A comparison of three treatment strategies for locally advanced and borderline resectable pancreatic cancer
Introduction
Pancreatic cancer is the fifth most common cause of cancer related death in the United States (1). It is a deadly disease that is found to be distantly metastatic by radiographic imaging in up to two-thirds of new diagnoses. When distant metastases are not found, surgical resection is the only potentially curative therapy, yet 80% of newly diagnosed patients are not eligible for surgery because of metastatic or locally advanced disease at presentation (2,3). Even when patients with clinically localized pancreatic cancer undergo surgical resection there is still a high rate of treatment failure due to local tumor regrowth, incomplete resection, or metastatic disease.
Non-metastatic but locally unresectable pancreatic cancer can be divided into two categories: (I) borderline resectable and (II) locally advanced disease. Borderline resectable pancreatic cancer can involve the superior mesenteric vein (SMV) or portal vein (PV), the gastroduodenal or hepatic arteries, or less than half the circumference of the superior mesenteric artery (SMA). Locally advanced pancreatic cancer includes disease that encases more that 50% of the superior mesenteric artery (SMA) or celiac artery (CA), or invades or encases the aorta or involves lymph nodes that are outside of the resection field (4).
While surgery remains the only potentially curative option for localized pancreatic cancer, the optimal initial treatment strategy when surgery is not possible is unknown. Three treatment strategies commonly employed in the current era include chemotherapy alone (C), concurrent chemoradiation therapy (CRT), or induction chemotherapy followed by chemoradiation therapy (CCRT). Trials examining the inclusion of radiation have mostly examined up-front CRT and have had mixed results. Emerging data suggests that CCRT is a valuable strategy for patients with borderline resectable or locally advanced disease because it allows more time for more aggressive or micrometastatic disease to declare itself before the addition of local therapy (5,6). The primary aim of this study was to compare overall survival (OS), metastasis free survival (MFS), local control (LC), and percent of patients who were able to undergo margin-negative resection for these three treatment strategies. We also conducted univariable and multivariable analyses to determine factors associated with better survival.
Methods
We retrospectively reviewed 115 sequentially treated cases of borderline resectable (T3 but unresectable) or locally advanced (T4) pancreatic adenocarcinoma who were treated at our institution between the years 2000 and 2010. Pathologic diagnosis was obtained for every patient. Workup included a computed tomography (CT) scan of the chest, abdomen, and pelvis with oral and IV contrast, endoscopic ultrasound, complete blood count, basic metabolic panel, and CA 19-9. Patients had a performance status of less than three according to the Eastern Cooperative Oncology Group (ECOG) scale. Patients were evaluated by a multi-disciplinary team which consisted of a medical oncologist, radiation oncologist, and a surgeon and all patients were felt to have locally unresectable, non-metastatic disease at the time of diagnosis.
Patients were treated with either chemotherapy alone (C), up-front chemoradiation therapy (CRT), or chemotherapy followed by chemoradiation therapy (CCRT). Patients who were treated with radiation therapy received between 45 and 54 Gy in 1.8 to 2 Gy fractions using 3D conformal radiation therapy, usually with a 3-field or 4-field technique. Following initial therapy, most patients who remained ineligible for surgery were treated with maintenance chemotherapy until disease progression or toxicity.
Of the patients who received up-front chemotherapy, 16/92 (17.4%) received gemcitabine alone, and 67/92 (72.8%) received gemcitabine combined with another(other) drug(s) including oxaliplatin (32/92, 34.8%), cisplatin (13/92, 14.1%), erlotinib (7/92, 7.6%), oxaliplatin and cetuximab (5/92, 5.4%), AVN-944 (3/92, 3.3%), docetaxel (2/92, 2.2%), S-1 (2/92, 2.2%), oxaliplatin and erlotinib (1/92, 1.1%), oxaliplatin and bevacizumab (1/92, 1.1%), and capecitabine (1/92, 1.1%). Nine patients did not receive gemcitabine including 4/92 (4.3%) patients who received irinotecan and docetaxel, 3/92 (3.3%) patients who received Genexol-PM, and 2/92 (2.2%) patients who received FOLFIRINOX. During concurrent chemoradiation therapy, patients received either 5-fluoruracil (5-FU) (21%), capecitabine (72%), or gemcitabine (7%). In patients who received CCRT the median time from the start of chemotherapy to the start of radiation therapy was 4.6 months with a range of 1.0 to 26.1 months.
