Does histology really influence gastric cancer prognosis?
Introduction
In the past decade, advances in systemic therapy for gastric cancer (GC) have led to improved survival. Despite this, five-year overall survival (OS) ranges from 36–53% in patients with stage II or higher treated GC (1-3). In western countries where routine screening is not performed, patients are often diagnosed at an advanced stage. Currently, clinical parameters such as tumor-node-metastasis (TNM) staging have been the most reliable predictors of survival. Other prognostic factors include positive lymph node ratio and neutrophil-lymphocyte ratio (NLR), with higher positive lymph node ratio and higher NLR both suggestive of worse survival (4,5). Response to chemotherapy, as measured by tumor regression grade, has also been correlated with survival, though reports are conflicting (6). Predictive biomarkers under investigation include expression of human epidermal growth factor receptor 2 (HER2), a tyrosine kinase receptor involved in cell proliferation, apoptosis, and differentiation (7). HER-2 overexpression, which occurs more commonly in intestinal-type GC, was found to be associated with decreased survival (8). More recently, Li et al. demonstrated that a seven micro-RNA signature was associated with improved relapse-free survival and OS (9).
Histology has been one of the earliest prognostic markers in GC. The Lauren classification divided GC into intestinal and diffuse subtypes (10). Intestinal-type GC occurs more frequently in elderly male patients and is thought to be associated with improved survival (11,12). In 2010, the World Health Organization (WHO) re-classified gastric adenocarcinoma into five major subtypes: tubular, papillary, mucinous, poorly cohesive, and mixed. Signet-ring cell gastric carcinoma, which is grouped with diffuse-type in the Lauren classification and poorly cohesive subtype in the WHO classification, has generally been associated with a worse prognosis, although some studies cite a more favorable prognosis. In addition, the impact of signet-ring cell on prognosis may be stage dependent (13-16).
Linitis plastica (LP) is a well-recognized clinical entity which is not part of any staging or classification schema but which has important implications for survival. LP is characterized by macroscopic thickening and rigidity of the gastric wall, lymph node metastasis, and peritoneal spread (17-19). Histologically, LP is a subset of diffuse-type GC. In addition, foci of signet-ring cells are often present (20). Five-year OS rates for this disease range from 0 to 20% despite multimodal therapy (21,22). The purpose of this study is to determine whether the reported impact of histology on prognosis is related to the association between LP and diffuse and signet-ring cell GC. Secondly, the management of gastric LP patients is controversial; by examining our institutional experience, we can assess long term outcomes for optimally treated LP patients.
Methods
Study population
Prior to study initiation, approval from the institutional review board was obtained. The medical records of patients undergoing surgery for gastric adenocarcinoma from 2000 to 2015 at a single institution were evaluated. Clinicopathologic characteristics were examined and patients were divided into four groups of patients based on Lauren classification: intestinal (n=93), diffuse (n=20), diffuse with signet-ring cell features (n=57), and LP (n=40). LP was defined as patients who had diffuse GC by histology but also presented with circumferential infiltration and thickening of the gastric wall for at least a third of the stomach length on endoscopy or by imaging. Endoscopically, these patients were classified as Borrmann type IV with infiltrative tumors and indistinct borders (23,24).
Staging work-up and treatment
Preoperative staging consisted of a combination of esophagogastroduodenoscopy (EGD) with or without endoscopic ultrasound (EUS) and imaging by computed tomography (CT) and/or positron emission tomography (PET). Biopsies performed at an outside hospital were reviewed by pathologists at our institution to confirm the diagnosis of gastric adenocarcinoma. Eighty-four patients (40%) received neoadjuvant therapy. Patients underwent gastrectomy with a D1 or modified D2 (with preservation of the spleen and distal pancreas except in cases of continuous spread) lymphadenectomy. After surgery patients who underwent neoadjuvant chemotherapy had their tumors evaluated by the pathologist for response to therapy and tumor regression was assessed. The scoring system for tumor response was as follows: no residual tumor [complete response, 0], marked response [minimal residual tumor, 1], moderate response [2], and no definite response identified [3]. For statistical evaluation, patients with scores of 0 and 1 were combined and 2 and 3 were combined.
