
Regorafenib with or without chemotherapy/immunotherapy in second-line treatment of metastatic colorectal cancer during the COVID-19 pandemic: a single-center retrospective analysis
Highlight box
Key findings
• This single-center retrospective study shows that regorafenib, alone or combined with capecitabine or immune checkpoint inhibitors (ICIs), is a feasible and safe second-line treatment option for patients with metastatic colorectal cancer (mCRC) during the coronavirus disease 2019 (COVID-19) pandemic. Patients receiving combination therapy had improved progression-free survival and overall survival compared to those treated with regorafenib monotherapy.
What is known and what is new?
• It is known that regorafenib is effective as a third-line treatment for mCRC.
• This study offers new insights into the potential benefits of using regorafenib as a second-line treatment, particularly when combined with capecitabine or ICIs, in events such as the COVID-19 pandemic.
What is the implication, and what should change now?
• The findings suggest that regorafenib, especially in combination with other therapies, could be an important second-line option for patients with mCRC, particularly when intravenous chemotherapy is not feasible.
• Further prospective studies are needed to confirm these findings and guide optimal treatment strategies for mCRC in the context of limited hospital access.
Introduction
In 2022, colorectal cancer (CRC) ranked as the third most commonly diagnosed cancer and the second leading cause of cancer-related mortality worldwide (1). In China, CRC ranks second in incidence and fourth in mortality, with approximately 517,000 new cases and 240,000 deaths each year (2). The current standard treatments for CRC in both first and second line include a combination of fluoropyrimidines with either oxaliplatin or irinotecan, supplemented by bevacizumab or cetuximab (3). Metastatic colorectal cancer (mCRC) presents a significant treatment challenge, with limited effective options for patients who progress after front-line therapy (4). For third-line treatment, regorafenib, fruquintinib, and trifluridine-tipiracil (TAS-102), with or without bevacizumab, are recommended (3,4).
The phase III CORRECT trial evaluated regorafenib, a small-molecule inhibitor of multiple kinases involved in tumor growth and angiogenesis, in pretreated mCRC patients (5-8). This trial randomized 760 patients with progressive disease on standard therapy to receive either best supportive care with placebo or regorafenib. The trial met its primary endpoint, yielding an overall survival (OS) of 6.4 months for regorafenib and of 5.0 months for placebo [hazard ratio (HR) =0.77, 95% confidence interval (CI) 0.64–0.94; P=0.005] and also demonstrated a modest improvement in progression-free survival (PFS) (9-11). Similarly, the CONCUR trial in Asia (12) as well as other studies (13-15) showed that regorafenib prolonged OS compared to placebo in patients with mCRC who have experienced disease progression following standard therapies.
Despite the well-defined efficacy in further lines of therapy, the role of regorafenib as a second-line treatment remains to be fully determined. In this context, the STREAM trial, an academic, multicenter, single-arm, Italian phase II study (16), revealed that regorafenib monotherapy, when used as a second-line treatment for rat sarcoma viral oncogene homolog (RAS)-mutant advanced colorectal cancer, exhibited an objective response rate (ORR) of 10.9% with a disease control rate (DCR) of 54.6% and a median PFS (mPFS) of 3.6 months and median OS (mOS) of 18.9 months. Although the study did not achieve its primary endpoint, the results are clinically meaningful, particularly in those patients or in those situations where a reduction in the number of hospital admissions is required.
A similar scenario involved the coronavirus disease 2019 (COVID-19) pandemic which profoundly impacted healthcare delivery worldwide, limiting patients’ ability to access regular hospital-based chemotherapies (17). This situation highlighted the need for oral therapies that can be administered at home, reducing hospital visits and the associated risk of COVID-19 exposure. Regorafenib, with its oral administration and broad antitumor activity, offers a promising alternative to traditional intravenous chemotherapies (13). Moreover, combining regorafenib, a multi-kinase inhibitor also targeting the vascular endothelial growth factor receptors (VEGFR)1–3 (5), with capecitabine, a fluoropyrimidine cytotoxic drug, sounds like using other combinations, including 5-fluorouracil/capecitabine with the antiangiogenic bevacizumab that have been widely explored in AVEX (18) and AVF2107g trials (19). Besides, studies like REGONIVO have shown encouraging results for regorafenib combined with anti-programmed death 1 (PD-1) antibody in later-line treatments, suggesting the potential of this combination for enhanced therapeutic efficacy (20). Due to the relatively short infusion times, immune checkpoint inhibitors (ICIs) like anti-PD-1 antibodies can be administered conveniently in outpatient clinics or community health settings, making them accessible even under pandemic constraints. This study evaluated the efficacy and safety of regorafenib, alone or combined with capecitabine or ICIs, as a second-line treatment for patients with mCRC during the COVID-19 pandemic. Although the COVID-19 pandemic has ended, challenges such as minimizing hospital visits and addressing barriers to care for mCRC patients remain highly relevant. Oral therapeutic regimens like regorafenib offer a convenient alternative to intravenous chemotherapy, reducing logistical burdens and psychological stresses for patients. This study aims to explore the potential of regorafenib, alone or combined with capecitabine or ICIs, as a second-line treatment to address these unmet needs and inform future clinical strategies. We present this article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-2024-891/rc).
