Update on antiangiogenic therapy in colorectal cancer: aflibercept and regorafenib
Background
Colorectal cancer is a major cause of morbidity and mortality throughout the world. It is the third most common cancer diagnosis worldwide and affects men and women equally (1). In the United States, colorectal cancer accounted for 9% of all cancer mortality in 2012 (2). The survival of patients with metastatic colorectal cancer (mCRC) has markedly improved since the 1990s when 5-fluorouracil (5FU) based chemotherapy achieved an overall survival (OS) of 12 months. The addition of oxaliplatin and Irinotecan increased the OS to approximately 18 months (3-6). The survival was further augmented with anti-angiogenic agents and bevacizumab, in combination with chemotherapy, was the first of the drug class to receive regulatory approval for use in mCRC therapy (7,8). Recently, 2 other anti-angiogenic drugs, aflibercept and regorafenib, were found to improve the survival of mCRC patients in randomized trials which further reiterates the importance of targeting angiogenesis in CRC therapy (9,10). This article will review the development of aflibercept and regorafenib and their current role in the treatment of colorectal cancer (Table 1).
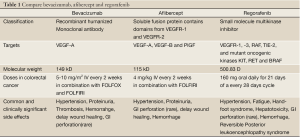
Full table
Tumor angiogenesis and VEGF signaling pathway
Angiogenesis refers to a multi-step process leading to the formation of new blood vessels to supply nutrients and oxygen to the tissues (11). The process begins with vasodilatation, increased vessel permeability, stromal degradation and endothelial cell proliferation and migration, resulting in the formation of a new or extended capillary (12). Whilst angiogenesis is ordered and occur only during wound repair, tissue remodeling or inflammation under normal physiologic conditions, the process is chaotic in neoplasms resulting in leaky, tortuous and inefficient vessels (13-15).
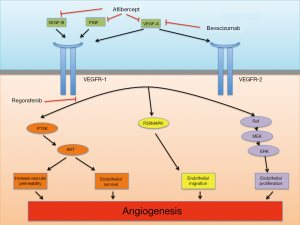
The VEGF/VEGFR signaling is a well studied pro-angiogenic pathway and the ligands include VEGF-A, VEGF-B, VEGF-C, VEGF-D and placental growth factor (PIGF) that interact with membrane bound tyrosine kinase receptors VEGFR-1 (FLT-1), VEGFR-2 (FLK-1/KDR) and VEGFR-3 (FLT4); and other co-receptors include neurophilin (NRP)-1 and NRP-2 (16-18). The binding of VEGF-A (or VEGF) to VEGFR-2 had been found to be key mediator of angiogenesis (17). VEGF-A (commonly known as VEGF) is expressed in many human cancers and binding with VEGFR-2 in tumor microenvironment triggers a number of intracellular signaling cascades in endothelial cells leading to formation and enhancement of tumor microvasculature (18,19).
Bevacizumab
Bevacizumab is a recombinant humanized monoclonal IgG1 antibody that binds to and inhibits the biologic activity of VEGF by preventing its binding to VEGFR-1 and VEGFR-2 (Figure 1). The therapeutic role of bevacizumab in treating metastatic CRC patients is well established and supported by well-conducted randomized trials (7,8,20-22). These topics had been well reviewed in the literature and we refer readers to those articles (23,24). Recently, the benefit of continuing angiogenetic suppression beyond first disease progression in mCRC patients was confirmed recently by the ML18147 study. In this randomized phase III trial, bevacizumab beyond disease progression while switching the cytotoxic chemotherapy improved the PFS (5.7 vs. 4.1 months) and OS (11.2 vs. 9.8 months) in the group that continued bevacizumab compared to those who didn’t (25).
