
Regional trends in colorectal cancer mortality in people aged 45–84 years in the US, 1999–2022
Highlight box
Key findings
• Age-adjusted mortality rate (AAMR) decreased across all United States census regions.
• The Midwest consistently had the highest AAMR, while the West had the lowest.
• Males in the Northeast had the highest AAMR, while females in the West had the lowest.
• Black or African Americans in the Midwest experienced the highest AAMR among racial groups, while Asian or Pacific Islanders in the Midwest had the lowest.
What is known and what is new?
• Adult obesity and smoking are known major factors contributing to cancer-related deaths but regional factors have lacked attention.
• This study analyzes the correlation between different regions of the United States and their corresponding colorectal cancer mortality rates.
What is the implication, and what should change now?
• Persistent differences in mortality rates across regions based on gender and race raise the need to address these disparities with targeted public health interventions.
• Future policymaking and resource allocation need to consider these differences to help minimize disparities in mortality trends and improve healthcare outcomes for all population groups.
Introduction
Colorectal cancer (CRC) ranks as the second most fatal cancer in the United States as of 2023 (1). The risk of developing CRC increases with age, but other significant risk factors include family history, male sex, genetic polyposis syndromes, dietary habits like eating red meat or consuming excessive amounts of alcohol, conditions such as inflammatory bowel disease, and smoking (2). Colonoscopy plays a critical role as a screening tool for detecting CRC. The United States Preventive Services Task Force (USPSTF) recommends colonoscopy screening for all adults aged 45 to 75 years, and even earlier for individuals deemed to be at high risk. Screening for individuals 75 to 85 years of age is done at the discretion of the clinician and patient based on the patient’s overall health, history, and preferences (3). This initiative, among others, such as the Colorectal Cancer Control Program sponsored by the Center for Disease Control, aims to increase early detection, and thus decrease mortality from CRC (4).
In 2020, national spending on CRC medical care reached $24.3 billion, up from $22.3 billion in 2015. This makes CRC-related expenses the second highest among cancer treatments, following only those for female breast cancer (5). Given the significant financial burden of CRC treatment and its position as a major cause of cancer mortality, it is crucial to not only examine changes in mortality trends but also to understand how various risk factors, demographic elements, and medical advancements are influencing these trends. Moreover, studying mortality trends may elucidate disparities among races and regions and provide insight into how CRC screening can garner more attention in these populations. Across the nation, adult obesity and smoking are major factors contributing to cancer-related deaths, except in the Midwest, where access to the United States Department of Agriculture’s Supplemental Nutrition Assistance Program has shown to be the most influential (6). Furthermore, a study done by Rogers et al. using the Centers for Disease Control and Prevention as well as The Surveillance, Epidemiology, and End Results databases indicates that the South and Midwest may be particularly concerning regions for CRC mortality among men diagnosed before the age of 50 years (6). These regions have been noted as geographical hotspots for CRC mortality by Siegel et al. as well (1). National estimates of differences in the contemporary trends of CRC mortality rates based on geographic regions are less well established for screening age population groups. Therefore, we analyzed the publicly available national database of recorded deaths in the United States related to CRC to further explore the impact of geographic location on CRC mortality for the screening age group population. We present this article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-624/rc).
Methods
Study design
The Centers for Disease Control and Prevention Wide-ranging Online Data for Epidemiologic Research (CDC WONDER) was used to identify CRC-related deaths in the United States, specifically the Multiple Cause of Death section (Multiple Cause of Death Data on CDC WONDER, n.d.). This database has been used by other researchers in the past to analyze national trends in mortality of diseases such as chronic lower lung disease (7). CRC-related mortality was identified using the International Classification of Diseases, 10th Revision, Clinical Modification codes C18, C19, and C20 in patients aged 45–84 years. The age restriction was chosen to align with the age range for Class A, B, and C of CRC screening recommended by the USPSTF (3). The USPSTF is a panel of experts in disease prevention and evidence-based medicine who are nationally recognized for providing screening guidelines to physicians. The USPSTF sets recommendations by considering the effectiveness of screening strategies in reducing incidence, mortality, or both, as well as their accuracy, potential harm, and whether effects vary demographically (8). This study was exempt from institutional review board approval because the CDC WONDER database contains anonymous data available to the public. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Demographic and regional study groups
Data on CRC-related deaths and population sizes from 1999 to 2022 were gathered and categorized. The data was stratified by demographics including gender, race/ethnicity, age groups (45–54, 55–64, 65–74, and 75–84 years), region, and state. Racial/ethnic groups were classified as non-Hispanic White, non-Hispanic Black, non-Hispanic American Indian/Alaska Native, non-Hispanic Asian/Pacific Islander, and Hispanic as reported on death certificates. Regions were delineated according to the four census regions established by the Census Bureau divisions.