Methods
Study design
This retrospective study included patients with mCRC treated at Peking University Third Hospital between January 2020 and September 2023. The eligibility criteria were histologically confirmed mCRC, progression after first-line treatment, and administration of regorafenib alone or in combination with capecitabine/anti-PD-1 antibodies (sintilimab or tislelizumab) as second-line therapy. Patients who could not regularly visit the hospital due to COVID-19 restrictions were included. Meanwhile, the exclusion criteria included patients with incomplete medical records or those who received other investigational drugs during the study period. Patients were followed up regularly through electronic medical records and scheduled clinical visits until death or the end of the study period.
Data collection
Data were obtained from electronic medical records, including baseline clinical factors such as age, sex, Eastern Cooperative Oncology Group (ECOG) performance status, metastatic sites, RAS/B-Raf proto-oncogene, serine/threonine kinase (BRAF) mutation status, and prior treatment regimens. These factors were assessed to evaluate their potential impact on treatment outcomes. Treatment regimens, PFS, OS, and adverse drug reactions (ADRs) were also collected. PFS was defined as the interval from regorafenib initiation to either disease progression or death. OS was defined as the duration from regorafenib initiation to death from any cause. ADRs were assessed using the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0.
Ethical approval
This study adhered to the Declaration of Helsinki (as revised in 2013) and received approval from the Ethics Committee of Peking University Third Hospital (approval No. IRB00006761-M2023746). The Ethics Committee waived the requirement for informed consent given the retrospective design of the study. Patient data were anonymized for confidentiality.
Statistical analysis
Due to the retrospective nature of this study, no prior sample size estimation was performed. The sample size was determined based on the available patient population meeting the inclusion criteria during the study period from January 2020 to September 2023. Kaplan-Meier estimates were used to assess PFS and OS, with group differences evaluated via the log-rank test. Multivariate Cox regression analysis was performed to identify independent prognostic factors for PFS and OS. HRs and their 95% CIs were calculated. All statistical tests were two-sided, and a P value <0.05 was considered statistically significant. Statistical analyses were conducted using SPSS 27 (IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
The study included 31 patients with a median age of 65 years (range, 29–91 years), predominantly male, and with most having an ECOG performance status of 1. The metastatic sites were primarily in the liver, lungs, and peritoneum or abdominal cavity. Most patients had mutated RAS/BRAF V600E status and received chemotherapy combined with bevacizumab as their first-line regimen. For second-line treatment, regorafenib was administered as monotherapy or in combination with capecitabine or ICIs. Detailed baseline characteristics are summarized in Table 1.
Table 1
| Characteristic | Value (n=31) |
|---|---|
| Age | |
| Median [range], years | 65 [29–91] |
| <65 years, n (%) | 14 (45.2) |
| ≥65 years, n (%) | 17 (54.8) |
| Sex, n (%) | |
| Male | 18 (58.1) |
| Female | 13 (41.9) |
| ECOG performance status, n (%) | |
| 0 | 14 (45.2) |
| 1 | 17 (54.8) |
| Metastatic sites, n (%) | |
| Liver | 12 (38.7) |
| Lung | 18 (58.1) |
| Peritoneum or abdominal cavity | 11 (35.5) |
| RAS/BRAF V600E status, n (%) | |
| Mutated | 20 (64.5) |
| Wild type | 11 (35.5) |
| First-line regimen, n (%) | |
| Chemotherapy only | 8 (25.8) |
| Chemotherapy plus bevacizumab | 19 (61.3) |
| Chemotherapy plus cetuximab | 4 (12.9) |
| Second-line regimen, n (%) | |
| Regorafenib only | 19 (61.3) |
| Regorafenib plus chemotherapy | 9 (29.0) |
| Regorafenib plus ICIs | 3 (9.7) |
| Regorafenib maintenance dose, n (%) | |
| 80 mg | 24 (77.4) |
| 120 mg | 7 (22.6) |
ECOG, Eastern Cooperative Oncology Group; RAS, rat sarcoma viral oncogene homolog; BRAF, B-Raf proto-oncogene, serine/threonine kinase; ICI, immune checkpoint inhibitor.
