
Development and nomogram prediction of early postoperative recurrence in hepatocellular carcinoma based on preoperative CT imaging radiomic features and serum features related to microvascular infiltration
Highlight box
Key findings
• A predictive nomogram model was established that combines preoperative serum biomarkers and computed tomography radiomic features. This model was shown to effectively predict early postoperative recurrence in hepatocellular carcinoma (HCC) patients.
• The key predictive factors included Kiel 67 (Ki-67) levels, tumor diameter, alpha fetoprotein and vascular endothelial growth factor levels, the fibrosis-4 index, multifocality, abnormal enhancement around the tumor, and a specifically derived radiomics score.
• The model demonstrated excellent predictive accuracy with area under the curve values of 0.9265 and 0.9255 for the training and internal test sets, respectively.
What is known and what is new?
• Early postoperative recurrence of HCC is a major factor affecting patient survival, and traditional prognostic models have limited accuracy.
• This study introduced a novel nomogram that incorporates advanced radiomics and serum biomarkers related to microvascular infiltration, offering significant improvements in predicting early recurrence of HCC.
What is the implication, and what should change bow?
• The introduction of this nomogram that identifies patients at high risk of early recurrence into clinical practice could enable personalized surgical planning and follow-up strategies.
• Clinical guidelines may need to be updated to incorporate radiomic and biomarker analyses as standard preoperative assessments in HCC treatment protocols.
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common malignant tumor worldwide, and the fourth most common cancer in China, and thus poses a severe threat to the health and lives of the Chinese population (1,2). Current treatment options for liver cancer include radical surgery, local therapies (e.g., radiofrequency ablation, and transarterial chemoembolization), radiotherapy, and chemotherapy (3,4). However, due to the complex histological features, high tumor heterogeneity, and diverse invasive behavior of HCC, the recurrence rate remains high post-treatment. Indeed, research has reported that the recurrence rate after radical surgery is as high as 70% (5,6). Early recurrence of liver cancer typically refers to the reappearance or metastasis of HCC within two years of treatment (7). Early recurrence as a significant risk factor for a poor prognosis in HCC patients, who have worse outcomes than those with late recurrence (8). Therefore, risk assessment, appropriate monitoring, and the treatment of patients with postoperative recurrence are crucial for improving HCC patient outcomes.
Currently, predicting HCC recurrence relies primarily on pathological data and traditional imaging, such as tumor staging, infiltration degree, and microvascular infiltration (9). Microvascular infiltration is a research hotspot in the early recurrence of HCC, and is usually confirmed through postoperative pathological examination (9-11). Preoperatively, it can only be indirectly inferred from imaging features, such as unclear tumor boundaries or infiltrative growth. Recent studies have also identified serum markers associated with microvascular infiltration, including alpha fetoprotein (AFP), vascular endothelial growth factor A (VEGF-A), Speckled Protein 100 (SP100), and the fibrosis-4 (FIB-4) index (12-14). However, these markers alone cannot directly diagnose microvascular infiltration and offer limited predictive accuracy when used in isolation.
Radiomics, a rapidly evolving field, transforms medical imaging data into high-throughput quantitative features that capture tumor heterogeneity beyond what is observable through conventional imaging (15). By integrating radiomic features derived from preoperative computed tomography (CT) imaging, clinicians can non-invasively assess tumor biology and prognosis. However, radiomic features alone may not fully reflect the systemic and biochemical environment of the tumor, which is captured by serum markers. Therefore, combining radiomic features with serum biomarkers associated with microvascular infiltration offers a complementary approach to enhance the prediction of early recurrence.
This study addresses the limitations of existing models by developing a multidimensional predictive model that integrates preoperative CT radiomic features with serum biomarkers related to microvascular infiltration. This novel combination leverages the strengths of both modalities: radiomics provides a non-invasive, quantitative assessment of tumor heterogeneity, while serum markers reflect underlying biological processes, such as angiogenesis and tumor invasiveness. By bridging these complementary domains, the proposed model aims to improve the accuracy and clinical utility of early recurrence prediction, providing clinicians with a robust, personalized tool to guide postoperative management and improve patient outcomes. We present this article in accordance with the TRIPOD and STROBE reporting checklists (available at https://jgo.amegroups.com/article/view/10.21037/jgo-2024-914/rc).