Statistical analysis
CRC-related crude and age-adjusted mortality rates (AAMR) were computed for the study period. Crude mortality rates were calculated by dividing the number of CRC-related deaths by the corresponding United States population at each respective time point. As previously outlined, AAMRs were standardized using the 2000 United States population (9).
We used the Join point Regression Program to analyze trends over the study period (Join point version 4.9.0.0, available from the National Cancer Institute, Bethesda, Maryland). This program employs Join point regression, which identifies significant temporal changes in annual mortality trends by fitting models consisting of linear segments, where significant time-dependent variations were observed. The Monte Carlo permutation test was used to link a Join point and thus calculate annual percent change (APC) with a 95% confidence interval (CI) for the AAMR (10). The weighted average of the APCs was calculated and reported as AAPCs and respective 95% CI: as a summary of the reported mortality trend for the studied period. APC and AAPCs were considered decreasing or increasing if the slope describing the variation in mortality over the years was significantly different from zero using 2-tailed t-test. Statistical significance was set at P≤0.05. This study used “*” to signify statistical significance.
Results
Overall
From 1999 to 2022, there were 1,106,085 deaths due to CRC in the United States.
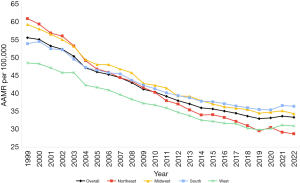
Overall AAMR decreased during this period (Figure 1, Table S1) with an AAPC of −2.31 (95% CI: −2.43 to −2.21). The APC in AAMR was −2.99* (95% CI: −3.19 to −2.85) from 1999–2014, which increased to −1.01* (95% CI: −1.50 to −0.28) from 2014–2022 (Figure S1). Overall, the AAMR decreased to the lowest at 33.00 in 2019 and increased to 33.20 in 2020 (Figure 1, Table S1).
Region stratified
The Midwest had the highest AAMR over the study period (Figure 1, Table S1) and an AAPC of −2.36* (96% CI: −2.48 to −2.25). The APC was −3.00* (95% CI: −3.19 to −2.85) in 1999–2015 and increased to −0.87* (95% CI: −1.43 to −0.05) in 2015–2022 (Figure S2).
The West had the lowest AAMR over the years (Figure 1, Table S1) and an AAPC of −1.99* (95% CI: −2.13 to −1.87). The APC was −2.69* (95% CI: −3.52 to −1.86) in 1999–2014 and increased to −1.79* (95% CI: −2.86 to −0.66) in 2014–2019. The APC further increased to 1.27 (95% CI: −0.31 to 3.36) in 2019–2022 (Figure S2).
The Northeast had an AAMR of 60.90 (95% CI: 59.80 to 62.01) in 1999, decreasing to 28.78 (95% CI: 28.11 to 29.45) in 2022 (Figure 1, Table S1), and an AAPC of −3.27* (95% CI: −3.42 to −3.14). The APC was −3.87* (95% CI: −4.18 to −3.67) from 1999–2013 and then increased to −2.33* (95% CI: −2.80 to −1.51) (Figure S2).
The South had an AAMR of 53.95 (95% CI: 53.15 to 54.74) in 1999, decreasing to 36.45 (95% CI: 35.94 to 36.97) in 2022 (Figure 1, Table S1), with an AAPC of −1.77* (95% CI: −1.87 to −1.67). The APC was −2.57* (95% CI: −2.84 to −2.42) in 1999–2012, increasing to −1.44* (95% CI: −2.07 to −0.91) in 2012–2019, and increasing even further to 1.03 (95% CI: −0.09 to 2.68) in 2019–2022 (Figure S2).