Treatment outcomes
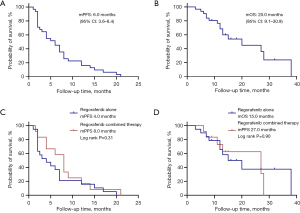
The average follow-up time was 20.4 months (95% CI: 15.6–25.2). The mPFS for all patients was 6.0 months (95% CI: 3.6–8.4), and the mOS was 20.0 months (95% CI: 9.1–30.9) (Figure 1A,1B). Patients treated with regorafenib plus capecitabine or immunotherapy (combination therapy) tended to have a longer PFS compared to those treated with regorafenib alone, with an mPFS of 8.0 versus 4.0 months, respectively (P=0.31). Similarly, OS was longer in the combination therapy group than in the regorafenib-alone group, with an mOS of 27.0 versus 15.0 months, respectively (P=0.90) (Figure 1C,1D).
Subgroup analysis
Subgroup analysis was conducted to clarify the influence of various clinical and demographic factors on treatment outcomes (Table 2). Patients with liver metastases at the initiation of regorafenib therapy exhibited a trend toward shorter PFS and OS compared to those without liver metastases. The mPFS was 2.5 months for patients with liver metastases versus 7.0 months for those without (P=0.09). Similarly, the mOS was 13.0 months for patients with liver metastases, compared to 27.0 months for those without (P=0.22) (Figure 2A,2B).
Table 2
| Subgroup | PFS | OS | |||
|---|---|---|---|---|---|
| Median survival, months | P value | Median survival, months | P value | ||
| Age | 0.50 | 0.19 | |||
| <65 years | 7.0 | 28.0 | |||
| ≥65 years | 4.0 | 15.0 | |||
| Sex | 0.98 | 0.37 | |||
| Male | 4.0 | 27.0 | |||
| Female | 6.0 | 20.0 | |||
| ECOG performance status | 0.42 | 0.93 | |||
| 0 | 4.0 | 28.0 | |||
| 1 | 6.0 | 20.0 | |||
| Liver metastasis | 0.09 | 0.22 | |||
| With | 2.5 | 13.0 | |||
| Without | 7.0 | 27.0 | |||
| RAS/BRAF V600E status | 0.62 | 0.33 | |||
| Mutated | 4.0 | 20.0 | |||
| Wild type | 7.0 | NA | |||
| Prior treatment | 0.01 | 0.17 | |||
| With bevacizumab | 4.0 | 13.0 | |||
| Without bevacizumab | 9.0 | 27.0 | |||
| Second-line regimen | 0.31 | 0.90 | |||
| Regorafenib only | 4.0 | 15.0 | |||
| Regorafenib combined therapy | 8.0 | 27.0 | |||
| Regorafenib maintenance dose | 0.95 | 0.67 | |||
| 80 mg | 6.0 | 20.0 | |||
| 120 mg | 5.0 | 15.0 | |||
PFS, progression-free survival; OS, overall survival; ECOG, Eastern Cooperative Oncology Group; RAS, rat sarcoma viral oncogene homolog; BRAF, B-Raf proto-oncogene, serine/threonine kinase; NA, not available.
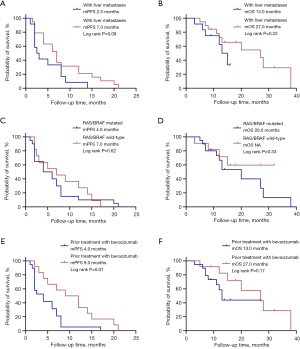
The presence of RAS/BRAF V600E mutations was not significantly associated with PFS or OS. Patients with mutated RAS/BRAF had an mPFS of 4.0 months, while those with wild-type RAS/BRAF had an mPFS of 7.0 months (P=0.62) (Figure 2C). The mOS was 20.0 months in patients with mutated RAS/BRAF. However, the mOS for the wild-type RAS/BRAF group could not be calculated and was recorded as “not available” (NA) because fewer than half of the patients in this group had experienced mortality at the time of analysis. This observation suggests a potential survival advantage for the wild-type RAS/BRAF group compared to the mutated group (Figure 2D).