Methods
Research subjects
This study was a single-center, retrospective analysis. A total of 156 HCC patients who were hospitalized and underwent radical surgery at the Tumor Hospital Affiliated to Nantong University between January 2021 and January 2022 were included in the study. The inclusion criteria for the study were: (I) have pathologically confirmed HCC; (II) have undergone preoperative CT imaging within 2 weeks prior to surgery; (III) have CT images of adequate quality; and (IV) have provided informed consent. Patients were excluded from the study if they met any of the following exclusion criteria: (I) had received preoperative HCC treatment; (II) had incomplete clinical data; and/or (III) were lost to follow-up within 2 years. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the Tumor Hospital Affiliated to Nantong University (No. 2024-029), and informed consent was obtained from all the patients.
Follow-up assessment
Patients were regularly followed up by outpatient visits, and telephone calls, and reference to their medical records. Postoperative imaging included ultrasound, CT, magnetic resonance imaging, or hepatic artery angiography to detect recurrence. Early recurrence was defined as new liver lesions or metastases within 2 years after surgery, confirmed by imaging and pathology.
Clinical data collection
The following preoperative data were collected for this study: (I) basic information: gender, age, liver cirrhosis history, hepatitis history, etc.; (II) pathological data: Barcelona Clinic Liver Cancer (BCLC) stage, Kiel 67 (Ki-67), and Edmondson grade; (III) Preoperative imaging data: CT images and CT signs (e.g., tumor diameter, number of lesions, tumor location, abnormal enhancement around the tumor, and hepatosplenomegaly); and (IV) preoperative AFP, VEGF-A, SP100, FIB-4 index levels.
CT examination method
CT imaging of the upper abdomen was performed with both unenhanced and contrast-enhanced scans. The specific scanning parameters were as follows: slice thickness: 5 mm; slice spacing: 5 mm; matrix: 512×512; tube voltage: 120 kV; tube current: 210 mAs; and reconstruction slice thickness: 1.25 mm. A routine unenhanced scan was first conducted, followed by contrast injection. A Ulrich Missouri dual-channel power injector (Ulrich Medical, Germany) was used to inject iopromide (1.1 mL/kg) at a flow rate of 2.4 m/s into the antecubital vein. Subsequently, 20 mL of saline was injected at the same flow rate to flush the catheter. Scans were performed at arterial phase, venous phase, and delayed phase at 25, 60, and 120 seconds after contrast injection, respectively.
Radiomics analysis
Feature extraction
The collected CT image data were resampled and standardized for gray scale. A senior radiologist with over five years of experience in abdominal imaging manually delineated the regions of interest (ROI) along the edges of the target lesions on the CT plain, arterial, venous, and delayed phase images using the open-source software 3D Slicer. Subsequently, the Radiomics plugin in 3D Slicer was used for feature extraction, which included: (I) first-order statistics: these provide basic information about tumor density and heterogeneity. (II) Shape features (3D): these offer geometric information about the tumor, such as maximum diameter and volume, which are crucial for assessing the tumor’s growth pattern and its invasive properties. (III) Texture analysis, including the gray level co-occurrence matrix (GLCM), gray level size zone matrix (GLSZM), gray level run length matrix (GLRLM), gray level dependence matrix (GLDM), and neighboring gray tone difference matrix (NGTDM). These texture features are closely associated with the tumor’s microvascular structure. Microvascular invasion often correlates with higher image texture heterogeneity, indicating a more disordered and complex tumor microenvironment.