Region and gender stratified
Northeastern males had the highest average AAMR over the study period of both genders in all regions while Western females had the lowest (Figure 2, Table S2). Males in the Northeast had the highest average AAMR of all males while Western males had the lowest. Females in the Northeast had the highest average AAMR of all females while Western females had the lowest (Figure 2, Table S2).
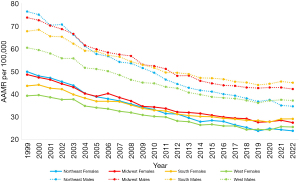
In the Midwest, males had a higher AAMR than females, while females had a higher AAPC (Figure 2, Table S2). Midwest males had an AAPC of −2.44* (95% CI: −2.58 to −2.30). The APC in 1999–2015 was −3.18* (95% CI: −3.42 to −2.99) and increased to −0.71 (95% CI: −1.37 to 0.46) in 2015–2022 (Figure S3). Comparatively, Midwest females had a lower AAMR of 48.50 (95% CI: 47.26 to 49.75) over the studied period (Figure 2, Table S2) and an AAPC of −2.51* (95% CI: −2.65 to −2.38). The APC in 1999–2012 was −3.14* (95% CI: −3.50 to −2.90) and increased to −1.69* (95% CI: −2.09 to −1.06) in 2012–2022 (Figure S3).
In the West, males once again had a higher AAMR than females while females had a higher AAPC (Figure 2, Table S2). Males had an AAPC of −2.09* (95% CI: −2.22 to −1.99) and the APC was −2.82* (95% CI: −3.46 to −2.18) in 1999–2014 and −1.60* (95% CI: −3.00 to −0.70) in 2014–2019. The APC further increased to 0.83 (95% CI: −0.68 to 2.65) in 2019–2022 (Figure S3). Western females had a lower AAMR (Figure 2, Table S2) and an AAPC of −1.92* (95% CI: −2.14 to −1.77). The APC was −2.55* (95% CI: −2.73 to −2.41) in 1999–2019 and increased to 2.40 (95% CI: −0.06 to 5.84) in 2019–2022 (Figure S3).
In the Northeast, males had a higher AAMR than females while females had a higher AAPC (Figure 2, Table S2). Northeastern males had an AAPC of −3.41* (95% CI: −3.59 to −3.26). The APC was −4.13* (95% CI: −4.53 to −3.87) in 1999–2013 and increased to −2.30* (95% CI: −2.86 to −1.22) in 2013–2022 (Figure S3). Comparatively, Northeastern females had a lower AAMR (Figure 2, Table S2) and an AAPC of −3.25* (95% CI: −3.46 to −3.07). The APC was −3.72* (95% CI: −4.36 to −3.48) in 1999–2014 and increased to −2.36 (95% CI: −3.09 to 0.09) in 2014–2022 (Figure S3).
In the South, males had a higher AAMR than females (Figure 2, Table S2) and an AAPC of −1.82* (95% CI: −1.92 to −1.74). The APC changed twice over the study period, measuring −2.67* (95% CI: −2.94 to −2.51) in 1999–2011, −1.53* (95% CI: −2.02 to −1.09) in 2011–2019, and then 0.83 (95% CI: −0.25 to 2.44) in 2019–2022 (Figure S3). On the other hand, Southern females had a lower AAMR (Figure 2, Table S2) and an AAPC of −1.81* (95% CI: −1.98 to −1.70). The APC changed twice over the study period, measuring −2.67* (95% CI: −3.07 to −2.47) in 1999–2013, −1.28* (95% CI: −2.47 to −0.62) in 2013–2020, and 2.49 (95% CI: −0.32 to 4.08) in 2020–2022 (Figure S3).