Patients with prior treatment with bevacizumab had significantly shorter PFS compared to those without prior bevacizumab treatment. The mPFS was 4.0 months in patients with prior bevacizumab compared to 9.0 months in those without (P=0.01) (Figure 2E). For OS, there was a trend toward shorter survival in patients with prior bevacizumab treatment (13.0 vs. 27.0 months), although this difference did not reach statistical significance (P=0.17) (Figure 2F).
Multivariate analysis
Multivariate analysis was performed to identify independent prognostic factors for PFS and OS. Liver metastases (HR =2.515, 95% CI: 1.037–6.100; P=0.04) and prior treatment with bevacizumab (HR =2.613, 95% CI: 1.168–5.846; P=0.02) were identified as independent prognostic factors for PFS in the second-line treatment. No factors were identified as independent prognostic factors for OS in the second-line setting. These findings highlight the significant impact of these factors on the prognosis of patients with mCRC receiving regorafenib.
Safety
ADRs were common but generally manageable. The most common grade 3/4 ADRs included hand-foot skin reactions (HFSRs), fatigue, hypertension, and proteinuria. Specifically, the incidence of grade 3/4 ADRs was as follows: HFSRs, 19.4%; fatigue, 16.1%; hypertension, 12.9%; and proteinuria, 9.6%. Due to the retrospective nature of this real-world study, most grade 1–2 ADRs, which are typically tolerable, were not systematically documented in the medical records. Instead, documentation primarily focused on grade 3–4 ADRs, as these are more likely to result in dose modifications or treatment discontinuation. Three patients discontinued treatment due to ADRs, underscoring the need for careful monitoring and management of these side effects (Table 3).
Table 3
| Adverse event | Grade 3/4 adverse event, n (%) |
|---|---|
| Hand-foot skin reactions | 6 (19.4) |
| Fatigue | 5 (16.1) |
| Hypertension | 4 (12.9) |
| Proteinuria | 3 (9.7) |
Discussion
The COVID-19 pandemic raised the issue of quickly adapting oncological treatment protocols due to the need to drastically reduce the number of hospital admissions. The availability of oral medications and easily manageable combination therapies at home therefore represents an important unmet need in the treatment of patients with mCRC. The findings of this retrospective analysis suggest that regorafenib, particularly when combined with capecitabine, offered a viable second-line oral treatment option for patients with mCRC during the COVID-19 pandemic. This study also includes a small subset of patients treated with regorafenib combined with ICIs, given the relatively short infusion time that make ICIs feasible for outpatient or community-based administration. The observed mPFS of 6 months and OS of 20 months in the entire cohort are consistent with previously reported outcomes for regorafenib in patients with mCRC (16,21). Despite the lack of statistical significance in PFS and OS improvements with combination therapy (P=0.31 and P=0.90, respectively), the indication of potential benefit merits further research.
Previous studies have established regorafenib as an effective third-line treatment for mCRC in heavily pretreated populations (9,10,12). The CORRECT trial, a pivotal phase III study, reported an mPFS of 1.9 months and an OS of 6.4 months in patients receiving regorafenib compared to placebo (9). The CONCUR trial, which included Asian patients, also demonstrated a similar efficacy profile, with an mPFS of 3.2 months and an OS of 8.8 months (12). In contrast, our study, which examined second-line treatment with regorafenib alone or combined with capecitabine/ICIs, observed a mPFS of 6 months and an mOS of 20 months, indicating a potential benefit when used earlier in the treatment continuum. However, unlike the prospective STREAM trial (16) evaluating regorafenib second-line monotherapy in RAS-mutant mCRC patients pretreated with oxaliplatin, fluoropyrimidines and bevacizumab, our study was a retrospective analysis without restrictions on RAS status or kind of first-line treatment protocol. This key methodological difference should be considered when comparing outcomes, despite similarities in PFS and OS data between the two studies. Our findings were within context of a real-world setting during the COVID-19 pandemic, highlighting the feasibility and safety of regorafenib in combination with other therapeutic modalities in a second-line context.