Feature reduction and selection
After feature extraction, we used the Mann-Whitney U test for statistical analysis to select radiomic features significantly associated with early recurrence of HCC. We employed the Spearman rank correlation coefficient to detect correlations between features, thereby avoiding redundancy in the model. Additionally, we standardized all features to ensure they were on the same scale, facilitating comparison and analysis. Subsequently, we utilized the least absolute shrinkage and selection operator (LASSO) algorithm for feature selection and dimensionality reduction. This method helped us identify the key features that best predict early HCC recurrence from a large set of radiomic features. This process optimized our predictive model by minimizing unnecessary information, ensuring the model was both accurate and practical. Finally, based on the importance of the selected features, we calculated each patient’s radiomic score using a formula (referred to as the radiomic scoring formula). This score integrates multiple imaging features, aiding physicians in assessing the risk of early postoperative recurrence in patients.
Statistical analysis
Data analysis was performed using IBM SPSS 26.0 (International Business Machines Corporation, USA) Normality was tested. Non-normally distributed continuous data are presented as [median (M25, M75)], and comparisons between groups were conducted using the Mann-Whitney rank-sum test. Categorical data are presented as n (%), and comparisons between groups were performed using the Chi-squared test. Logistic regression analysis was performed to identify independent risk factors for early postoperative recurrence. The nomogram model was developed using R language (R Foundation for Statistical Computing, Austria), and its area under the curve (AUC) value was obtained by receiver operating characteristic (ROC) curve to evaluate its performance. The threshold interpretation of AUC values was as follows: AUC (<0.6) indicates poor differentiation. AUC (0.6 to <0.7) indicates average differentiation. AUC (0.7to <0.8) indicates moderate discrimination. AUC (0.8 to <0.9) indicates good discrimination. AUC 0.9–1.0 indicates excellent discrimination. A calibration curve was generated to evaluate the goodness of fit of the model, and the consistency of the calibration curve was evaluated using Hosmer-Lemeshow test, with P>0.05 indicating a good fit. The net benefit of the model was evaluated using decision curve analysis.
Results
Comparison of clinical data between non-early recurrence and early recurrence patients
In this study, 60 patients (38.46%) experienced early recurrence, and 96 patients (61.54%) did not. The early recurrence patients had a significantly higher tumor diameter, Ki-67, AFP, and VEGF-A levels, FIB-4 index, number of lesions, and abnormal enhancement around tumors than the non-recurrence patients (P<0.05). Differences in the Edmondson grading distribution were also significant (P<0.05) (Tables 1-3).
Table 1
| Clinical features | Non-early relapse group (n=96) | Early relapse group (n=60) | Z/χ2 | P |
|---|---|---|---|---|
| Age (years) | 60.00 (55.00, 65.00) | 61.00 (54.75, 66.00) | –1.5191 | 0.13 |
| Gender | ||||
| Male | 28 (29.17) | 18 (30.00) | 0.0123 | 0.91 |
| Female | 68 (70.83) | 42 (70.00) | ||
| Cirrhosis | ||||
| No | 74 (77.08) | 50 (83.33) | 0.8846 | 0.35 |
| Yes | 22 (22.92) | 10 (16.67) | ||
| History of hepatitis | ||||
| No | 67 (69.79) | 38 (63.33) | 0.6999 | 0.40 |
| Yes | 29 (30.21) | 22 (36.67) | ||
| Drinking history | ||||
| No | 74 (77.08) | 42 (70.00) | 0.9716 | 0.32 |
| Yes | 22 (22.92) | 18 (30.00) | ||
| BCLC staging | ||||
| 0 | 40 (41.67) | 21 (35.00) | 3.624 | 0.16 |
| A | 54 (56.25) | 34 (56.67) | ||
| B | 2 (2.08) | 5 (8.33) | ||
| Ki-67 | ||||
| ≤20 | 71 (73.96) | 27 (45.00) | 13.2568 | <0.001 |
| >20 | 25 (26.04) | 33 (55.00) | ||
| Edmondson grade | ||||
| I | 73 (76.04) | 7 (11.67) | 61.26 | <0.001 |
| II | 21 (21.88) | 48 (80.00) | ||
| III | 2 (2.08) | 5 (8.33) |
Data are presented as median (M25, M75) or n (%). BCLC, Barcelona Clinic Liver Cancer.