Region and race stratified
AAMR from 1999–2022 was lowest in Asians and Pacific Islanders in the Northeast (Figure 3, Table S3), who had an AAPC of −1.63* (95% CI: −2.13 to −1.04). APC was the same as the AAPC (Figure S4). AAMR was highest for Asians and Pacific Islanders in the West (Figure 3, Table S3), who had an AAPC of −1.44* (95% CI: −1.93 to −1.13). The APC for Western Asians and Pacific Islanders was initially −2.01* (95% CI: −3.17 to −1.64) from 1999–2019. From 2019–2022, the APC increased to 2.48 (95% CI: −1.59 to 7.11) (Figure S4). AAPC was −1.70* (95% CI: −2.51 to −0.69) for Asians and Pacific Islanders in the Midwest and −1.19* (95% CI: −1.95 to −0.39) in the South. The APC for the Midwest was the same as the AAPC, while the APC for the South was −2.53* (95% CI: −8.75 to −1.18) in 1999–2013 before increasing to 0.94 (95% CI: −0.65 to 6.99) in 2013–2022 (Figure S4).
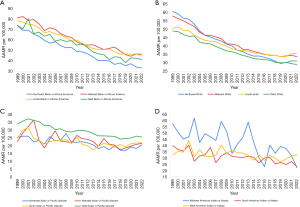
AAMR over the study period was highest in Midwestern Black people (Figure 3, Table S4), who had an AAPC of −2.69* (95% CI: −2.97 to −2.43). The APC was −3.15* (95% CI: −5.25 to −2.71) in 1999–2012 and increased to −2.09 (95% CI: −2.72 to 0.81) in 2012–2022 (Figure S5). AAMR was lowest for Black people in the Northeast (Figure 3, Table S4), who had an AAPC of −3.41* (95% CI: −3.61 to −3.23). The APC was steady at −3.41* (95% CI: −3.61 to −3.23) from 1999–2022 (Figure S5). Black people in the South had an AAPC of −2.48* (95% CI: −2.68 to −2.30) while those in the West had an AAPC of −2.55* (95% CI: −3.16 to −2.16). The APC for Black people in the South was −2.86* (95% CI: −3.93 to −2.59) from 1999–2014 and increased to −1.75 (95% CI: −2.38 to 0.42) in 2014–2022 (Figure S5). The APC for Black people in the West was −3.11* (95% CI: −5.49 to −0.62) in 1999–2019 and increased to 1.29 (95% CI: −3.01 to 7.34) in 2019–2022 (Figure S5).
Average AAMR for White people was highest in the Midwest (Figure 3, Table S5). They had an AAPC of −2.30* (95% CI: −2.45 to −2.17). The APC was −3.02* (95% CI: −3.25 to −2.84) in 1999 to 2015 and increased to −0.62 (95% CI: −1.29 to 0.47) in 2015–2022 (Figure S6). AAMR for White people was lowest in the West (Figure 3, Table S5) and they had an AAPC of −1.99* (95% CI: −2.12 to −1.86). The APC was −2.72* (95% CI: −3.48 to −2.36) in 1999–2014 and increased to −1.62* (95% CI: −2.82 to −0.60) in 2014–2019. The APC rose once again to 1.20 (95% CI: −0.36 to 3.30) in 2019–2022 (Figure S6). The AAPC for White people in the Northeast and in the South were −3.20* (95% CI: −3.36 to −3.05) and −1.59* (95% CI: −1.73 to 1.48) respectively. The APC for Northeastern White people was −3.88* (95% CI: −4.24 to −3.64) in 1999–2013 and increased to −2.13* (95% CI: −2.68 to −1.13) in 2013–2022 (Figure S6). For Southern White people, the APC was −2.60* (95% CI: −3.12 to −2.38) in 1999–2011, increased to −1.38* (95% CI: −2.06 to −0.64) in 2011–2019, and increased again to 1.98* (95% CI: 0.38 to 4.21) in 2019–2022 (Figure S6).
AAMR for American Indian or Alaska Natives was highest in the Midwest (Figure 3, Table S6) where they had an AAPC and APC of −2.51* (95% CI: −3.89 to −0.96) (Figure S7). AAMR for American Indian or Alaska Natives was lowest in the South (Figure 3, Table S6) where the AAPC and APC were −1.72* (95% CI: −2.36 to −0.96) (Figure S7). In the West, American Indian or Alaska Natives had an AAPC and APC of −0.92* (95% CI: −1.59 to −0.14) (Figure S7). There was no American Indian or Alaska Native Northeast AAMR data available for the studied period.