Local failure was defined as findings of local disease progression on CT or MRI consisting of at least a 20% increase in the sum of the longest diameter of the lesion taking as reference the smallest longest diameter recorded since the treatment started (7). One- and two-year metastasis free survival (MFS) was calculated as defined by the proportion of patients alive without distant metastasis at those time points. One- and two-year local control (LC) was calculated as defined by the proportion of patients with no local progression with all other events including death being censored.
We calculated OS, MFS, and LC using Kaplan-Meier analysis and used the two-tailed log-rank test to compare survival between the three treatment groups. Time zero was defined as the day of the start of therapy. We repeated the log-rank analysis for the comparison of C and CCRT excluding patients who died or progressed before three, six, and nine months in order to test whether potential advantages in the CCRT group were due to selection of patients with less aggressive disease. We also calculated OS, MFS, and LC for the subsets of patients with (I) borderline resectable disease and (II) locally advanced disease using Kaplan-Meier analysis and used two-tailed log-rank analysis to compare outcomes for these two groups. Univariable and multivariable survival analyses were performed using Cox-proportional hazards models. The input variables for multivariable analysis were those found to be statistically significant on univariable analysis. ANOVA was used to compare means in age and pretreatment CA 19-9 among the treatment groups. Chi-square was used to test for differences in categorical parameters among the treatment groups. Chi-square was also used to test for differences in patterns of failure. Statistical analyses were conducted using Stata 12.0. This study was approved by an institutional review board.
Results
Median follow-up was 18.7 months. Twelve of 115 patients were still alive at the time of last follow-up. There were no statistically significant differences in the baseline characteristics of the treatment groups (Table 1). Fifty-seven patients (49%) had locally advanced disease and 58 patients (51%) had borderline resectable disease and there was no difference in the distribution of treatment strategies between these two groups. There was a trend toward older age and higher CA 19-9 in patients receiving chemotherapy alone. However, there was considerable variation in the CA 19-9. The mean age was 64 years. Surgical resection was ultimately attained in 8/58 (14%) patients with borderline resectable disease and 2/57 (4%) patients with locally advanced disease. Likewise, surgical resection was attained in 6/50 (12%) patients treated with radiation therapy (CRT or CCRT) and 4/65 (6%) of patients treated with chemotherapy alone (C). There was no statistically significant difference in the rate of margin-negative resection by treatment type (P=0.406). Patients with borderline resectable disease were more likely to undergo margin-negative resection than patients with locally advanced disease, although this finding was not statistically significant (P=0.094). Of the patients receiving C alone, 11/65 (17%) were diagnosed with distant metastases or died before 3 months.
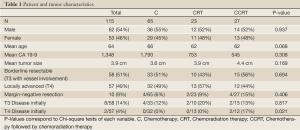
Full table
Values for median OS and MFS, and 1- and 2-year OS, MFS, and LC are found in Table 2. Patients treated with CCRT experienced improved median OS compared to C alone (21.5 vs. 13.9 months, P=0.003) (Figure 1). Patients treated with CCRT also experienced improved median MFS compared to C alone (16.1 vs. 10.2 months, P=0.012) (Figure 2). There was no statistically significant difference in OS between CRT and C (P=0.441) or CCRT and CRT (P=0.544). Likewise, there was no statistically significant difference in MFS between CRT and C (P=0.971), or CCRT and CRT (P=0.231). There was no statistically significant difference in LC between any of the treatment groups (CCRT vs. C, P=0.193; CRT vs. C, P=0.330; CCRT vs. C, P=0.870) (Figure 3). The improvement in OS in patients receiving CCRT compared to chemotherapy alone was more pronounced in patients with locally advanced disease (P=0.010) than in patients with borderline resectable disease (P=0.089). Likewise, the improvement in MFS in patients receiving CCRT compared to chemotherapy alone was more pronounced in patients with locally advanced disease (P=0.020) than in patients with borderline resectable disease (P=0.218). Median OS for the eight patients with borderline resectable disease achieving margin-free resection was 47.1 months (95% CI, 9.0 months - undefined). Median OS for the two patients with locally advanced disease achieving margin-free resection was 29.7 months.