Several studies have evaluated the efficacy and safety of combining regorafenib with cytotoxic agents. For example, a multicenter phase II study evaluated regorafenib combined with FOLFIRI as second-line therapy, and revealed a mPFS of 6.1 months compared to 5.3 months with FOLFIRI alone, suggesting that regorafenib’s efficacy could be enhanced in combination with chemotherapy (22). Furthermore, a network meta-analysis comparing regorafenib in combination with various chemotherapy agents suggests that regorafenib may perform better in terms of PFS than some other biological agents such as aflibercept and panitumumab, albeit with manageable adverse events (21). Globally, these studies and our single center experience claim the potential benefits of combining regorafenib with traditional cytotoxic agents, though larger, randomized studies are necessary to validate these findings across different combinations. Our study, however, mainly focuses on the combination of regorafenib with capecitabine, an approach that remains underexplored in the literature. The scarcity of data on this combination limits direct comparisons, yet highlighting the need for additional studies to fully understand its clinical utility in mCRC management. Given that both regorafenib and capecitabine are differently approved for CRC treatment, exploring their combined effect could provide a meaningful alternative for patients unable to access regular intravenous chemotherapy, as was necessary during the pandemic.
Administering regorafenib at home, in fact, offers significant advantages, particularly during pandemics or other situations where regular hospital visits are challenging (23). The ability to maintain effective cancer treatment while minimizing exposure to healthcare settings is crucial for vulnerable patient populations (24). Although infection rates, hospitalization rates, and COVID-related treatment delays were not analyzed due to the study’s retrospective nature, the observations in our study suggest that combining regorafenib with capecitabine or ICIs may enhance treatment efficacy without significantly increasing toxicity, making it a promising approach for mCRC management especially under pandemic constraints (25-29). The safety profile of regorafenib observed in our study aligns previously reported adverse effects (13,29-31), with HFSR, fatigue, hypertension, and proteinuria being the most common. The incidence of grade 3/4 ADRs was manageable, leading to treatment discontinuation in only three patients, indicating that regorafenib is generally well-tolerated when administered in a real-world setting. Due to the small sample size, a sub-group analysis for safety exploring the association with capecitabine or ICIs was not possible. The retrospective nature of this study resulted in limited documentation of grade 1–2 ADRs, which are generally tolerable and less likely to lead to treatment modifications. Instead, the focus was on grade 3–4 ADRs, which are clinically significant and more likely to require intervention. While this approach aligns with routine clinical practice, it limits the ability to fully assess the overall safety profile of regorafenib-based therapies. Future studies should aim to comprehensively capture data on all grades of ADRs to provide a more complete understanding of the safety of regorafenib in combination with other therapies.
Subgroup analysis revealed that patients with liver metastases at the initiation of regorafenib therapy showed a trend toward worse outcomes compared to those without liver metastases. Presence of liver metastases was identified as an independent predictor of PFS in the multivariate analysis. This finding underscores the aggressive nature of liver metastases in mCRC (32) and highlights the need for tailored therapeutic strategies for this subgroup of patients. Future studies with larger sample sizes are needed to confirm the impact of liver metastases on treatment outcomes.
Similarly, patients with prior treatment with bevacizumab also showed significantly poorer PFS compared to those without such treatment. For OS, a trend toward shorter survival was observed but did not reach statistical significance (P=0.17). The poorer outcomes in this subgroup may be attributed to the high RAS/BRAF mutation rate, which is known to confer resistance to certain therapies and is associated with a worse prognosis (33,34). This highlights the need for novel therapeutic approaches for patients with a history of bevacizumab treatment.
The main limitations of this study are its retrospective nature and the small sample size, which potentially affected the generalizability of the results. Additionally, the study did not account for all potential confounding factors, and the results should be interpreted with caution. Moreover, the COVID-19 pandemic itself might have adversely influenced treatment adherence and follow-up, further complicating the interpretation of results. Future prospective, randomized controlled trials are necessary to confirm these results and offer stronger evidence for the use of regorafenib combined with chemotherapy or immunotherapy as a second-line treatment for mCRC. Such studies should also explore the potential biomarkers that predict response to regorafenib and combination therapies, enhancing the personalization of treatment strategies. Additionally, long-term follow-up studies are required to evaluate the sustainability of responses and the long-term safety profile of regorafenib, particularly when used in combination with other agents. The ongoing impact of the COVID-19 pandemic on cancer treatment access and outcomes should continue to be monitored, with a focus on developing flexible and effective treatment strategies that can be administered with minimal hospital visits.