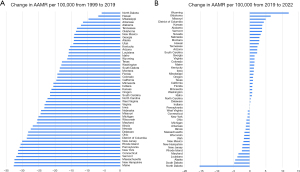
State-level difference
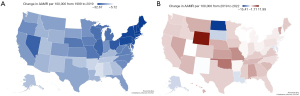
In 1999, AAMR varied from West Virginia where it was the highest to Hawaii where it was the lowest (Table S7). In 2022, AAMR varied from the highest in Mississippi to the lowest in Massachusetts (Table S7). New Hampshire had the largest decrease in AAMR from 1999–2022 (Table S7). Hawaii had the smallest decrease in AAMR (Table S7). States in the greater 90th percentile of CRC-related mortality from 1999–2019 included North Dakota, Mississippi, and Arkansas. Conversely, states in the less than 10th percentile of CRC-related mortality included Pennsylvania, New York, New Jersey, and most Northeastern states (Figures 4,5). From 2019–2022, Colorado showed the greatest increase in AAMR while North Dakota had the greatest decrease in AAMR (Figures 4,5).
Discussion
Our study offers significant insights into the 23-year trends of AAMR for CRC in the United States. Overall, there was a consistent annual decrease in AAMR throughout the study period. However, we observed an increase in the APC after 2014, a trend that has been documented previously (11). Additionally, there was a non-significant upward trend observed during the COVID-19 pandemic years from 2020 to 2022.
In 2018, screening guidelines for CRC were changed to begin at age 45 instead of 50 due to a perceived increase in CRC diagnosis in young people. This year alone, screening rates in people aged 40–49 years doubled (5–12%). Despite the increase in screening rates, the anticipated clinically meaningful earlier detection of CRC was not observed in this population. This age group also had equal odds of being diagnosed with late-stage disease instead of the expected migration towards earlier stages. Southern states had the highest rate of CRC diagnosis before and after the change in policy while the West had the lowest. No statistically significant changes in diagnosis rate before or after guideline change were observed in any region (12). This likely explains why despite a large policy change in 2018, a statistically significant overall change in AAMR did not occur.
An overall change in APC was observed in the year 2014, as well as in each region individually from the years 2012–2015. Investigations done by Murphy et al. into this trend revealed that as for White and Black individuals, incidence of CRC sharply decreased from 2012–2013, likely due to colonoscopy and polypectomy becoming the favored method of screening over sigmoidoscopy and fecal occult blood testing in the early 2000’s (13). They also mention that despite the decrease in risk factors in the general population such as smoking and red meat consumption, the increasing prevalence of obesity in the United States makes risk factors less of a factor in this time period for decreasing incidence and mortality (14).
Our study highlights important regional trends in CRC. The Midwest and South were the regions with the highest mortality from 2005–2022. Interestingly, both regions are hotspots for early-onset CRC (EOCRC), which refers to cases occurring in individuals under 50, between 1999–2017 (6). In these hotspot areas, survival rates for men with EOCRC were significantly lower (6). While Siegel et al. found similar hotspot regions (15), the reasons behind these trends in EOCRC remain unclear. Such increased mortality from EOCRC likely contributes to the overall CRC mortality rates in these two regions.
The Northwest had the largest decrease in AAMR over the study period; comparatively, the South had the smallest decrease in AAMR. These trends are in part due to screening rates in each region as the Northeast has the highest screening rate while the South has the lowest (13). A further increase in APC occurred between 2019–2020, potentially exacerbated by the lack of colorectal screening and treatment capability during the COVID-19 pandemic (16). The South and Midwest may be concerning regions for CRC mortality among men diagnosed before the age of 50, indicating that outcomes in screening in this age group have not been explored thoroughly before and are essential for future recommendations.
Throughout the study period, males consistently exhibited a higher AAMR compared to females in all regions. The AAPC was larger for males than females in the Midwest, West, and Northeast regions. Overall, the slowing or reversal of AAPC in recent years, particularly in the South and West, raises concern of emerging disparities and the potential need for renewed public health efforts.