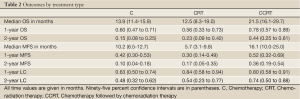
Full table
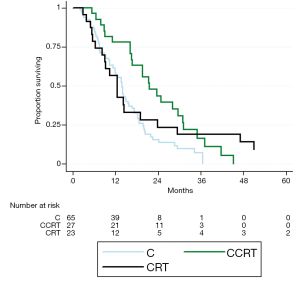
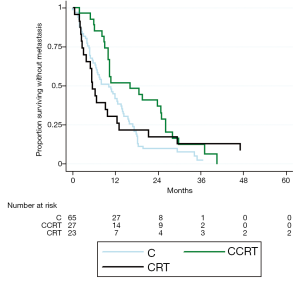
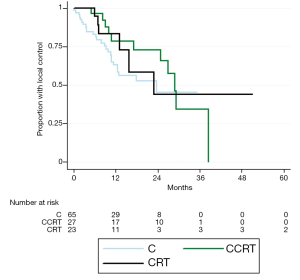
The statistically significant improvement in OS of CCRT compared to chemotherapy alone persisted when limiting the analysis to patients who were still alive with no progression at three months (P=0.015), six months (P=0.015), and nine months (P=0.011). The improvement in MFS of CCRT compared to chemotherapy alone was still statistically significant when limited the analysis to patients who were still alive with no progression at three months (P=0.042), but not at six months (P=0.198), or nine months (P=0.242).
In patients with borderline resectable disease median OS was 16.7 months (95% CI, 12.7-20.4 months) and median MFS was 10.5 months (95% CI, 8.1-14.5 months). In patients with locally advanced disease median OS was 13.7 (95% CI, 10.5-16.1 months) and median MFS was 9.2 months (95% CI, 5.0-13.2 months). OS and MFS were improved in patients with borderline resectable disease compared to locally advance disease by log-rank analysis (P=0.032 and P=0.039 respectively). There was no difference in LC between patients with borderline resectable and locally advanced disease (P=0.318).
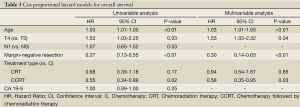
On univariable survival analysis, younger patients had improved overall survival (P=0.001) (Table 3). Patients with locally advanced disease had worse overall survival than patients with borderline resectable disease (HR 1.53, P=0.033). Patients who received chemotherapy followed by chemoradiation therapy and patients who were able to undergo margin-negative resection had better survival (P=0.015, and P<0.001 respectively). Nodal status at diagnosis did not affect overall survival. There was also no difference in survival based on the CA 19-9 level prior to treatment. On multivariable analysis younger age (P=0.009), borderline resectable disease (P=0.035), margin-negative resection (P=0.002), and receiving chemotherapy followed by chemoradiation therapy (P=0.035) were all associated with improved OS.
Full table
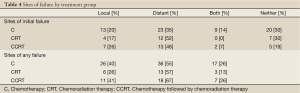
More patients experienced distant metastasis than local progression for the overall group, and for all three treatment groups (Table 4). There was no difference in the overall percent of patients experiencing local progression among the three treatment groups (P=0.46). Isolated local progression without distant metastasis at any time before death occurred in 9 patients (14%) in the C group, 3 patients (13%) of the CRT group, and 4 patients (15%) in the CCRT group (P=0.73). Distant metastasis without local progression at any time before death occurred in 19 patients (33%) in the C group, 10 patients (43%) of the CRT group, and 11 patients (41%) in the CCRT group (P=0.38). Most distant recurrences occurred in the liver, lung, or peritoneum.
Full table
Discussion
We report our experience treating a large series of patients with borderline resectable and locally advanced pancreatic cancer using three treatment strategies including chemotherapy alone, concurrent chemoradiation therapy, or induction chemotherapy followed by chemoradiation therapy. Patients treated with induction chemotherapy followed by chemoradiation therapy had an improved OS and MFS compared to patients treated with chemotherapy alone. The use of induction chemotherapy followed by chemoradiation therapy was associated with improved survival compared to chemotherapy alone on multivariable survival analysis as well.
The optimal strategy for upfront treatment of borderline resectable and locally advanced pancreatic cancer has not been elucidated by prospective clinical trials. Both early (8,9), and more modern (10,11) randomized trials of C vs. CRT have produced conflicting results. CCRT has been compared to CRT in a retrospective review of 323 patients that showed improved OS (8.5 vs. 11.9 months) and progression free survival (4.2 vs. 6.4 months) in the CCRT group (6).