Statistical analysis
Clinicopathologic factors were described by means and standard deviation for continuous variables and frequencies and percentages for categorical variables. Differences in variables between the four groups were evaluated by Fisher’s exact test and analysis of variance (ANOVA). Kaplan-Meier survival was used to determine median OS and log rank test was used to determine statistical significance between staging and histological groups. Univariate and multivariate Cox proportional hazard regression analysis was used to evaluate factors associated with survival and quantified by hazard ratio with 95% confidence interval (CI). Only variables with P value less than 0.15 from the univariate analysis were selected as candidate variables for Cox regression. Variable selection of Cox regression model was performed based on AIC criterion. A two-sided P≤0.05 was considered statistically significant. Statistical analysis was performed with R version 3.3.2 software (R Foundation for Statistical Computing, Vienna, Austria).
Results
From 2000 to 2015, 210 patients underwent resection for gastric adenocarcinoma. Of the cohort, there were 40 patients who were clinically diagnosed with LP. These patients all had diffuse-type histology, though 72.5% also had foci of signet-ring cells. Table 1 compares clinicopathological variables between four groups of patients: intestinal (n=93), diffuse (n=20), diffuse with signet-ring cell (n=57), and LP (n=40). Patients with intestinal histology tended to be older (P<0.001) but there were no differences between the groups in terms of other clinical variables such as gender, ethnicity, preoperative body mass index, and preoperative stage. Administration of neoadjuvant chemotherapy differed among the four groups, with preoperative therapy occurring in 32%, 35%, 50%, and 70% of intestinal, signet-ring cell, LP, and diffuse GC patients, respectively (P=0.008). Approximately 50% of patients received adjuvant therapy, with the majority (63%) receiving adjuvant chemotherapy only. There were no differences in receipt of adjuvant therapy between the four groups. Seventy-five percent of LP patients underwent total gastrectomy compared to 32%, 42%, and 55% for signet-ring, intestinal, and diffuse, respectively (P=0.006). On final surgical pathology, LP patients had a higher rate (27.5%) of positive surgical margins, perineural invasion (PNI), and lymph node metastasis (P<0.001). None of the LP patients had stage I disease but 70% were stage III compared to 41% with stage I and 12% with stage III for intestinal patients (P<0.001).
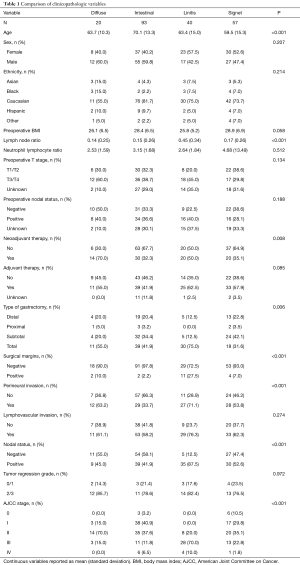
Full table
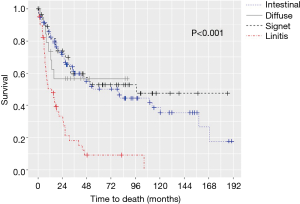
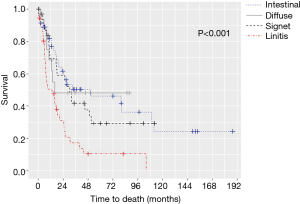
The median OS for all pathologic stages, stratified by histology, was 14 months for LP, 79 months for intestinal, 97 months for signet-ring cell, and not reached for diffuse GC patients (P<0.001) (Figure 1). Survival was then assessed for stage II and III patients who underwent surgery for curative-intent. LP patients still fared the worst with median OS of 13.7 months compared to 15.8, 30.7, and 50.9 months for diffuse, signet-ring, and intestinal GC, respectively (P<0.001) (Figure 2). Kaplan-Meier survival curves were also constructed for LP patients. Median OS for stage II and III patients were 20.1 and 15.1 months, respectively (P=0.672) (Figure 3A). When survival was stratified by resection status, there was no difference in survival for R0, R1, and R2 resections (Figure 3B). Survival was also stratified by tumor regression grade (Figure 3C). Though there was a trend for improved survival in patients who had a complete or nearly complete response to neoadjuvant therapy, there were no statistically significant differences in survival based on treatment response.

