Neoadjuvant chemoradiotherapy for esophageal/gastroesophageal carcinoma
Introduction
According to the American Cancer society, the estimated number of new esophageal cancer diagnoses in 2012 will approach 17,500, approximately 500 more cases than in 2011, with a male preponderance (1). Over the past decade, the rate of esophageal adenocarcinoma has risen significantly, specifically among the Caucasian population (2). Risk factors include higher rates of gastroesophageal reflux and obesity. The standard treatment modality for local and locoregional disease had primarily been surgery. Treatment has evolved to combine additional therapeutic modalities in conjunction with surgery, specifically with varying combinations of radiation and chemotherapy (3-6).
Improvement in outcomes seen with multimodality therapy has prompted further investigation into alternative chemotherapies and radiation protocols (7,8). The rate of complete pathological response (pCR) is increasingly used as a measure of efficacy of neoadjuvant therapies and predictor of outcome (9). Tepper et al. reported in 2008 a pCR of 33% after undergoing neoadjuvant therapy consisting of cisplatin, fluorouracil, and conformal radiation to 50.4 Gy (4). More recently in the CROSS study, a pCR rate of 29% was observed utilizing neoadjuvant paclitaxel/carboplatin and concurrent radiotherapy to a total dose of 41.4Gy (6,10). Following initial presentation of this data, our institution implemented a similar neoadjuvant concurrent chemoradiotherapy regimen using paclitaxel and carboplatin beginning in July 2010. In contrast to the CROSS study, the radiation total dose prescribed was 50.4 Gy. Patients then proceeded to surgical resection. In this study, we evaluated patient, tumor, imaging, and treatment characteristics and response in consecutive patients treated using this trimodality regimen.
Materials and methods
Eligibility
Patients with histologically documented adenocarcinoma of the distal esophagus (thoracic esophagus below 25 cm) or gastroesophageal junction (GEJ) were eligible for review on this IRB approved retrospective study. Those patients who received all aspects of their trimodality therapy at our institution were included for evaluation. All tumors were staged with preoperative imaging and considered resectable (T2-3, N0-1, Stage IB-IIIA).
Positron emission tomography/computed tomography (PET/CT) was obtained prior to and after completion of chemoradiotherapy (CRT). The majority of patients underwent evaluation with computed tomography (CT) with oral and intravenous contrast of the chest, abdomen and pelvis. For patients who underwent endoscopy at outside institutions, repeat endoscopy was performed on the discretion of the surgeon as was endoscopic ultrasound (EUS) with or without biopsy. All outside pathology and radiology was reviewed. All patients were discussed at a multidisciplinary conference with participation of all sub-specialty disciplines involved in the care of esophageal and GEJ carcinomas and treatment recommendations reviewed.
All patients were screened and high risk anesthesia consults were obtained for those patients with significant co-morbidities. Preoperative cardiac stratification and pulmonary function tests were obtained when indicated. Patients were excluded if they were considered non-surgical candidates on the basis of medical co-morbidities, were previously treated with chemotherapy or radiation within the treatment area, were considered unresectable or had metastatic disease, or if they had lymphadenopathy outside the area of planned resection.
Patient data reviewed included complete history/physical examination, upper endoscopy/EUS, biopsy results, CT chest/abdomen and pelvis with oral and IV contrast, PET/CT, and laboratory results including albumin and protein.
Treatment
All patients received concurrent CRT followed by Ivor-Lewis esophagogastrectomy (ILE). Chemotherapy consisted of weekly administration of paclitaxel 50 mg/m2 and carboplatin AUC =2 given intravenously with total infusion time of 2 hours for an average of 6 weeks. These were administered on days 1, 8, 15, 22, 29 and 36. Patients were premedicated with dexamethasone 10 mg, diphenhydramine 50 mg, famotidine 20 mg, and palonosetron 0.25 mg as well as hydrated with intravenous fluid prior to the administration of chemotherapeutic medications.