No prospective randomized trials directly comparing CCRT to chemo alone have been reported. The Groupe Coopérateur Multidisciplinaire en Oncologie (GERCOR) retrospectively analyzed patients treated on prospective phase II and III GERCOR studies (5) to compare the survival of patients treated with C vs. CCRT. This analysis included patients with both borderline resectable or locally advanced disease according to the NCCN definition (4). Patients treated with CCRT had improved progression free survival (10.8 vs. 7.4 months, P=0.005), and improved overall survival (15.0 vs. 11.7 months, P=0.0009). Our data are consistent with the GERCOR’s prospectively gathered data in showing a survival benefit of CCRT over chemotherapy alone. The GERCOR LAP 07 phase III trial (12) is a randomized prospective phase III trial that will examine the role of CCRT after chemotherapy alone and the benefit of adding erlotinib for locally advanced pancreatic cancer.
Induction chemotherapy prior to chemoradiation therapy allows for the selection of patients for local radiation therapy who are less likely to have more aggressive or micrometastatic disease and therefore have a better prognosis. The success of this strategy in pancreatic cancer may result from better systemic control or possible eradication of micrometastatic disease from newer gemcitabine based therapy compared to older fluoropyrimidine-based therapy (13,14). FOLFIRINOX has recently been shown to confer a survival advantage compared to gemcitabine in the setting of metastatic pancreatic cancer and is receiving attention as a way to further improve induction chemotherapy in locally unresectable disease (15).
Other mechanisms of screening for patients who are more likely to benefit from localized therapy are being investigated. The expression of Smad4(Dpc4), a tumor suppressor gene activated in more than half of pancreatic cancers, has been shown to be associated with local rather than distant tumor progression (16,17). Testing for Smad4(Dpc4) status at initial diagnosis may help individualize treatment regimens to either focus on local control with radiation for Smad4(Dpc4) activated tumors versus systemic control with chemotherapy and/or targeted agents for non-Smad4(Dpc4) activated tumors. A phase II clinical trial, RTOG 1201, will attempt to assess the validity of Smad4(Dpc4) as a method of determining the optimal treatment for patients with locally unresectable pancreatic cancer.
Our analysis suggests that the OS and MFS benefits of CCRT vs. C are not entirely due to metastatic disease or death that occurs in the first few months before radiation is started. In this series, patients who survived without metastatic disease for three, six, or nine months on chemotherapy alone still benefitted from the addition of chemoradiation therapy. However, other unrecorded factors such as performance status and cancer or non-cancer related comorbidities may have pushed healthier patients into the CCRT group and accounted for the better survival in this group.
Surgery remains the only treatment of localized pancreatic cancer that offers the possibility of a cure. In our analysis, undergoing margin-negative resection was associated with improved OS on both univariable and multivariable analysis. Twelve percent of patients who received radiation therapy (CRT or CCRT) were able to undergo margin-negative resection. In the subset of patients with locally advanced (T4) disease, only 2/53 patients (4%) achieved margin-negative resection. Both of these patients were treated with CCRT. This very small percentage of the patients is slightly higher, yet perhaps trivially so, than that shown in a prospective study attempting to convert LAPC to resectable disease where only 1/87 patients (1%) achieved a margin-negative resection (18). Until better therapies are developed, this small group of patients is the only group that we can hope to offer durable survival.
The rate of distant metastases before three months in patients receiving chemotherapy alone is low in our study (17%) compared to previously reported results (29-35%) (19). While patients were restaged before starting chemoradiation therapy in the CCRT group, there was no uniform policy requiring restaging at three months. Such a policy might have resulted in a higher percentage of disease progression at that time. The median time to the start of chemoradiation therapy in the CCRT group was 4.6 months.
The strengths of this study are that it examines a recent series of patients treated by a multidisciplinary gastrointestinal oncology group using modern therapeutics and supportive measures to directly compare three treatment strategies. The patients underwent uniform staging techniques, and had thorough follow-up. While much of the published data about the treatment of locally unresectable pancreatic cancer compares two strategies (C vs. CRT or CRT vs. CCRT), our study benefits from the comparison of all three strategies in the same setting. While our study is retrospective and hypothesis-generating, the inclusion of three treatment strategies provides important perspective given the inconsistent and confusing results of past studies.
Among the weaknesses of this study are that it was conducted retrospectively. Though available staging and patient characteristics were controlled for in our analysis, there is a possibility of selection bias in that patients with a poor functional status or greater comorbidities might not have been offered radiation therapy as often. While there were no statistical differences in baseline characteristics, there was a trend toward higher initial CA 19-9, and older age in the group that received chemotherapy alone. The benefits of CCRT shown here should be validated in a randomized clinical trial.