Univariate analysis was used to assess preoperative and pathologic variables associated with survival (Table 2). Patient characteristics and clinical parameters such as age, gender, ethnicity, BMI, and NLR did not influence survival. Preoperative nodal disease was associated with a worse survival (HR 1.86, 95% CI, 1.15–3.01, P=0.002). Interestingly, neither neoadjuvant nor adjuvant therapy were not associated with survival. Tumor regression grade also did not affect outcome. Histopathologic characteristics such as positive surgical margins, PNI, lymphovascular invasion (LVI), nodal metastasis, and higher pathologic stage were associated with worse survival (P<0.001). LP was also associated with worse survival (HR 3.45, 95% CI, 2.19–5.44, P<0.001).
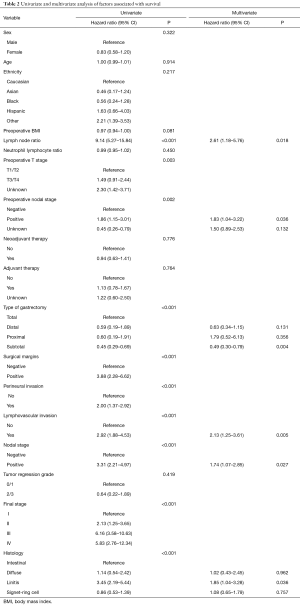
Full table
In the Cox regression model, LVI and pathologically positive lymph nodes were associated with worse survival. Compared to total gastrectomy, subtotal gastrectomy was associated with better survival (HR 0.49, 95% CI, 0.30–0.79, P=0.004). For histology, when compared to intestinal-type, only LP was associated with worse survival (HR 1.85, 95% CI, 1.04-3.28, P=0.036).
Discussion
Histology is a frequently cited prognostic factor in GC, with worse survival reported in diffuse-type and signet-ring cell carcinoma. A concept related to histology is gastric LP, which occurs in up to 20% of GC cases (25,26). LP has a variable histologic and clinical definition and is not a component of GC staging. We sought to examine whether the poorer survival reported in diffuse and signet-ring cell types may be partially attributed to the presence of LP. By separating LP from other histologic subgroups, we could better evaluate the individual impact of histology and of LP on survival. In comparing the four groups, expected differences were present. The majority of LP patients had stage III disease. The LP group also had a higher rate of microscopically positive resection margins despite a higher rate of total gastrectomy. Clinicopathologic parameters were similar among intestinal, diffuse, and signet-ring cell tumors. Pathologic factors of notable difference included low rate of PNI in intestinal tumors (34%) compared to high rates in LP (71%), and high rates of nodal metastasis in LP. After accounting for all clinicopathologic variables, gastric LP remained significantly associated with decreased survival on multivariate analysis. On the other hand, diffuse and signet-ring cell GC did not portend a worse survival compared to intestinal-type GC. These findings demonstrate that while there was a higher incidence of poor prognostic indicators in patients with LP, the presence of LP itself remained independently associated with worse outcomes. On the other hand, histology did not influence survival after controlling for other confounding variables.
Neutrophilia and lymphopenia, and the resulting elevated NLR, has been shown to be associated with worse outcomes in early and advanced GC (4,25,26). Interestingly, our study did not demonstrate survival difference associated with NLR. One possible explanation is that NLR was evaluated as a continuous variable in our study. Perhaps there is a cutoff value or stratification in which NLR significantly affects survival. Additional work is needed to clarify the impact of NLR on prognosis in our study.