Conformal radiotherapy to a total dose of 50.4 Gy in 28 fractions was delivered. All patients were treated using volumetric modulated arc therapy (VMAT) with 6 MV photons. Volumes were designed to include gross tumor and nodal disease as noted on endoscopy and on imaging studies, regional nodes and the celiac axis with margin. Organs at risk for treatment planning included lungs, heart, spinal cord, uninvolved esophagus and stomach, liver, and kidneys. Heterogeneity corrections were used in treatment planning using Eclipse Treatment Planning System version 8.5 (Varian Medical Systems, Palo Alto, CA). Dose was prescribed to the planning target volume (PTV) so that at least 95% of the PTV received 99% of prescription dose with dose constraint of 93%≤ PTV ≤107%. One or two arcs were used as needed to meet the above target constraints. Normal tissue dose constraints were consistent with current standard practice with priority on maximum spinal cord dose and volumetric heart and lung dose (11).
Repeat PET/CT and CT imaging with contrast were obtained for restaging following completion of CRT and prior to resection. Surgery was optimally performed 6 to 8 weeks after completion of concurrent CRT. Resection of all patients was performed via midline laparotomy and right posterior lateral thoracotomy (ILE). Prior to proceeding with resection, every surgery started with a small upper midline incision and exploration of the abdominal cavity to rule out metastatic disease. All perigastric, periesophageal, subcarinal and celiac axis nodes that were technically accessible were removed. A gastric conduit with a stapled anastomosis was utilized for all patients and an intraoperative leak test was performed routinely. A feeding jejunostomy was performed in all patients for feeding access. Frozen section analysis of the proximal margin and gross examination of the distal resection margin was analyzed intraoperatively as were any suspicious peritoneal and/or liver lesions.
Data collection
Medical records of consecutive patients diagnosed and treated for distal esophageal or GEJ adenocarcinoma from July 2010 to October 2011 were reviewed. Patient characteristics including age, Eastern Cooperative Oncology Group (ECOG) performance status, gender, weight (pre and post CRT), and past medical history were abstracted. Initial tumor characteristics including histology, grade, clinical stage (based on preoperative CT, PET/CT, EUS), length of tumor, proximal/distal extent of tumor, and standardized uptake values (SUVs) pre and post CRT PET/CTs were reviewed. Chemotherapy characteristics including number of neoadjuvant and adjuvant cycles, toxicities, and treatment delays were recorded. Similarly, radiation treatment characteristics were collected. Time interval data included time of diagnosis to completion of CRT, diagnosis to surgery, and completion of CRT to surgery. Laboratory data prior to and following completion of neoadjuvant treatment was reviewed.
Pathologic evaluation included analysis of the resection specimen and frozen sections, resection status (R0-2), histologic features, presence of perineural and lymphovascular invasion, and nodal involvement. Patients were considered to have a complete pathologic response (pCR) if no tumor cells were identified in either the primary tumor or nodes. Patients were considered to have minimal residual disease if the tumor was <2 mm or isolated tumor cells were identified. Gross residual disease within the pathologic specimen was categorized as macroscopic. Comparisons were made between preoperative biopsy and resection pathology and PET/CT change pre and post CRT to assess response to neoadjuvant therapy. Length of hospital stay, in hospital mortality and postoperative complications were recorded.
Statistical methods
Descriptive statistics such as frequencies and relative frequencies were computed for all categorical variables. Numeric variables were summarized using simple descriptive statistics such as the mean, standard deviation, range, and percentages. Associations between tumor response measures, pCR and SUV, and covariates were tested using the Fisher Exact Test. A 0.05 nominal significance level was used in all hypothesis testing. All statistical analyses were performed using SAS, version 9.3, statistical software (SAS Institute Inc., Cary, NC).
Results
A total of 18 consecutive patients who received trimodality therapy between July 2010 and October 2011 were evaluated. Two patients were excluded who received chemotherapy and/or radiation therapy at an outside institution despite undergoing operative intervention at our institution. The remaining 16 patients received all aspects of their care at our institution.
Patient and tumor characteristics
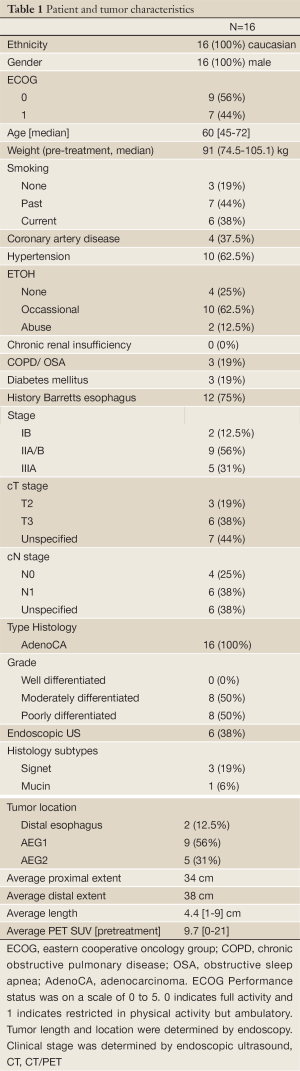
Patient and tumor characteristics are summarized in Table 1. All patients were Caucasian males with a median age of 60 years and ECOG performance status (PS) of 0-1. Tobacco use was common among our patient population with 82% of patients reporting current or past history of usage.