Table 2
| CT signs | Non-early relapse group (n=96) | Early relapse group (n=60) | Z/χ2 | P |
|---|---|---|---|---|
| Tumor diameter | ||||
| ≤5 cm | 69 (71.88) | 24 (40.00) | 15.5820 | <0.001 |
| >5 cm | 27 (28.12) | 36 (60.00) | ||
| Number of lesions | ||||
| Single | 88 (91.67) | 43 (71.67) | 10.97 | <0.001 |
| Multiple | 8 (8.33) | 17 (28.33) | ||
| Abnormal enhancement around the tumor | ||||
| No | 87 (90.62) | 34 (56.67) | 24.4673 | <0.001 |
| Yes | 9 (9.38) | 26 (43.33) | ||
| Tumor location | ||||
| Left lobe | 38 (39.58) | 23 (38.33) | 0.2614 | 0.88 |
| Right lobe | 48 (50.00) | 32 (53.33) | ||
| Left lobe + right lobe | 10 (10.42) | 5 (8.33) | ||
| Lesion morphology and margin | ||||
| Regular | 57 (59.38) | 26 (43.33) | 3.816 | 0.051 |
| Irregular | 39 (40.62) | 34 (56.67) | ||
| Tumor pseudocapsule | ||||
| Yes | 49 (51.04) | 22 (36.67) | 3.077 | 0.08 |
| None | 47 (48.96) | 38 (63.33) | ||
| Hepatosplenomegaly | ||||
| No | 64 (66.67) | 40 (66.67) | 0.0000 | >0.99 |
| Yes | 32 (33.33) | 20 (33.33) | ||
Data are presented as n (%). CT, computed tomography.
Table 3
| Index | Non-early relapse group (n=96) | Early relapse group (n=60) | Z/χ2 | P |
|---|---|---|---|---|
| AFP (μg/L) | 304.20 (245.14, 420.22) | 417.03 (251.93, 525.42) | –2.8213 | 0.005 |
| VEGF-A (pg/mL) | 127.71 (95.49, 159.95) | 121.35 (86.60, 152.61) | –2.5372 | 0.01 |
| S100P (μg/L) | 14.40 (11.78, 16.86) | 14.00 (11.44, 16.60) | –1.9580 | 0.050 |
| FIB-4 Index | 2.06 (1.69, 2.39) | 1.96 (1.64, 2.33) | –2.2713 | 0.02 |
Data are presented as median (M25, M75). AFP, alpha fetoprotein; VEGF-A, vascular endothelial growth factor A; FIB-4, fibrosis-4.
Radiomic features
After the dimensionality reduction and LASSO regression, four arterial-phase and 10 venous-phase radiomic features associated with early postoperative recurrence were identified. The lambda with the smallest standard error of the distance was 0.03 (Figure 1). The imaging score formula corresponding to the model variables is expressed as follows:

−7.372−0.325 × V-Shape-Maximum − 3Ddiameter + 2.015 × A-Shape-Sphericity –1.421 × A-Firstorder-12Percentile – 1.587 × V-Firstorder-Kurtosis + 0.4 × V-Firstorder-Median + 0.898 × V-Firstorder-Robust Mean TAbsolute Deviation + 2.118 × V-GLCM – Difference Variance + 0.067 × A-GLCM – Inverse Variance + 5.168 × V-GLCM – Joint Energy + 1.159 × GLCM – Maximum Probability + 4.72 × V-GLSZM – Gray-Level Non Uniformity – 0.299 × V-GLSZM – High Gray-Level Zone Emphasis + 2.702 × V-GLSZM –Small Area Emphasis – 0.897 × A-GLSZM – Small Area High Gray-Level Emphasis + 2.39 × V-GLSZM – Small Area Low Gray-Level Emphasis.
