. Recommendations on Fecal Immunochemical Testing to Screen for Colorectal Neoplasia: A Consensus Statement by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2017;152:1217-1237.e3. [Crossref] [PubMed]
- Pignone M, Saha S, Hoerger T, et al. Cost-effectiveness analyses of colorectal cancer screening: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 2002;137:96-104. [Crossref] [PubMed]
- Podmore C, Selby K, Jensen CD, et al. Colorectal Cancer Screening After Sequential Outreach Components in a Demographically Diverse Cohort. JAMA Netw Open 2024;7:e245295. [Crossref] [PubMed]
- Rubin L, Okitondo C, Haines L, et al. Interventions to increase colorectal cancer screening adherence in low-income settings within the United States: A systematic review and meta-analysis. Prev Med 2023;172:107522. [Crossref] [PubMed]
- Kívés Z, Endrei ND, Vajda R, et al. Experience and Attitude of Colorectal Screening Pilot Program Participants Regarding Screening and Screening Programs in Hungary. Iran J Public Health 2022;51:2733-41. [PubMed]
- Wang H, Roy S, Kim J, et al. Barriers of colorectal cancer screening in rural USA: a systematic review. Rural Remote Health 2019;19:5181. [Crossref] [PubMed]
- Lee R, Holmes D. Barriers and recommendations for colorectal cancer screening in Africa. Glob Health Action 2023;16:2181920. [Crossref] [PubMed]
- O'Leary MC, Reuland DS, Correa SY, et al. Uptake of colorectal cancer screening after mailed fecal immunochemical test (FIT) outreach in a newly eligible 45-49-year-old community health center population. Cancer Causes Control 2023;34:125-33. [Crossref] [PubMed]
- Lee B, Keyes E, Rachocki C, et al. Increased Colorectal Cancer Screening Sustained with Mailed Fecal Immunochemical Test Outreach. Clin Gastroenterol Hepatol 2022;20:1326-1333.e4. [Crossref] [PubMed]
- Davis MM, Coury J, Larson JH, et al. Improving colorectal cancer screening in rural primary care: Preliminary effectiveness and implementation of a collaborative mailed fecal immunochemical test pilot. J Rural Health 2023;39:279-90. [Crossref] [PubMed]
- Dougherty MK, Brenner AT, Crockett SD, et al. Evaluation of Interventions Intended to Increase Colorectal Cancer Screening Rates in the United States: A Systematic Review and Meta-analysis. JAMA Intern Med 2018;178:1645-58. [Crossref] [PubMed]
- Gupta S, Halm EA, Rockey DC, et al. Comparative effectiveness of fecal immunochemical test outreach, colonoscopy outreach, and usual care for boosting colorectal cancer screening among the underserved: a randomized clinical trial. JAMA Intern Med 2013;173:1725-32. [Crossref] [PubMed]
- Kapinos KA, Halm EA, Murphy CC, et al. Cost Effectiveness of Mailed Outreach Programs for Colorectal Cancer Screening: Analysis of a Pragmatic, Randomized Trial. Clin Gastroenterol Hepatol 2022;20:2383-2392.e4. [Crossref] [PubMed]
- Cooper CP, Gelb CA, Hawkins NA. How many "Get Screened" messages does it take? Evidence from colorectal cancer screening promotion in the United States, 2012. Prev Med 2014;60:27-32. [Crossref] [PubMed]