When stratified by region, Black or African American populations exhibited significant disparities in CRC mortality in all regions, with the highest AAMR in the Midwest census region and lowest in the West. Geographic trends between Black or African Americans and White individuals were similar in this regard, underscoring the disparity of socioeconomic status, access to healthcare, and difference in genetic predisposition between these two groups (17). A higher AAMR for Black or African Americans in the Midwest has also been attributed to higher birth rates (6). Interestingly, no one region resulted in either the highest or lowest AAMR across all races. This finding stresses the importance of region-specific public health interventions tailored to the needs of racial groups in varying geographical areas. Understanding this difference will require further investigation to address more region-specific challenges to each population.
State-wide variations in CRC AAMR largely correspond with census data by region. Interestingly, much of the difference between AAMR variation between states may be a result of screening rates. In fact, simulation modeling suggests that 46%–63% of CRC deaths in the U.S. are due to missed screening (18). Lansdorp-Vogelaar et al. found that screening rates were an important factor in projecting CRC associated mortality when comparing Louisiana to New Jersey (19). State-specific participation rate in organized screening programs may also contribute to differences in screening and therefore mortality. Screen to Save is an initiative aimed at increasing screening rates in minority populations. This program sees the lowest rate of participation in the South, which coincides with the higher mortality rates found in this study in Southern populations (20). Additionally, barriers to screening have been observed in rural areas, such as a lack of prevention attitude toward cancer, perceived lack of privacy, shortage of specialists, and distance to test facilities (21). For this reason, many organized screening programs have been aimed towards rural populations, a possible explanation for the drastic decrease in CRC mortality in rural states such as Vermont, Maine, and West Virginia (17,22). However, Lansdorp-Vogelaar et al. also found that controlling for smoking and obesity prevalence in addition to screening rates eliminated differences in CRC mortality between the two states, suggesting that other variables beyond screening rates may also be having a considerable effect on AAMR (19).
States with the most pronounced increases in AAMR were typically located in Appalachia and parts of the South and Midwest (1). However, overall, there was an increase in AAMR in nearly all states except for North Dakota. This pattern aligns with findings from Shah et al., who noted that the AAPC has risen across all regions (23). These trends underscore significant concerns regarding the escalating rates of colorectal carcinoma despite the availability of robust screening tools, highlights the need for readiness within the United States healthcare system to manage this rise effectively.
Because CDC WONDER data is collected from a public health database, this study may have some limitations. Variables such as social determinants of health could have contributed to the patients’ deaths but were not reported on the website or death certificate. Another limitation of this study was the inability to differentiate between Native Americans and Alaskan Natives, who may have differing mortality rates.
Conclusions
The overall mortality related to CRC has decreased significantly in the past two decades. However, there are persistent differences in CRC-associated mortality rates across regions based on gender and race. It’s crucial to address these disparities with targeted public health interventions. To promote health equity throughout the United States, it’s essential to consider these differences and current trends for future policymaking and resource allocation. This will help minimize disparities in mortality trends and improve healthcare outcomes for all population groups.