Conclusions
This single-center, retrospective study conducted under pandemic-specific conditions indicates that regorafenib, either as monotherapy or in combination with chemotherapy/immunotherapy, may be considered a safe and feasible second-line treatment option for mCRC in situations such as the COVID-19 pandemic. Prospective studies are warranted to explore the benefits of combination therapies and to optimize treatment strategies for this patient population.
Acknowledgments
The authors would like to thank the staff and patients at Peking University Third Hospital for their participation and support in this study. We also acknowledge the contributions of our colleagues in the oncology department for their invaluable assistance and insights during the data collection and analysis phases.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-2024-891/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-2024-891/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-2024-891/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-2024-891/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study adhered to the Declaration of Helsinki (as revised in 2013) and received approval from the Ethics Committee of Peking University Third Hospital (approval No. IRB00006761-M2023746). The Ethics Committee waived the requirement for informed consent given the retrospective design of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024;74:229-63. [Crossref] [PubMed]
- Han B, Zheng R, Zeng H, et al. Cancer incidence and mortality in China, 2022. J Natl Cancer Cent 2024;4:47-53. [Crossref] [PubMed]
- Cervantes A, Adam R, Roselló S, et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 2023;34:10-32. [Crossref] [PubMed]
- Benson AB, Venook AP, Al-Hawary MM, et al. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2021;19:329-59. [Crossref] [PubMed]
- Schmieder R, Hoffmann J, Becker M, et al. Regorafenib (BAY 73-4506): antitumor and antimetastatic activities in preclinical models of colorectal cancer. Int J Cancer 2014;135:1487-96. [Crossref] [PubMed]
- Wilhelm SM, Dumas J, Adnane L, et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer 2011;129:245-55. [Crossref] [PubMed]
- Chen D, Wei L, Yu J, et al. Regorafenib inhibits colorectal tumor growth through PUMA-mediated apoptosis. Clin Cancer Res 2014;20:3472-84. [Crossref] [PubMed]
- Wang YJ, Zhang YK, Zhang GN, et al. Regorafenib overcomes chemotherapeutic multidrug resistance mediated by ABCB1 transporter in colorectal cancer: In vitro and in vivo study. Cancer Lett 2017;396:145-54. [Crossref] [PubMed]
- Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303-12. [Crossref] [PubMed]
- Yoshino T, Komatsu Y, Yamada Y, et al. Randomized phase III trial of regorafenib in metastatic colorectal cancer: analysis of the CORRECT Japanese and non-Japanese subpopulations. Invest New Drugs 2015;33:740-50. [Crossref] [PubMed]
- Xu J, Xu RH, Qin S, et al. Regorafenib in Chinese patients with metastatic colorectal cancer: Subgroup analysis of the phase 3 CONCUR trial. J Gastroenterol Hepatol 2020;35:1307-16. [Crossref] [PubMed]
- Li J, Qin S, Xu R, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2015;16:619-29. [Crossref] [PubMed]
- Ducreux M, Petersen LN, Öhler L, et al. Safety and effectiveness of regorafenib in patients with metastatic colorectal cancer in routine clinical practice in the prospective, observational CORRELATE study. Eur J Cancer 2019;123:146-54. [Crossref] [PubMed]
- Van Cutsem E, Martinelli E, Cascinu S, et al. Regorafenib for Patients with Metastatic Colorectal Cancer Who Progressed After Standard Therapy: Results of the Large, Single-Arm, Open-Label Phase IIIb CONSIGN Study. Oncologist 2019;24:185-92. [Crossref] [PubMed]
- Xu D, Liu Y, Tang W, et al. Regorafenib in Refractory Metastatic Colorectal Cancer: A Multi-Center Retrospective Study. Front Oncol 2022;12:838870. [Crossref] [PubMed]