For many tumors, including esophageal and colorectal carcinoma, response to neoadjuvant therapy, particularly a complete pathologic response, improves long term survival (27,28). Tumor regression grade as a prognostic marker in GC has been variably reported. One of the earlier studies by Becker et al. did not demonstrate any patients with complete pathologic responses after treatment, however tumor regression was correlated with survival (6). In the current study, 12/62 (19.4%) patients had a complete or marked response to neoadjuvant chemotherapy, and only 4/62 (6.5%) exhibited a complete response on final pathology. In other studies, tumor responses were also low, with less than 25% of patients with a significant response while >50% of patients had minimal response (29-31). Many of these reports also failed to show a statistically significant relationship between response to neoadjuvant therapy and survival. As with the current study, one possible explanation is the low complete or near complete response rate to neoadjuvant therapy in GC.
The management of LP remains controversial. Some authors argue that long term survival is rarely possible in this disease (18,32). However, a recent study by Blackham et al. found comparable survival between optimally resected LP patients and non-LP patients with advanced disease, with median OS of 25–56 months (33). Our cohort consisted of 40 LP patients, which though small is comparable to other series. We did not find survival differences among LP patients based on stage and completeness of resection. Among gastric LP patients, only 50% received preoperative chemotherapy. Patients who did not receive neoadjuvant therapy were often treated in the earlier period of the study, which is also true for the entire cohort. A higher percentage of patients (63%) did received adjuvant chemotherapy or chemoradiation. It is unclear which factors, such as patient refusal, poor functional status, or lack of access contributed to the low rates of administration of neoadjuvant and adjuvant therapy and to what extent they contributed to the lower survival. Therefore, despite optimal resection, the low rate of multimodal therapy in this aggressive disease likely partially contributed to the decreased survival in this study.
Another potential contributor of decreased survival is the presence of occult metastatic disease. In resected stage II and III patients, linitis plastic still fared the worst with median OS of only 13.7 months. Though patients were explored for metastatic disease prior to resection, routine peritoneal washings were not performed in the earlier study period. Many studies have demonstrated the association between free intraperitoneal tumor cells and poor survival and peritoneal recurrence (34-36). It is possible that LP patients had a greater likelihood of undiagnosed peritoneal disease and hence survival rates were lowered. Within the entire cohort, not just LP, there were also a few patients who had negative peritoneal nodule biopsies that were found to be metastatic disease on final pathology. These patients were included because we also wanted to examine their outcomes. Interestingly these patients had similar survival to those with advanced disease without peritoneal metastasis, though there are too few patients to make adequate comparisons.
This study attempts to differentiate between the effect of histology and of LP on GC outcomes. Here, we defined LP as diffuse GC of Borrmann IV classification with circumferential thickening for at least a third of the stomach length. By a more uniform characterization of gastric LP, comparisons can be made between studies regarding LP. This will hopefully facilitate a greater understanding of this disease. Our data analysis was limited by the retrospective nature of this study and its inherent biases. Also, in addition to limitations mentioned above, we were limited by our ability to abstract data from patients to what was available from chart reviews. As such, information such as reasons for omission of chemotherapy were lacking for some patients. Lastly, the time interval of the study was relatively long, so there was heterogeneity in the management of patients.
This study demonstrates that outcomes in GC remain poor despite multimodal therapy and improvement in systemic therapy. Still, it is clear that too often LP patients are not diagnosed at an early and potentially curable stage. The biological behavior of LP may be different from other subtypes. As yet, there are no biological markers to distinguish LP from other types of GC. With continued advances, perhaps a molecular characterization of GC will help to diagnose the disease at an earlier stage and allow for better selection of optimal therapy.