Conclusions
In conclusion, our retrospective results strongly suggest that, until a randomized controlled clinical trial is reported, patients who have been treated with chemotherapy alone with no progression may benefit from the addition of chemoradiation therapy if they can tolerate it. Providers should plan to add chemoradiation therapy after a trial period of chemotherapy alone for any patient who doesn’t progress and can tolerate combined therapy. Treatment with CCRT is associated with improved median OS and MFS compared to chemotherapy alone. This is a strategy that selects for patients who are less likely to develop early metastases and therefore have a better prognosis. A prospective randomized study is needed to confirm these findings. Our analysis suggests that other factors that portend improved survival include younger age, borderline resectable disease, and margin-negative resection.
Acknowledgements
This data was presented as an oral presentation at the American Society for Radiation Oncology Annual Meeting, Oct 30, 2012.
Disclosure: The authors declare no conflict of interest.
References
- Department of Health and Human Services, Centers for Disease Control and Prevention, National Program of Cancer Registries (NPCR). Available online: http://apps.nccd.cdc.gov/uscs/toptencancers.aspx. Accessed Sept 7, 2012.
- Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas-616 patients: Results, outcomes, and prognostic indicators. J Gastrointest Surg 2000;4:567-79.
- Winter JM, Cameron JL, Campbell KA, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J Gastrointest Surg 2006;10:1199-210; discussion 1210-1.
- National Comprehensive Cancer Network. Pancreatic Adenocarcinoma, Version 2.2012. Accessed October 12, 2012. Available online: http://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf
- Huguet F, André T, Hammel P, et al. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol 2007;25:326-31.
- Krishnan S, Rana V, Janjan NA, et al. Induction chemotherapy selects patients with locally advanced, unresectable pancreatic cancer for optimal benefit from consolidative chemoradiation therapy. Cancer 2007;110:47-55.
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205-16.
- Treatment of locally unresectable carcinoma of the pancreas: comparison of combined-modality therapy (chemotherapy plus radiotherapy) to chemotherapy alone. Gastrointestinal Tumor Study Group. J Natl Cancer Inst 1988;80:751-5.
- Klaassen DJ, MacIntyre JM, Catton GE, et al. Treatment of locally unresectable cancer of the stomach and pancreas: a randomized comparison of 5-fluorouracil alone with radiation plus concurrent and maintenance 5-fluorouracil--an Eastern Cooperative Oncology Group study. J Clin Oncol 1985;3:373-8.
- Loehrer PJ Sr, Feng Y, Cardenes H, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol 2011;29:4105-12.
- Chauffert B, Mornex F, Bonnetain F, et al. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000-01 FFCD/SFRO study. Ann Oncol 2008;19:1592-9.
- Groupe Cooperateur Multidisciplinaire en Oncologie (GERCOR). Gemcitabine With or Without Capecitabine and/or Radiation Therapy or Gemcitabine With or Without Erlotinib in Treating Patients With Locally Advanced Pancreatic Cancer That Cannot Be Removed by Surgery. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2008- [cited 2013 Jan 17]. Available online: http://clinicaltrials.gov/ct2/show/NCT00634725?term=nct00634725&rank=1, NLM Identifier: NCT00634725
- Burris HA 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997;15:2403-13.
- Li CP, Chao Y, Chi KH, et al. Concurrent chemoradiotherapy treatment of locally advanced pancreatic cancer: gemcitabine versus 5-fluorouracil, a randomized controlled study. Int J Radiat Oncol Biol Phys 2003;57:98-104.
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N Engl J Med 2011;364:1817-25.
- Crane CH, Varadhachary GR, Yordy JS, et al. Phase II trial of cetuximab, gemcitabine, and oxaliplatin followed by chemoradiation with cetuximab for locally advanced (T4) pancreatic adenocarcinoma: correlation of Smad4(Dpc4) immunostaining with pattern of disease progression. J Clin Oncol 2011;29:3037-43.
- Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol 2009;27:1806-13.
- Kim HJ, Czischke K, Brennan MF, et al. Does neoadjuvantchemoradiation downstage locally advanced pancreatic cancer? J Gastrointest Surg 2002;6:763-9.
- Mishra G, Bulter J, Ho C, et al. Phase II trial of induction gemcitabine/CPT-11 followed by a twice-weekly infusion of gemcitabine and concurrent external beam radiation for the treatment of locally advanced pancreatic cancer. Am J Clin Oncol 2005;28:345-50.