Note: V, volume-based; A, area-based; GLCM: gray-level co-occurrence matrix; GLSZM: gray-level size zone matrix.
Calculations showed that the radiomic scores of the patients without early recurrence were significantly lower than those of the patients with early recurrence [–1.35 (–2.29, 1.21) vs. 0.94 (–0.40, 1.87), P=0.0001].
Logistic multi-factor analysis
The logistic analysis results showed that the number of lesions, Edmondson grade, AFP and VEGF-A levels, and radiomics score were independent risk factors for early postoperative recurrence in HCC patients (P<0.05) (Table 4).
Table 4
| Variables | β | SE | Z | P | OR (95% CI) |
|---|---|---|---|---|---|
| Intercept | –7.2647 | 1.7147 | –4.2367 | <0.001 | 0.0007 (0.0000–0.0202) |
| Number of lesions | 1.5285 | 0.6772 | 2.2570 | 0.02 | 4.6111 (1.2228–17.3884) |
| Abnormal enhancement around the tumor | 1.0073 | 0.6131 | 1.6430 | 0.10 | 2.7382 (0.8234–9.1056) |
| Edmondson stage | 2.8924 | 0.6151 | 4.7022 | <0.001 | 18.0362 (5.4021–60.2179) |
| Ki-67 | 0.7350 | 0.5139 | 1.4301 | 0.16 | 2.0854 (0.7616–5.7104) |
| Tumor diameter | 0.3703 | 0.5346 | 0.6927 | 0.49 | 1.4482 (0.5079–4.1292) |
| AFP | 0.0042 | 0.0021 | 2.0330 | 0.042 | 1.0042 (1.0001–1.0082) |
| VEGF-A | 0.0132 | 0.0063 | 2.0833 | 0.04 | 1.0133 (1.0008–1.0259) |
| FIB-4 | 0.3872 | 0.4693 | 0.8250 | 0.41 | 1.4728 (0.5870–3.6950) |
| Radiomics score | 0.3876 | 0.1232 | 3.1464 | 0.002 | 1.4734 (1.1574–1.8757) |
HCC, hepatocellular carcinoma; SE, standard error; OR, odds ratio; CI, confidence interval; AFP, alpha fetoprotein; VEGF-A, vascular endothelial growth factor A; FIB-4, fibrosis-4.
Nomogram model establishment
Based on the results of the logistic multivariate analysis, 156 subjects were randomly divided into a training set and an internal test set at a ratio of 8:2 using the R language rms package (R Foundation for Statistical Computing, Austria), and a nomogram model for predicting the risk of early postoperative recurrence in HCC patients was constructed (Figure 2A). In the nomogram, “0” on the “number of lesions” axis represents a single lesion, and “1” represents multiple lesions; “0” on the “Edmondson stage” axis represents Edmondson stage I, “1” represents Edmondson stage II, and “2” represents Edmondson stage III; the “VEGF-A” axis represents the actual preoperative VEGF-A level of the patient; the “AFP” axis represents the actual preoperative AFP level of the patient; the “radiomics score” axis represents the radiomics score of the patient’s preoperative CT image; and the “risk” axis represents the risk of early postoperative recurrence in HCC patients predicted by the model.

The ROC curve of the nomogram model was plotted (Figure 2B), and the results showed that the AUC value of the training set was 0.9265 (0.8799–0.9731). The internal validation of the nomogram was performed by 1000-bootstrap analyses, and the AUC value was 0.9255 (0.8376–1.0000), indicating that the model has good accuracy (Figure 2B, b-d). In addition, the ROC curves of simple clinical characteristics, microvascular infiltration serological characteristics, and the radiomics score for predicting early postoperative recurrence of HCC were plotted simultaneously. The results showed that the AUC value of the nomogram prediction model was greater than the AUC value of the simple clinical characteristics, microvascular infiltration serological characteristics, and radiomics score (Figure 2B), indicating that the nomogram model has better predictive efficiency. As Table 5 shows, the cut-off value of the model was 0.4610. In this study, risk scores ≥0.4610 points indicated a high risk of early postoperative recurrence in HCC patients, and risk scores <0.4610 points indicated a low risk of early postoperative recurrence in HCC patients.