Conclusions
In contrast to prior reports, this study suggests that histology has minimal impact on GC outcomes. The presence of LP and nodal metastasis appear to be more important prognostic variables. Despite improvement in multimodal therapy, novel prognostic and predictive markers, as well as a greater understanding of the biology of LP need to be developed to improve survival in this aggressive malignancy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the institutional review board of H. Lee Moffitt Cancer Center (No. Pro00004978) and since this was a retrospective review patient informed consent was not necessary.
References
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Ychou M, Boige V, Pignon J-P, et al. Perioperative Chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: An FNCLCC and FFCD multicenter phase III Trial. J Clin Oncol 2011;29:1715-21. [Crossref] [PubMed]
- Tsuburaya A, Mizusawa J, Tanaka Y, et al. Neoadjuvant chemotherapy with S‐1 and cisplatin followed by D2 gastrectomy with para‐aortic lymph node dissection for gastric cancer with extensive lymph node metastasis. Br J Surg 2014;101:653-60. [Crossref] [PubMed]
- Shimada H, Takiguchi N, Kainuma O, et al. High preoperative neutrophil-lymphocyte ratio predicts poor survival in patients with gastric cancer. Gastric Cancer 2010;13:170-6. [Crossref] [PubMed]
- Xu DZ, Geng QR, Long ZJ, et al. Positive lymph node ratio is an independent prognostic factor in gastric cancer after d2 resection regardless of the examined number of lymph nodes. Ann Surg Oncol 2009;16:319-26. [Crossref] [PubMed]
- Becker K, Mueller JD, Schulmacher C, et al. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer 2003;98:1521-30. [Crossref] [PubMed]
- Gravalos C, Jimeno A. HER2 in gastric cancer: A new prognostic factor and a novel therapeutic target. Ann Oncol 2008;19:1523-9. [Crossref] [PubMed]
- Park DI, Yun JW, Park JH, et al. HER-2/neu amplification is an independent prognostic factor in gastric cancer. Dig Dis Sci 2006;51:1371-9. [Crossref] [PubMed]
- Li X, Zhang Y, Zhang Y, et al. Survival prediction of gastric cancer by a seven-microRNA signature. Gut 2010;59:579-85. [Crossref] [PubMed]
- Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. Acta Pathol Microbiol Scand 1965;64:31-49. [Crossref] [PubMed]
- Bringeland EA, Wasmuth HH, Mjones P, et al. A population-based study on incidence rates, Lauren distribution, stage distribution, treatment, and long-term outcomes for gastric adenocarcinoma in Central Norway 2001-2011. Acta Oncol 2017;56:39-45. [Crossref] [PubMed]
- Zheng HC, Zheng YS, Xia P, et al. The pathobiological behaviors and prognosis associated with Japanese gastric adenocarcinomas of pure WHO histological subtypes. Histol Histopathol 2010;25:445-52. [PubMed]
- Kwon KJ, Shim KN, Song EM, et al. Clinicopathological characteristics and prognosis of signet ring cell carcinoma of the stomach. Gastric Cancer 2014;17:43-53. [Crossref] [PubMed]
- Hyung WJ, Noh SH, Lee JH, et al. Early gastric carcinoma with signet ring cell histology. Cancer 2002;94:78-83. [Crossref] [PubMed]
- Piessen G, Messager M, Leteurtre E, et al. Signet ring cell histology is an independent predictor of poor prognosis in gastric adenocarcinoma regardless of tumoral clinical presentation. Ann Surg 2009;250:878-87. [Crossref] [PubMed]
- Gronnier C, Messager M, Robb WB, et al. Is the negative prognostic impact of signet ring cell histology maintained in early gastric adenocarcinoma? Surgery 2013;154:1093-9. [Crossref] [PubMed]
- Kim DY, Kim HR, Kim YJ, et al. Clinicopathological features of patients with Borrmann type IV gastric carcinoma. ANZ J Surg 2002;72:739-42. [Crossref] [PubMed]
- Aranha GV, Georgen R. Gastric linitis plastica is not a surgical disease. Surgery 1989;106:758-62; discussion 62-3. [PubMed]
- Yokota T, Teshima S, Saito T, et al. Borrmann's type IV gastric cancer: clinicopathologic analysis. Can J Surg 1999;42:371-6. [PubMed]
- Mastoraki A, Papanikolaou IS, Sakorafas G, et al. Facing the challenge of managing linitis plastica--review of the literature. Hepatogastroenterology 2009;56:1773-8. [PubMed]
- Pedrazzani C, Marrelli D, Pacelli F, et al. Gastric linitis plastica: which role for surgical resection? Gastric Cancer 2012;15:56-60. [Crossref] [PubMed]
- Schauer M, Peiper M, Theisen J, et al. Prognostic factors in patients with diffuse type gastric cancer (linitis plastica) after operative treatment. Eur J Med Res 2011;16:29-33. [Crossref] [PubMed]
- Borchard F. Classification of gastric carcinoma. Hepatogastroenterology 1990;37:223-32. [PubMed]
- Borrmann R. Geschwülste des Magens und Duodenums. Verdauungsschlauch. Springer, 1926:812-1054.