Despite benefit in metastatic setting, the addition of bevacizumab had not improved clinical outcome in adjuvant setting in CRC (26,27). The AVANT trial randomized curatively resected stage III or high risk stage II colon cancer to 3 arms: FOLFOX4 for 12 cycles, bevacizumab 5 mg/kg plus FOLFOX4 for 12 cycles or bevacizumab 7.5 mg/kg plus oxaliplatin and capecitabine (XELOX); both bevacizumab arm will receive additional bevacizumab 7.5 mg/kg monotherapy every 3 weeks for eight cycles after completing combination therapy. The hazard ratio (HR) for disease-free survival (DFS) and OS for bevacizumab-FOLFOX4 versus FOLFOX4 were 1.17 (95% CI: 0.98-1.39; P=0.07) and 1.27 (95% CI: 1.03-1.57; P=0.02) respectively; and for bevacizumab-XELOX versus FOLFOX4 was 1.07 (95% CI: 0.9-1.28; P=0.44) and 1.15 (95% CI: 0.93-1.42; P=0.21) respectively (27). In summary, in the AVANT trial, the addition of bevacizumab did not improve DFS including subset analysis according to baseline VEGF-A or VEGFR-1 or 2 levels. Interestingly, the data suggested potential detrimental effect in the bevacizumab-containing arms from more relapses and deaths due to disease progression (27). One hypothesis proposed to explain the failure of bevacizumab in adjuvant setting was that established CRC metastatic tumors were more dependent on angiogenesis than micrometastases, which were more sensitive to cytotoxic chemotherapy (28,29).
Aflibercept
Aflibercept (or VEGF Trap) is a recombinant fusion protein consisting of the extracellular domains of human VEGFR-1 and 2 fused to the Fc portion of human IgG1 (30). The decoy protein binds to VEGF-A, VEGF-B and PIGF and prevents the activation of VEGFR-1 and VEGFR-2 by these ligands, in contrast to bevacizumab in which binds VEGF-A only (Figure 1). VEGF-A is a key regulator of tumor angiogenesis and most human malignancies express high VEGF-A level (14,17). PIGF also plays an important role in angiogenesis by enhancing VEGF-A expression (31). Furthermore, patients with metastatic renal cell cancer previously treated with anti-VEGF therapy had increased PIGF level suggesting that PIGF may play a role in resistance to anti-VEGF treatment (32,33). In addition, compared to bevacizumab, aflibercept has a higher affinity for VEGF-A and its native receptor (34). Preclinically, aflibercept inhibited tumor growth, angiogenesis, metastases and improved the survival of tumor-bearing mice for various cancer types including pancreas, ovarian and renal cell carcinoma (30). Aflibercept in combination with cytotoxic drugs (Irinotecan, 5FU, paclitaxel, docetaxel), transtuzumab or radiotherapy exerted greater inhibition of tumor vasculature and growth than aflibercept alone in tumor xenograft models (35-40).
In the phase I trial, 47 patients with refractory solid tumors or non-Hodgkin’s lymphoma were enrolled to receive aflibercept intravenously every 2 weeks at doses ranging from 0.3 to 7.0 mg/kg (41). Dose-limiting toxicities (DLT) were rectal ulceration and proteinuria at 7.0 mg/kg dose. Aflibercept was also evaluated in combination with various chemotherapeutic agents including FOLFOX4 (42,43), irinotecan with 5FU and leucoverin (44), docetaxel (45) alone and with cisplatin (46), and gemcitabine (47) in advanced solid tumors patients. In combination with FOLFOX4, aflibercept doses 2, 4 and 5 mg/kg were explored in patients with advanced solid tumors and no DLT was encountered in the phase I trial (42). Grade 3 or worse toxicities included neutropenia, thrombocytopenia, hypertension, proteinuria, hemorrhagic events (include 1 Grade 5 hemorrhagic stroke at 4 mg/kg), febrile neutropenia and deep vein thrombosis. In subset of mCRC, partial response was observed.
Aflibercept was also evaluated in combination with irinotecan, 5FU and leucovorin in a dose-escalation study. Aflibercept doses 2, 4, 5 and 6 mg/kg doses every 2 weeks were explored and DLTs observed were Grade 3 proteinuria lasting >2 weeks, acute nephrotic syndrome and thrombotic microangiopathy at 4 mg/kg; Grade 3 stomatitis, esophagitis reflux at 5 mg/kg; and, febrile neutropenia, Grade 3 stomatitis and Grade 3 abdominal pain due to intestinal obstruction at 6 mg/kg (44). As such, aflibercept 4 mg/kg dose level was selected as for further development in combination with irinotecan, 5-FU and leucovorin (41,42,44). The pharmacokinetic studies showed that aflibercept’s elimination half-life ranged from less than 1-3 days for free aflibercept and was approximately 18 days for VEGF-bound aflibercept (41,48).