Full table
All patients had moderately to poorly differentiated adenocarcinomas. Three patients had signet ring features and one was found to have mucin production. Over half of the esophageal tumors were considered AEG 1 as defined by the Siewert classification with the tumor epicenter located between 1-5 cm above the GEJ (12). The average length of the tumor was 4.4 cm (1-9 cm). EUS was performed in 38% of patients for staging. All patients underwent pretreatment PET/CT revealing a mean SUV of 9.7 [0-21]. Utilizing the American Joint Committee on Cancer AJCC seventh addition, most patients were stage IIA/B or IIIA. All patients were at least a clinical/radiographic T2 or with clinically/radiographically positive nodes which was documented in 6 patients.
Treatment characteristics
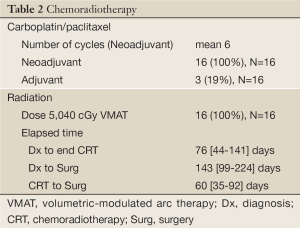
All patients received conformal radiation to 50.4 Gy using a VMAT technique with concurrent carboplatin and paclitaxel, with a mean of 6 cycles. Treatment parameters are summarized in Table 2. Median elapsed time from diagnosis to completion of concurrent chemoradiaton was 76 days (44-141 days), from diagnosis to surgery was 143 days (99-224 days), and from completion of concurrent chemoradiation to surgery was 66 days (35-92 days). Four patients did require a break from treatment secondary to fever/bronchitis, body rash, thrombocytopenia, and an unspecified reason.
Full table
Pathologic and SUV response
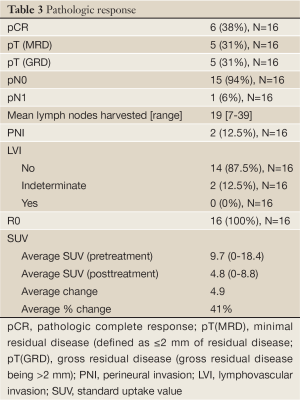
Pathologic and SUV response to neoadjuvant therapy was reviewed (Table 3). All patients received R0 resections. The mean number of lymph nodes harvested was 19 [7-39]. pCR was achieved in 6 (38%) patients with an additional 5 (31%) patients having only minimal residual disease. The remaining 5 patients (31%) had macroscopic residual disease. One patient had pathologic nodal disease seen at resection. Of those 5 patients with gross residual disease, 3 had signet ring features (60%). The sole patient with residual nodal disease (ypN1) had a poorly differentiated adenocarcinoma with signet ring features.
Full table
The average SUV reduction seen post neoadjuvant therapy was 41%. Of the 11 patients with SUV reductions of >35%, 5 had a complete pathologic response and 3 others had minimal residual disease. Of the three patients with signet ring features, 2 had no SUV reduction and all had gross residual disease. The only patient with residual nodal disease (ypN1) had signet ring features and was without a SUV reduction following CRT. Response results are listed in Table 3.
Tumor factors that trended toward significance for a negative association with pathologic response (pCR and minimal residual disease) were lymphovascular/perineural invasion and signet ring/mucin histology (P=0.063). Signet ring/mucin features were also associated with a PET/CT SUV responses of ≤35% (P=0.063).
Treatment tolerance and follow up
Nutritional status was evaluated prior to and following the completion of neoadjuvant CRT (Table 4). Median decrease in albumin, protein and weight were 0.25, 0.1 g/dL and 3.9 kg respectively. Supplemented enteral nutrition via a percutaneous endoscopic gastrostomy tube was utilized preoperatively during neoadjuvant chemoradiotherapy in 3 (19%) of 16 patients, suggesting the tolerance of this regimen.