- Kitamura K, Beppu R, Anai H, et al. Clinicopathologic study of patients with Borrmann type IV gastric carcinoma. J Surg Oncol 1995;58:112-7. [Crossref] [PubMed]
- Issam Beyrouti M, Beyrouti R, Ben Amar M, et al. Linitis plastica. Presse Med 2007;36:1782-6. [Crossref] [PubMed]
- Berger AC, Farma J, Scott WJ, et al. Complete response to neoadjuvant chemoradiotherapy in esophageal carcinoma is associated with significantly improved survival. J Clin Oncol 2005;23:4330-7. [Crossref] [PubMed]
- García-Aguilar J, de Anda EH, Sirivongs P, et al. A pathologic complete response to preoperative chemoradiation is associated with lower local recurrence and improved survival in rectal cancer patients treated by mesorectal excision. Dis Colon Rectum 2003;46:298-304. [Crossref] [PubMed]
- Becker K, Langer R, Reim D, et al. Significance of histopathological tumor regression after neoadjuvant chemotherapy in gastric adenocarcinomas: a summary of 480 cases. Ann Surg 2011;253:934-9. [Crossref] [PubMed]
- Fujitani K, Mano M, Hirao M, et al. Posttherapy nodal status, not graded histologic response, predicts survival after neoadjuvant chemotherapy for advanced gastric cancer. Ann Surg Oncol 2012;19:1936-43. [Crossref] [PubMed]
- Blackham AU, Greenleaf E, Yamamoto M, et al. Tumor regression grade in gastric cancer: Predictors and impact on outcome. J Surg Oncol 2016;114:434-9. [Crossref] [PubMed]
- Jafferbhoy S, Shiwani H, Rustum Q. Managing gastric linitis plastica: keep the scalpel sheathed. Sultan Qaboos Univ Med J 2013;13:451-3. [Crossref] [PubMed]
- Blackham AU, Swords DS, Levine EA, et al. Is linitis plastica a contraindication for surgical resection: A multi-institution study of the U.S. gastric cancer collaborative. Ann Surg Oncol 2016;23:1203-11. [Crossref] [PubMed]
- Pecqueux M, Fritzmann J, Adamu M, et al. Free intraperitoneal tumor cells and outcome in gastric cancer patients: a systematic review and meta-analysis. Oncotarget 2015;6:35564-78. [PubMed]
- Bentrem D, Wilton A, Mazumdar M, et al. The value of peritoneal cytology as a preoperative predictor in patients with gastric carcinoma undergoing a curative resection. Ann Surg Oncol 2005;12:347-53. [Crossref] [PubMed]
- Badgwell B, Cormier JN, Krishnan S, et al. Does neoadjuvant treatment for gastric cancer patients with positive peritoneal cytology at staging laparoscopy improve survival? Ann Surg Oncol 2008;15:2684-91. [Crossref] [PubMed]