Acknowledgments
Part of this manuscript has been presented in Omaha, NE at Creighton University School of Medicine Department of Medicine Research Day in May 2024.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-624/rc
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-624/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-624/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Wagle NS, Cercek A, et al. Colorectal cancer statistics, 2023. CA Cancer J Clin 2023;73:233-54. [Crossref] [PubMed]
- Sninsky JA, Shore BM, Lupu GV, et al. Risk Factors for Colorectal Polyps and Cancer. Gastrointest Endosc Clin N Am 2022;32:195-213. [Crossref] [PubMed]
- A and B Recommendations | United States Preventive Services Taskforce [Internet]. [cited 2024 Aug 13]. Available online: https://www.uspreventiveservicestaskforce.org/uspstf/recommendation-topics/Uspstf-a-and-b-recommendations
- CDC. Colorectal Cancer Control Program. 2024 [cited 2024 Aug 13]. About Colorectal Cancer Control Program. Available online: https://www.cdc.gov/colorectal-cancer-control/about/index.html
- Financial Burden of Cancer Care | Cancer Trends Progress Report [Internet]. [cited 2024 Aug 13]. Available online: https://progressreport.cancer.gov/after/economic_burden
- Rogers CR, Moore JX, Qeadan F, et al. Examining factors underlying geographic disparities in early-onset colorectal cancer survival among men in the United States. Am J Cancer Res 2020;10:1592-607. [PubMed]
- Baral N, Jabbar ABA, Noor A, et al. Demographic and geographical trends in chronic lower respiratory diseases mortality in the United States, 1999 to 2020. Respir Res 2024;25:258. [Crossref] [PubMed]
- Colorectal cancer: Screening [Internet]. US Preventive Services Taskforce; 2021 [cited 2024 Nov 12]. Available online: https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/colorectal-cancer-screening
- Age standardization of death rates; implementation of the year 2000 standard [Internet]. [cited 2024 Aug 13]. Available online: https://stacks.cdc.gov/view/cdc/13357
- Kim HJ, Fay MP, Feuer EJ, et al. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 2000;19:335-51. [Crossref] [PubMed]
- Ilyas F, Ahmed E, Ali H, et al. Temporal trends in colorectal cancer mortality rates (1999-2022) in the United States. Cancer Rep (Hoboken) 2024;7:e2012. [Crossref] [PubMed]
- Shafique N, Susman CG, Tortorello GN, et al. Changing colon cancer screening guidelines to age 45: Has it made a difference? Surgery 2024;176:680-3. [Crossref] [PubMed]
- Murphy CC, Sandler RS, Sanoff HK, et al. Decrease in Incidence of Colorectal Cancer Among Individuals 50 Years or Older After Recommendations for Population-based Screening. Clin Gastroenterol Hepatol 2017;15:903-909.e6. [Crossref] [PubMed]
- Tan JY, Yeo YH, Ng WL, et al. How have US colorectal cancer mortality trends changed in the past 20 years? Int J Cancer 2024;155:493-500. [Crossref] [PubMed]
- Siegel RL, Medhanie GA, Fedewa SA, et al. State Variation in Early-Onset Colorectal Cancer in the United States, 1995-2015. J Natl Cancer Inst 2019;111:1104-6. [Crossref] [PubMed]
- Kadakuntla A, Wang T, Medgyesy K, et al. Colorectal cancer screening in the COVID-19 era. World J Gastrointest Oncol 2021;13:238-51. [Crossref] [PubMed]
- Colorectal Cancer Screening Evidence-Based Programs Listing | Evidence-Based Cancer Control Programs (EBCCP) [Internet]. [cited 2024 Nov 3]. Available online: https://ebccp.cancercontrol.cancer.gov/topicPrograms.do?topicId=102265&choice=default&displayStart=
- Kimura A, Bell-Brown A, Akinsoto N, et al. Implementing an Organized Colorectal Cancer Screening Program: Lessons Learned From an Academic-Community Practice. AJPM Focus 2024;3:100188. [Crossref] [PubMed]
- Lansdorp-Vogelaar I, Meester R, de Jonge L, et al. Risk-stratified strategies in population screening for colorectal cancer. Int J Cancer 2022;150:397-405. [Crossref] [PubMed]
- Whitaker DE, Snyder FR, San Miguel-Majors SL, et al. Screen to Save: Results from NCI's Colorectal Cancer Outreach and Screening Initiative to Promote Awareness and Knowledge of Colorectal Cancer in Racial/Ethnic and Rural Populations. Cancer Epidemiol Biomarkers Prev 2020;29:910-7. Erratum in: Cancer Epidemiol Biomarkers Prev 2022;31:298. [Crossref] [PubMed]
- Wang H, Roy S, Kim J, et al. Barriers of colorectal cancer screening in rural USA: a systematic review. Rural Remote Health 2019;19:5181. [Crossref] [PubMed]
- Most Rural States 2024 [Internet]. [cited 2024 Nov 3]. Available online: https://worldpopulationreview.com/state-rankings/most-rural-states
- Shah SK, Narcisse MR, Hallgren E, et al. Assessment of Colorectal Cancer Screening Disparities in U.S. Men and Women Using a Demographically Representative Sample. Cancer Res Commun 2022;2:561-9. [Crossref] [PubMed]