Full table
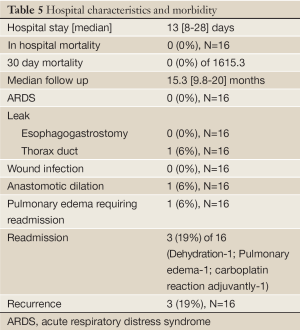
There was no in-hospital, peri-operative, or 30 day mortality. No anastomotic leaks occurred. Mean hospital stay was 13 days (8-28 days). One patient did develop a chyle leak requiring re-operation secondary to failure of medical management. An additional patient required postoperative anastomotic dilation for a stricture. Three patients required readmission within 30 days, one for dehydration, one for pulmonary edema, and the third related to additional adjuvant chemotherapy administration. Major morbidities are listed in Table 5.
Full table
With a median follow up was 15.3 months (9.8-20 months), three patients have developed recurrences (one anastomotic, one cervical lymph node, one supraclavicular lymph node). One of these patients has died from disease at 16.5 months from diagnosis. Two of the three patients with recurrences had tumors with signet ring/mucin features.
Discussion
Trimodality therapy is increasingly becoming the preferred regimen for the treatment of patients with localized/locally advanced esophageal and GEJ cancers (7,13). Our institution has adopted the regimen of neoadjuvant chemotherapy using paclitaxel 50 mg/m2 and carboplatin AUC=2 as per the CROSS study. In our study the radiotherapy differed as we utilized the standardly accepted Western CRT dose of 50.4 Gy and not the 41.4 Gy utilized by those investigators (3,9,10,14). Radiation treatments were delivered using an intensity modulated radiation therapy approach with VMAT versus 3D conformal fields as in the CROSS study. This neoadjuvant CRT regimen was followed by an R0 resection for all patients.
Our results as well as those from the CROSS study show these similar regimens to be well tolerated and achieve significant response rates. Various studies have shown that pCR after neoadjuvant chemoradiotherapy can be obtained in up to 13-33% of patients (4,5,10,15,16). Complete pathologic response has been shown to translate into an improvement in survival (17). The overall pCR rate in the CROSS study was 29%, but only 23% for the subset with adenocarcinoma (6). Our study showed a pCR rate of 38% with minimal residual disease present in an additional 31% of patients. Taken together, 69% of our studied population had minimal if any remaining viable cancer cells. The higher radiation dose might have contributed to the higher response rates observed however our limited sample size precludes any further conclusion and likely multiple patient, tumor, and treatment factors are influential. Additional follow up is needed to evaluate recurrence and survival outcomes.
An improvement in R0 resection rates can occur when neoadjuvant CRT is given prior to surgery compared to surgery alone (16). When compared to other similar studies, our 100% R0 resection rate is likely due to multiple factors including operative technique, case volume and high pathologic response rates (6).
All patients evaluated in this study had moderately to poorly differentiated adenocarcinomas. Subsets of adenocarcinomas are known to have mixed histology consisting of signet ring cells and mucin. This histology has been associated with a worse prognosis (18). We similarly found that those patients with signet ring/mucin features were less likely to have a good pathologic response both locally and regionally. Our results show that of patients with residual macroscopic disease, 60% were of the signet ring subset whereas none of those with pCR or minimal residual disease were identified as having this feature. Although six patients were clinically node positive prior to the initiation of neoadjuvant therapy, the single patient with residual nodal disease following CRT had a poorly differentiated tumor with signet ring features. Two of the 3 patients with recurrent disease had signet ring/mucin features, including the patient deceased of disease.
Lordick et al. evaluated metabolic response by PET/CT to neoadjuvant therapy using a SUV decrease of ≥35% to determine significance (19). Our average SUV decrease from pre to post neoadjuvant CRT was 41%. When evaluating those tumors with signet ring features, 2 of 3 patients had a 0% reduction in SUV. Tumors with signet ring and mucin features were less likely to have a ≥35% SUV reduction than those without these features. This too suggests that those with signet ring/mucin features may be less metabolically responsive to CRT. Our results correlate with the published data regarding more aggressive/less responsive characteristics of this histology but warrant further investigation. Patients with these histologies may better benefit from alternate systemic therapy regimens.
Our study revealed that this regimen of neoadjuvant carboplatin/paclitaxel with concurrent radiotherapy to 50.4 Gy was well tolerated as all patients completed therapy without significant course altering complications. A limitation of our study in assessing tolerance to therapy is that specific toxicity grading was not captured prospectively. We have evaluated tolerance by treatment breaks, weight loss, laboratory and nutritional parameters. Few treatment breaks were required and nutritional parameters prior to and after neoadjuvant CRT showed minimal detrimental effect.