The benefit of aflibercept in combination with FOLFIRI was confirmed in the pivotal phase III VELOUR trial. In the study, patients with metastatic CRC previously treated with oxaliplatin-containing regimen, irregardless of prior bevacizumab treatment, were randomly assigned to received aflibercept 4 mg/kg IV every 2 weeks or placebo combination with FOLFIRI. Overall response rate was 19.8% in the aflibercept arm compared to 11.1% in the placebo (P=0.0001). Compared to the control group, the aflibercept-containing arm had better PFS (6.9 vs. 4.67 months; HR 0.758; P<0.0001) and OS (13.5 vs. 12.06 months; HR 0.817; P=0.0032). Pre-planned subgroup analysis showed that prior bevacizumab use did not influence aflibercept’s effect on PFS and OS though the study was not powered to show a treatment difference between arms (9,18). Toxicities related to aflibercept were consistent with those expected from the anti-VEGF drug class (49). When compared to the bevacizumab-related toxicity profile reported in the phase III trial of IFL with or without bevacizumab, the frequency of grade 3 or 4 proteinuria seemed to be higher for aflibercept than bevacizumab (7.5% vs. 0.8%) though risks for Grade 3 or 4 bleeding (2.8% vs. 3.1%) and hypertension (11% vs. 11%) seemed similar (9,21).
Together with the results from ML18147 study, clinicians now have the option of using aflibercept or bevacizumab with FOLFIRI in mCRC patients who progressed following oxaliplatin containing regimen. The benefit achieved by aflibercept and bevacizumab in second-line setting seemed comparable: in ML18147 study, continuing bevacizumab into second-line while switching the cytotoxic chemotherapy achieved a median OS improvement of 1.4 months (HR 0.81, 95% CI: 0.69-0.94; P=0.0062) (25) whilst the addition of aflibercept to FOLFIRI in the VELOUR trial achieved a comparable median OS survival improvement of 1.4 months (HR 0.817, 95.34% CI: 0.713-0.937; P=0.0032) (9). The frequency of vascular-related adverse events seemed to be higher with aflibercept than bevacizumab treatment when comparing across trials. Cost is another consideration: aflibercept treatment costs, in average, $11,063 per month, which is more than twice as high as bevacizumab therapy. As such, aflibercept is not recommended routinely in metastatic CRC patients who progressed on oxaliplatin-containing treatment until more evidence available.
Regorafenib
Regorafenib is structurally related to sorafenib and differ from the latter by the presence of a fluorine atom in the center phenyl ring (50,51). The slight structural difference resulted in higher inhibitory potency against various pro-angiogenic receptors than sorafenib including VEGFR2 (IC50 3 vs. 90 nM respectively), FGFR1 (202 vs. 580 nM) though IC50s for PDGFRβ were similar (52,53). Other receptor kinases inhibited by regorafenib include VEGFR1, -3, RAF, TIE2, and mutant oncogenic kinases KIT, RET and BRAF (52,54). Interestingly, sorafenib did not demonstrate significant anti-tumor activity in CRC. The effect of sorafenib plus 5-FU in colorectal tumor xenograft strudy was not significantly better than treatment using either drugs alone (55). Two of the 66 refractory mCRC patient who received sorafenib in four phase I had best response as stable disease and no objective response was observed (56). In contrast, regorafenib showed significant anti-cancer efficacy in CRC. In preclinical colorectal tumor xenograft studies, regorafenib treatment reduced tumor microvasculature and inhibited tumor growth in a dose-dependent manner (57). N-Oxide (M-2) and N-Oxide/N-desmethyl metabolite (M-5) are 2 active metabolites of regorafenib with potent pharmacologic activities similar to but distinct from regorafenib (57).
In the phase I trial, 53 patients with advanced solid tumor received regorafenib at the dose levels from 10 to 220 mg daily, 21 days on followed by 7 days off in repeating cycle. The most frequent adverse events were voice changes, hand-foot skin reaction, mucositis, diarrhea and hypertension. DLTs at 160 mg were skin toxicity and vomiting; skin toxicity, abdominal pain and asthma at 220 mg. On the basis of these observations, 160 mg once daily orally was determined the maximum tolerated dose (MTD) and the recommended dose for future studies. For efficacy, one mCRC patient had partial response at 220 mg but stopped treatment after 5.3 months for treatment-related side effects (58). Pharmacokinetic studies showed that terminal half-life of regorafenib were 20-40 hours, thus supporting once daily dosing schedule. At the 160 mg dose, plasma exposure at steady state of M-2 and M-5 were similar to or slightly greater than parent drug. The terminal half-life of M2 was comparable to regorafenib but the elimination of M-5 was slower with an estimated half-life of 51-64 hours (58,59). The unbound plasma concentration of the pharmacologically active species at the 160 mg dose level exceeded the IC50 of many target kinases, therefore, plausible that M-2 and M-5 may contribute to the clinical activity of regorafenib (58).