Table 5
| Dataset | AUC (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | Cut-off value |
|---|---|---|---|---|
| Training set | 0.9265 (0.8799–0.9731) | 0.9136 (0.8524–0.9748) | 0.8140 (0.6976–0.9303) | 0.4610 |
| Internal test set | 0.9255 (0.8376–1.0000) | 0.8667 (0.6946–1.0000) | 0.7647 (0.5631–0.9663) | 0.4610 |
AUC, area under the curve; CI, confidence interval.
Performance evaluation
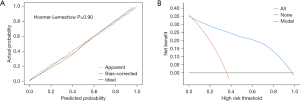
The Hosmer-Lemeshow test results (χ2=3.460, P=0.90) indicated that there was no significant difference between the predicted and actual values. The calibration curves also demonstrated that the predicted probabilities were generally consistent with the actual probabilities (Figure 3A). The clinical decision curves (Figure 3B) showed that within the threshold probability range of 0.01% to 75.00% (where the blue line was above the red and green lines), the model offered a net benefit and was effective at identifying patients at high risk of early postoperative recurrence for HCC surgery.
Discussion
HCC exhibits significant tumor heterogeneity, which contributes to its tendency for recurrence following treatment. A recent study has categorized HCC recurrence into early recurrence and late recurrence (14). Early recurrence is often associated with undetected micro-metastases or residual tumor cells prior to surgery, and may also be closely related to the tumor’s biological characteristics such as high invasiveness, active tumor angiogenesis, and genetic mutations. It is one of the key factors contributing to a poor prognosis in HCC patients (16). Therefore, reducing postoperative recurrence rates is a crucial component in improving overall treatment efficacy.
In recent years, researchers have focused on identifying risk factors for early recurrence to better predict recurrence risk. This study preliminarily identified the tumor diameter, Ki-67, AFP, and VEGF-A levels, FIB-4 index, lesion number, tumor-enhancing abnormalities, and Edmondson grading as factors associated with early HCC recurrence through the univariate analysis. These indicators are closely related to tumor invasiveness and biological behavior, further supporting their predictive ability for early HCC recurrence.
Radiomics is an emerging field that explores potential relationships between medical images and tumors non-invasively. In recent years, numerous researchers have identified and applied a variety of radiomics features related to HCC for early diagnosis and prognosis prediction (17-21). This is primarily because radiomics analysis facilitates the extraction of quantitative features from imaging data that cannot be observed with the naked eye, which are correlated with pathological characteristics of HCC, such as microvascular density and histological subtypes (22). In this study, after the dimensionality reduction, 10 venous-phase and four arterial-phase radiomic features associated with early recurrence were identified. These features describe tumor geometry, density distribution, and texture structure, helping to capture tumor heterogeneity and predict tumor biological behavior or clinical outcomes (e.g., recurrence risk and prognosis). The radiomics score formula derived from these features indicated that the imaging radiomics scores of patients without early recurrence were significantly lower than those with early recurrence. The radiomics score was identified as an independent risk factor for early postoperative recurrence of HCC. Thus, the radiomics score could potentially be used to predict early HCC recurrence.
Nomograms are statistical tools that combine radiomic features with other clinical variables (e.g., pathological features and serum markers) to construct individualized predictive models. By providing a simple graphical representation, nomograms enable the intuitive prediction of patient outcomes (e.g., survival rates and recurrence risks). For instance, Mao et al. (21) showed that radiomic features can be used to non-invasively indicate the potential relationship between CT images and HCC pathological grading. When the radiomics models were combined with clinical factors for training machine-learning models, the AUC value was 0.8014, indicating a significant increase in model performance and the effective prediction of preoperative pathological grading in HCC.