Reported rates of postoperative mortality after neoadjuvant chemoradiotherapy followed by surgery range from 0-12.3% (9). We had no in-hospital or 30 day mortality occurred in patients treated with this trimodality regimen and no anastomotic leaks occurred. Rates for intrathoracic anastomotic leaks vary in the literature and have been reported as high as 16% (20). We credit this low in hospital/30 day mortality and anastomotic leak rate to experienced meticulous technique and algorithmic postoperative care.
Conclusions
This study shows that neoadjuvant treatment with weekly administration of paclitaxel and carboplatin with concurrent radiotherapy to 50.4 Gy was well tolerated and resulted in significant rate of pathologic complete response or minimal residual disease. Patients with signet ring/mucin features appear to have a worse overall response rate and larger residual disease burden following neoadjuvant CRT. Our results suggest that this trimodality regimen can be successfully completed with minimal postoperative complications and mortality. Additional follow up is needed for analysis of recurrence and survival outcomes. Further investigation of predictive factors for response will aid in best tailoring therapy for patients with esophageal/GEJ adenocarcinoma.
Acknowledgements
Disclosure: Neoadjuvant carboplatin/paclitaxel with concurrent radiotherapy followed by surgery for esophageal/gastroesophageal junction adenocarcinoma: a single institution experience.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10-29.
- Simard EP, Ward EM, Siegel R, et al. Cancers with increasing incidence trends in the united states: 1999 through 2008. CA Cancer J Clin 2012. [Epub ahead of print].
- Herskovic A, Martz K, al-Sarraf M, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med 1992;326:1593-8.
- Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 2008;26:1086-92.
- Urba SG, Orringer MB, Turrisi A, et al. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol 2001;19:305-13.
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84.
- Gebski V, Burmeister B, Smithers BM, et al. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: A meta-analysis. Lancet Oncol 2007;8:226-34.
- Medical Research Council Oesophageal Cancer Working Group. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet 2002;359:1727-33.
- Juergens RA, Forastiere A. Combined modality therapy of esophageal cancer. J Natl Compr Canc Netw 2008;6:851-60; quiz 861.
- Gaast AV, van Hagen P, Hulshof M, et al. Effect of preoperative concurrent chemoradiotherapy on survival of patients with resectable esophageal or esophagogastric junction cancer: Results from a multicenter randomized phase III study. ASCO Meeting Abstracts 2010;28:4004.
- Marks LB, Ten Haken RK, Martel MK. Quantitative analyses of normal tissue effects in the clinic. Int J Radiat Oncol Biol Phys 2010;76:S1-S160.
- Siewert JR, Stein HJ. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg 1998;85:1457-9.
- Graham AJ, Shrive FM, Ghali WA, et al. Defining the optimal treatment of locally advanced esophageal cancer: A systematic review and decision analysis. Ann Thorac Surg 2007;83:1257-64.
- Minsky BD, Pajak TF, Ginsberg RJ, et al. INT 0123 (radiation therapy oncology group 94-05) phase III trial of combined-modality therapy for esophageal cancer: High-dose versus standard-dose radiation therapy. J Clin Oncol 2002;20:1167-74.
- Walsh TN, Noonan N, Hollywood D, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med 1996;335:462-7.
- Burmeister BH, Smithers BM, Gebski V, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol 2005;6:659-68.
- Meredith KL, Weber JM, Turaga KK, et al. Pathologic response after neoadjuvant therapy is the major determinant of survival in patients with esophageal cancer. Ann Surg Oncol 2010;17:1159-67.
- Matsuki A, Nishimaki T, Suzuki T, et al. Esophageal mucoepidermoid carcinoma containing signet-ring cells: three case reports and a literature review. J Surg Oncol 1999;71:54-7.
- Lordick F, Ott K, Krause BJ, et al. PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: the MUNICON phase II trial. Lancet Oncol 2007;8:797-805.
- Blewett CJ, Miller JD, Young JE, et al. Anastomotic leaks after esophagectomy for esophageal cancer: a comparison of thoracic and cervical anastomoses. Ann Thorac Cardiovasc Surg 2001;7:75-8.