In an expanded phase I study specific for relapsed or refractory mCRC patients, 38 patients received regorarefnib dose levels ranging from 60-220 mg daily administered on a “21 days on followed by 7 days off” dosing schedule. Enrolled patients had received a median of 4 previous lines of treatment. The most common adverse event leading to dose reduction was hand-foot skin reaction. Other treatment-related adverse events leading to regorafenib discontinuation included hypertension, fatigue, thrombocytopenia and diarrhea. Among 25 patients treated at 160 mg dose level, 6 patients permanently discontinued due to treatment-related adverse events including hand-foot skin reaction, hypertension, fatigue, thrombocytopenia and duodenal ulcer. In efficacy evaluation, 27 evaluable patients achieved 74% disease control rate with partial response in 1 patient (4%) and stable disease in 19 patients (70%). Overall, regorafenib was well tolerated and adverse events were manageable (59).
The multi-national phase III CORRECT trial enrolled mCRC patients who had received all locally-approved standard therapies and had progressed during or within 3 months after the last standard therapy (10). Patients were randomized in a 2:1 ratio to receive regorafenib or placebo. 500 patients received regorafenib at 160 mg orally 21 days on 7 days off and 253 patients received placebo. Median OS was 6.4 months in the regorafenib group versus 5.0 months in the placebo group (HR 0.77; 95% CI: 0.64-0.94; one-sided P=0.0052). Similar clinical benefit was observed in patient with colon cancer and rectal. The most common treatment-related Grade 3 or worse adverse events were hand-foot skin reaction (17%), fatigue (10%), diarrhea (7%), hypertension (7%), and rash or skin desquamation (6%), consistent with that observed in earlier phase trials. These adverse events were mostly manageable with dose reduction or interruption.
Conclusion
Angiogenesis is now a validated therapeutic target in CRC patients with macroscopic metastases. Recent development added 2 new anti-angiogenic drugs to the CRC treatment armamentarium and confirmed the advantage of continuing angiogenic suppression beyond first progression in metastatic CRC patients (60). Evidence so far supports the use of bevacizumab in both first- and second-line treatment of metastatic CRC patients. In comparison, the role of aflibercept in these settings remains unclear given the comparable efficacy but higher cost compared to bevacizumab. Aflibercept targets a broader set of pro-angiogenic growth factors than bevacizumab, and has the theoretical advantage of more effective angiogenic suppression and overcoming bevacizumab resistance. However, these hypotheses are yet to be confirmed in clinical studies. As the chemotherapeutic options and supportive care improve, more metastatic CRC patients nowadays have good performance status by the time they exhausted all standard therapy. For them, regorafenib is a welcomed option in addition to participation in clinical trials. Looking back, the overall survival of patients with metastatic CRC has increased several folds when compared to decades ago even though, it seemed, each drug achieved only incremental improvement individually. However, it is clear more novel treatment approaches are needed to continue this trend.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg 2009;22:191-7.
- American cancer Society: Cancer Facts & Figures 2012. Last accessed Janurary 5,2012. Available online: http://www.cancer.org/research/cancerfactsfigures/index
- André T, Louvet C, Maindrault-Goebel F, et al. CPT-11 (irinotecan) addition to bimonthly, high-dose leucovorin and bolus and continuous-infusion 5-fluorouracil (FOLFIRI) for pretreated metastatic colorectal cancer. GERCOR. Eur J Cancer 1999;35:1343-7.
- Cheeseman SL, Joel SP, Chester JD, et al. A ‘modified de Gramont’ regimen of fluorouracil, alone and with oxaliplatin, for advanced colorectal cancer. Br J Cancer 2002;87:393-9.
- Goldberg RM, Sargent DJ, Morton RF, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 2004;22:23-30.
- Grothey A, Sargent D, Goldberg RM, et al. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol 2004;22:1209-14.
- Fuchs CS, Marshall J, Mitchell E, et al. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C Study. J Clin Oncol 2007;25:4779-86.
- Saltz LB, Clarke S, Diaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 2008;26:2013-9.
- Van Cutsem E, Tabernero J, Lakomy R, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol 2012;30:3499-506.
- Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303-12.
- Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307:58-62, 2005.
- Tassi E, Wellstein A. The angiogenic switch molecule, secreted FGF-binding protein, an indicator of early stages of pancreatic and colorectal adenocarcinoma. Semin Oncol 2006;33:S50-6.