In this study, a nomogram model was established to predict early postoperative recurrence of HCC based on preoperative microvascular infiltration-related serological and CT radiomic features. This model demonstrated excellent classification performance not only in the training set but also in the internal validation set (AUC >0.9), significantly outperforming a model that only included conventional clinical and pathological data (23,24), proving its strong predictive and classificatory abilities. Moreover, the combined model significantly improved predictive efficacy over single factor-based models, indicating the synergistic effect of combining various risk factors for early HCC recurrence prediction. The model did not exhibit significant systemic bias. Its predicted risk probabilities closely aligned with the actual incidence rates, showing its reasonable and accurate prediction capability across different risk levels.
However, this study had some limitations. This study is a single-center retrospective study with a relatively small sample size, which may affect the external validity and generalizability of the results (25). Although the study demonstrated high predictive ability, the lack of external validation samples limits the model’s applicability and stability. Future multicenter studies and validation with larger sample sizes will help further assess the external validity of the model. Additionally, the study did not consider postoperative lifestyle factors, emotional status, or patient adherence to medical advice, which could significantly impact HCC recurrence. Future research should include these potential confounding factors to improve the model’s predictive capability. Therefore, while this model demonstrates good predictive performance in internal validation, further studies are needed to verify its external validity and facilitate its application in a broader clinical context.
Conclusions
This study developed a multidimensional nomogram model incorporating CT radiomic features, microvascular infiltration-related serological markers, and conventional clinical parameters. The integrative innovation of this model significantly enhances the prediction accuracy for early postoperative recurrence of HCC and holds the potential to provide strong support for the management and treatment decisions of patients with early recurrence of HCC.
Acknowledgments
We thank Dr. Riccardo Inchingolo (Interventional Radiology Unit, “F. Miulli” Regional General Hospital, Bari, Italy) for the critical comments and valuable advice on this study.
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the TRIPOD and STROBE reporting checklists. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-2024-914/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-2024-914/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-2024-914/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-2024-914/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the Tumor Hospital Affiliated to Nantong University (No. 2024-029), and informed consent was obtained from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Brown ZJ, Tsilimigras DI, Ruff SM, et al. Management of Hepatocellular Carcinoma: A Review. JAMA Surg 2023;158:410-20. [Crossref] [PubMed]
- Kinsey E, Lee HM. Management of Hepatocellular Carcinoma in 2024: The Multidisciplinary Paradigm in an Evolving Treatment Landscape. Cancers (Basel) 2024;16:666. [Crossref] [PubMed]
- Altaf S, Saleem F, Sher AA, et al. Potential Therapeutic Strategies to Combat HCC. Curr Mol Pharmacol 2022;15:929-42. [Crossref] [PubMed]
- Zakaria S, Ansary A, Abdel-Hamid NM, et al. Dantrolene Potentiates the Antineoplastic Effect of Sorafenib in Hepatocellular Carcinoma via Targeting Ca+2/PI3K Signaling Pathway. Curr Mol Pharmacol 2021;14:900-13. [Crossref] [PubMed]
- Nevola R, Ruocco R, Criscuolo L, et al. Predictors of early and late hepatocellular carcinoma recurrence. World J Gastroenterol 2023;29:1243-60. [Crossref] [PubMed]