- Cardone C, De Stefano A, Rosati G, et al. Regorafenib monotherapy as second-line treatment of patients with RAS-mutant advanced colorectal cancer (STREAM): an academic, multicenter, single-arm, two-stage, phase II study. ESMO Open 2023;8:100748. [Crossref] [PubMed]
- Blumenthal D, Fowler EJ, Abrams M, et al. Covid-19 - Implications for the Health Care System. N Engl J Med 2020;383:1483-8. [Crossref] [PubMed]
- Cunningham D, Lang I, Marcuello E, et al. Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): an open-label, randomised phase 3 trial. Lancet Oncol 2013;14:1077-85. [Crossref] [PubMed]
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335-42. [Crossref] [PubMed]
- Fukuoka S, Hara H, Takahashi N, et al. Regorafenib Plus Nivolumab in Patients With Advanced Gastric or Colorectal Cancer: An Open-Label, Dose-Escalation, and Dose-Expansion Phase Ib Trial (REGONIVO, EPOC1603). J Clin Oncol 2020;38:2053-61. [Crossref] [PubMed]
- Xie X, Zhang J, Hu H, et al. Efficacy and Safety of Regorafenib in Combination with Chemotherapy as Second-Line Treatment in Patients with Metastatic Colorectal Cancer: A Network Meta-Analysis and Systematic Literature Review. Adv Ther 2020;37:4233-48. [Crossref] [PubMed]
- Sanoff HK, Goldberg RM, Ivanova A, et al. Multicenter, randomized, double-blind phase 2 trial of FOLFIRI with regorafenib or placebo as second-line therapy for metastatic colorectal cancer. Cancer 2018;124:3118-26. [Crossref] [PubMed]
- Li XF, Luo H, Wang Z, et al. A multiple centers real-world study of regorafenib treatment modalities in Chinese metastatic colorectal cancer patients. J Clin Oncol 2022;40:e15543. [Crossref]
- Terracciano D, Buonerba C, Scafuri L, et al. Perspective: Cancer Patient Management Challenges During the COVID-19 Pandemic. Front Oncol 2020;10:1556. [Crossref] [PubMed]
- Doleschel D, Hoff S, Koletnik S, et al. Regorafenib enhances anti-PD1 immunotherapy efficacy in murine colorectal cancers and their combination prevents tumor regrowth. J Exp Clin Cancer Res 2021;40:288. [Crossref] [PubMed]
- Cousin S, Cantarel C, Guegan JP, et al. Regorafenib-Avelumab Combination in Patients with Microsatellite Stable Colorectal Cancer (REGOMUNE): A Single-arm, Open-label, Phase II Trial. Clin Cancer Res 2021;27:2139-47. [Crossref] [PubMed]
- Chen EX, Jonker DJ, Loree JM, et al. Effect of Combined Immune Checkpoint Inhibition vs Best Supportive Care Alone in Patients With Advanced Colorectal Cancer: The Canadian Cancer Trials Group CO.26 Study. JAMA Oncol 2020;6:831-8. [Crossref] [PubMed]
- Qu W, Liu Z, Chen X, et al. Regorafenib monotherapy or combined with an immune-checkpoint inhibitor as later-line treatment for metastatic colorectal cancer: a multicenter, real-world retrospective study in China. BMC Cancer 2024;24:22. [Crossref] [PubMed]
- Bekaii-Saab TS, Ou FS, Ahn DH, et al. Regorafenib dose-optimisation in patients with refractory metastatic colorectal cancer (ReDOS): a randomised, multicentre, open-label, phase 2 study. Lancet Oncol 2019;20:1070-82. [Crossref] [PubMed]
- Yan H, Liu J, Zhang Y, et al. Efficacy and safety of regorafenib in the treatment of metastatic colorectal cancer: a retrospective cohort study. J Gastrointest Oncol 2024;15:987-1001. [Crossref] [PubMed]
- Chen C, Luo X, Tang W, et al. Regorafenib combined with immune checkpoint inhibitors versus regorafenib monotherapy as a late-line treatment for metastatic colorectal cancer: a single-center, retrospective cohort study. J Gastrointest Oncol 2024;15:1497-507. [Crossref] [PubMed]
- Wang Y, Zhong X, He X, et al. Liver metastasis from colorectal cancer: pathogenetic development, immune landscape of the tumour microenvironment and therapeutic approaches. J Exp Clin Cancer Res 2023;42:177. [Crossref] [PubMed]
- Stahler A, Heinemann V, Ricard I, et al. Current treatment options in RAS mutant metastatic colorectal cancer patients: a meta-analysis of 14 randomized phase III trials. J Cancer Res Clin Oncol 2020;146:2077-87. [Crossref] [PubMed]
- Patelli G, Tosi F, Amatu A, et al. Strategies to tackle RAS-mutated metastatic colorectal cancer. ESMO Open 2021;6:100156. [Crossref] [PubMed]
(English Language Editor: J. Gray)