- El Zouhairi M, Charabaty A, Pishvaian MJ. Molecularly targeted therapy for metastatic colon cancer: proven treatments and promising new agents. Gastrointest Cancer Res 2011;4:15-21.
- Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer 2008;8:579-91.
- Holash J, Maisonpierre PC, Compton D, et al. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science 1999;284:1994-8.
- Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol 2005;23:1011-27.
- Kerbel RS. Tumor angiogenesis. N Engl J Med 2008;358:2039-49.
- Sun W. Angiogenesis in metastatic colorectal cancer and the benefits of targeted therapy. J Hematol Oncol 2012;5:63.
- Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol 2002;20:4368-80.
- Emmanouilides C, Sfakiotaki G, Androulakis N, et al. Front-line bevacizumab in combination with oxaliplatin, leucovorin and 5-fluorouracil (FOLFOX) in patients with metastatic colorectal cancer: a multicenter phase II study. BMC Cancer 2007;7:91.
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335-42.
- Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol 2007;25:1539-44.
- Koukourakis GV, Sotiropoulou-Lontou A. Targeted therapy with bevacizumab (Avastin) for metastatic colorectal cancer. Clin Transl Oncol 2011;13:710-4.
- Galfrascoli E, Piva S, Cinquini M, et al. Risk/benefit profile of bevacizumab in metastatic colon cancer: a systematic review and meta-analysis. Dig Liver Dis 2011;43:286-94.
- Bennouna J, Sastre J, Arnold D, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol 2013;14:29-37.
- Allegra CJ, Yothers G, O’Connell MJ, et al. Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: results of NSABP protocol C-08. J Clin Oncol 2011;29:11-6.
- de Gramont A, Van Cutsem E, Schmoll HJ, et al. Bevacizumab plus oxaliplatin-based chemotherapy as adjuvant treatment for colon cancer (AVANT): a phase 3 randomised controlled trial. Lancet Oncol 2012;13:1225-33.
- Norton L. Conceptual and practical implications of breast tissue geometry: toward a more effective, less toxic therapy. Oncologist 2005;10:370-81.
- Almog N. Molecular mechanisms underlying tumor dormancy. Cancer Lett 2010;294:139-46.
- Gaya A, Tse V. A preclinical and clinical review of aflibercept for the management of cancer. Cancer Treat Rev 2012;38:484-93.
- Roy H, Bhardwaj S, Babu M, et al. Adenovirus-mediated gene transfer of placental growth factor to perivascular tissue induces angiogenesis via upregulation of the expression of endogenous vascular endothelial growth factor-A. Hum Gene Ther 2005;16:1422-8.
- Fischer C, Mazzone M, Jonckx B, et al. FLT1 and its ligands VEGFB and PlGF: drug targets for anti-angiogenic therapy? Nat Rev Cancer 2008;8:942-56.
- Rini BI, Michaelson MD, Rosenberg JE, et al. Antitumor activity and biomarker analysis of sunitinib in patients with bevacizumab-refractory metastatic renal cell carcinoma. J Clin Oncol 2008;26:3743-8.
- Holash J, Davis S, Papadopoulos N, et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A 2002;99:11393-8.
- Le XF, Mao W, Lu C, et al. Specific blockade of VEGF and HER2 pathways results in greater growth inhibition of breast cancer xenografts that overexpress HER2. Cell Cycle 2008;7:3747-58.
- Hu L, Hofmann J, Holash J, et al. Vascular endothelial growth factor trap combined with paclitaxel strikingly inhibits tumor and ascites, prolonging survival in a human ovarian cancer model. Clin Cancer Res 2005;11:6966-71.
- Wachsberger PR, Burd R, Cardi C, et al. VEGF trap in combination with radiotherapy improves tumor control in u87 glioblastoma. Int J Radiat Oncol Biol Phys 2007;67:1526-37.
- Lejeune P CM, Le Moigne R, et al. Combination of the antiangiogenic agent aflibercept results in greater antitumor activity. In: Proceedings from the 99th American association for cancer research annual meeting. April 12-16, 2008: San Diego, CA. Abstract 1107.
- Abrahams C LB, Parveen A. et al. Combination of aflibercept (VEGF Trap) and docetaxel produces increased anti-tumor effects associated with enhanced changes to tumor vasculature. In: Proceedings from the 101st American association for cancer research annual meeting. Washington DC. April 17-21, 2010: Abstract 5427.