- Zhao QY, Liu SS, Fan MX. Prediction of early recurrence of hepatocellular carcinoma after resection based on Gd-EOB-DTPA enhanced magnetic resonance imaging: a preliminary study. J Gastrointest Oncol 2022;13:792-801. [Crossref] [PubMed]
- Yang X, Yuan C, Zhang Y, et al. Predicting hepatocellular carcinoma early recurrence after ablation based on magnetic resonance imaging radiomics nomogram. Medicine (Baltimore) 2022;101:e32584. [Crossref] [PubMed]
- Zhu Y, Gu L, Chen T, et al. Factors influencing early recurrence of hepatocellular carcinoma after curative resection. J Int Med Res 2020;48:300060520945552. [Crossref] [PubMed]
- Zhang S, Xu L, Dai F, et al. Construction of a predictive nomogram and bioinformatic investigation of the potential mechanism of postoperative early recurrence of hepatocellular carcinoma meeting the Milan criteria. Ann Transl Med 2022;10:866. [Crossref] [PubMed]
- He T, Zou J, Sun K. The efficiency of pathological response after preoperative transcatheter arterial chemoembolization for microvascular invasion and early tumor recurrence in hepatocellular carcinoma. Hepatobiliary Surg Nutr 2023;12:142-3. [Crossref] [PubMed]
- He J, Shi J, Fu X, et al. The Clinicopathologic and Prognostic Significance of Gross Classification on Solitary Hepatocellular Carcinoma After Hepatectomy. Medicine (Baltimore) 2015;94:e1331. [Crossref] [PubMed]
- Qi LN, Ma L, Wu FX, et al. S100P as a novel biomarker of microvascular infiltration and portal vein tumor thrombus in hepatocellular carcinoma. Hepatol Int 2021;15:114-26. [Crossref] [PubMed]
- Zhao X, Wang Y, Xia H, et al. Roles and Molecular Mechanisms of Biomarkers in Hepatocellular Carcinoma with Microvascular infiltration: A Review. J Clin Transl Hepatol 2023;11:1170-83. [PubMed]
- Yan WT, Li C, Yao LQ, et al. Predictors and long-term prognosis of early and late recurrence for patients undergoing hepatic resection of hepatocellular carcinoma: a large-scale multicenter study. Hepatobiliary Surg Nutr 2023;12:155-68. [Crossref] [PubMed]
- Zeng J, Zeng J, Lin K, et al. Development of a machine learning model to predict early recurrence for hepatocellular carcinoma after curative resection. Hepatobiliary Surg Nutr 2022;11:176-87. [Crossref] [PubMed]
- Hong SK, Jin XL, Suh S, et al. Different Risk Factors for Early and Late Recurrence After Curative Resection of Hepatocellular Carcinoma. World J Surg 2022;46:197-206. [Crossref] [PubMed]
- Li WF, Yen YH, Liu YW, et al. Preoperative predictors of early recurrence after resection for hepatocellular carcinoma. Am J Surg 2022;223:945-50. [Crossref] [PubMed]
- Jiang C, Cai YQ, Yang JJ, et al. Radiomics in the diagnosis and treatment of hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int 2023;22:346-51. [Crossref] [PubMed]
- Xia TY, Zhou ZH, Meng XP, et al. Predicting Microvascular infiltration in Hepatocellular Carcinoma Using CT-based Radiomics Model. Radiology 2023;307:e222729. [Crossref] [PubMed]
- Wang G, Ding F, Chen K, et al. CT-based radiomics nomogram to predict proliferative hepatocellular carcinoma and explore the tumor microenvironment. J Transl Med 2024;22:683. [Crossref] [PubMed]
- Mao B, Zhang L, Ning P, et al. Preoperative prediction for pathological grade of hepatocellular carcinoma via machine learning-based radiomics. Eur Radiol 2020;30:6924-32. [Crossref] [PubMed]
- Feng Z, Li H, Liu Q, et al. CT Radiomics to Predict Macrotrabecular-Massive Subtype and Immune Status in Hepatocellular Carcinoma. Radiology 2023;307:e221291. [Crossref] [PubMed]
- Wang Q, Qiao W, Zhang H, et al. Nomogram established on account of Lasso-Cox regression for predicting recurrence in patients with early-stage hepatocellular carcinoma. Front Immunol 2022;13:1019638. [Crossref] [PubMed]
- He Y, Luo L, Shan R, et al. Development and Validation of a Nomogram for Predicting Postoperative Early Relapse and Survival in Hepatocellular Carcinoma. J Natl Compr Canc Netw 2023;22:e237069. [PubMed]
- Seyfinejad B, Jouyban A. Importance of Method Validation in the Analysis of Biomarker. Curr Pharm Anal 2022;18:567-9. [Crossref]