- Chiron M VP, Lejeune P, et al. Synergistic activity of aflibercept (VEGF Trap) in combination with 5-fluorouracil and irinotecan in preclinical tumor models. In: Proceeding from AACR-NCI-EORTC: molecular targets and cancer therapeutics. San Francisco, CA. October 22-26, 2007: Abstract A13.
- Lockhart AC, Rothenberg ML, Dupont J, et al. Phase I study of intravenous vascular endothelial growth factor trap, aflibercept, in patients with advanced solid tumors. J Clin Oncol 2010;28:207-14.
- Limentani SA Jr, Purdham A, et al. A phase I dose escalation and pharmacokinetic (PK) study if intravenous (IV) aflibercept (VEGF-Trap) plus FOLFOX4 in patient (pts) with advanced solid tumor:preliminary result. J Clin Oncol 2008;26:abstract 3556.
- Mulay M, Limentani SA, Carroll M, et al. Safety and pharmacokinetics of intravenous VEGF trap plus FOLFOX4 in a combination phase I clinical trial of patients with advanced solid tumors. ASCO meeting Abstracts 2006;24:13061.
- Van Cutsem E, Khayat D, Verslype C, et al. Phase I dose-escalation study of intravenous aflibercept administered in combination with irinotecan, 5-fluorouracil and leucovorin in patients with advanced solid tumours. Eur J Cancer 2013;49:17-24.
- Isambert N, Freyer G, Zanetta S, et al. Phase I dose-escalation study of intravenous aflibercept in combination with docetaxel in patients with advanced solid tumors. Clin Cancer Res 2012;18:1743-50.
- Freyer G, Isambert N, You B, et al. Phase I dose-escalation study of aflibercept in combination with docetaxel and cisplatin in patients with advanced solid tumours. Br J Cancer 2012;107:598-603.
- Patnaik A, Pipas M, Rosen LS, et al. A phase I dose escalation and pharmacokinetic (PK) study of intravenous (iv) aflibercept (VEGF Trap) plus weekly gemcitabine (Gem) in patients (pts) with advanced solid tumors: preliminary results. ASCO Meeting Abstracts 2008;26:3558.
- Tew WP, Gordon M, Murren J, et al. Phase 1 study of aflibercept administered subcutaneously to patients with advanced solid tumors. Clin Cancer Res 2010;16:358-66.
- Jin K, Shen Y, He K, et al. Aflibercept (VEGF Trap): one more double-edged sword of anti-VEGF therapy for cancer? Clin Transl Oncol 2010;12:526-32.
- Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 2004;64:7099-109.
- Fabian MA, Biggs WH 3rd, Treiber DK, et al. A small molecule-kinase interaction map for clinical kinase inhibitors. Nat Biotechnol 2005;23:329-36.
- Wilhelm SM, Dumas J, Adnane L, et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinica antitumor activity. Int J Cancer 2011;129:245-55.
- Wilhelm S, Carter C, Lynch M, et al. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov 2006;5:835-44.
- Waddell T, Cunningham D. Evaluation of regorafenib in colorectal cancer and GIST. Lancet 2013;381:273-5.
- Wehler TC, Hamdi S, Maderer A, et al. Single-agent therapy with sorafenib or 5-FU is equally effective in human colorectal cancer xenograft-no benefit of combination therapy. Int J Colorectal Dis 2013;28:385-98.
- Strumberg D, Clark JW, Awada A, et al. Safety, pharmacokinetics, and preliminary antitumor activity of sorafenib: a review of four phase I trials in patients with advanced refractory solid tumors. Oncologist 2007;12:426-37.
- Zopf D, Heinig R, Thierauch KH, et al. Regorafenib (BAY73-4506): preclinical pharmacology and clinical identification and quantification of its major metabolites. Abstract presented at AACR 101st Annual meeting, Washington DC, April 17-21, Abs 1666,2010.
- Mross K, Frost A, Steinbild S, et al. A phase I dose-escalation study of regorafenib (BAY 73-4506), an inhibitor of oncogenic, angiogenic, and stromal kinases, in patients with advanced solid tumors. Clin Cancer Res 2012;18:2658-67.
- Strumberg D, Scheulen ME, Schultheis B, et al. Regorafenib (BAY 73-4506) in advanced colorectal cancer: a phase I study. Br J Cancer 2012;106:1722-7.
- NCCN guideline for Colon cancer version 3.2013. Last access Janurary 11, 2013. Available online: http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf


