Chemoradiotherapy versus chemotherapy alone for unresected intrahepatic cholangiocarcinoma: practice patterns and outcomes from the national cancer data base
Introduction
Despite its rarity in the United States, intrahepatic cholangiocarcinoma (IC) is associated with a poor prognosis, especially for unresected disease. The National Comprehensive Cancer Network (NCCN) offers a category 1 recommendation of chemotherapy (CT) alone for this population (1). Chemoradiotherapy (CRT) is a category 2a recommendation, largely owing to no randomized evidence to date.
The addition of local therapy for biliary neoplasms is appealing and an area of active investigation (2,3). Postoperative patterns of recurrence are largely locoregional prior to development of distant metastasis; in fact, initial failure occurs distantly in just 10–15% of cases (4). Locoregional recurrence is also the main cause of tumor-related mortality in these patients (4). Moreover, numerous non-comparative publications have highlighted the safety and efficacy of adding radiotherapy (RT) to CT; these have allowed for high local control, low toxicity rates, and/or numerically prolonged survival (5-12). Many of these studies underscore the importance of providing local therapy to prevent locoregional tumor progression, which can lead to symptomatic worsening and a deterioration in quality of life.
This comparative study of a large, contemporary national database sought to evaluate national practice patterns and outcomes of unresected IC receiving CT alone versus CRT. Although challenging to assess with single- or multi-institutional analyses owing to the relative rarity of this neoplasm, the National Cancer Data Base (NCDB) provides a unique resource with which to address this novel but clinically important issue.
Methods
The NCDB is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society, which consists of de-identified information regarding tumor characteristics, patient demographics, and patient survival for approximately 70% of the US population (13). All pertinent cases are reported regularly from CoC-accredited centers and compiled into a unified dataset, which is then validated. The NCDB contains information not included in the Surveillance, Epidemiology, and End Results (SEER) database, including details regarding use of systemic therapy. The data used in the study were derived from a de-identified NCDB file (2004–2013). The American College of Surgeons and the CoC have not verified and are neither responsible for the analytic or statistical methodology employed nor the conclusions drawn from these data by the investigators. As all patient information in the NCDB database is de-identified, this study was exempt from institutional review board evaluation.
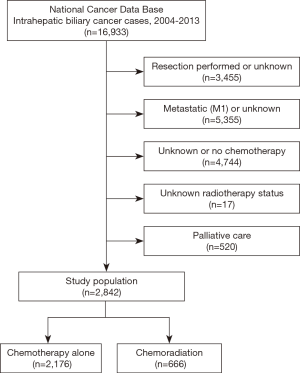
Inclusion criteria for this study were patients with newly-diagnosed primary IC. Other biliary neoplasms or hepatocellular carcinoma were not included in the assigned dataset given by the NCDB. Patients that underwent resection (lobectomy, hepatectomy, wedge/segmental resection, or surgery not otherwise specified) were excluded. Patients with M1 disease, unknown M classification, or in situ disease were excluded. Patients without known receipt of CT were eliminated (1,14). Those with missing RT status were also eliminated, as were those coded as palliative in the database. All patients were dichotomized into two groups based on receipt of CT alone versus CRT. In accordance with the variables in NCDB files, information collected on each patient broadly included demographic, clinical, and treatment parameters.
All statistical tests were performed with SAS software (Version 9.4, Cary, NC, USA); tests were two-sided, with a threshold of P<0.05 for statistical significance. Univariable and multivariable logistic regression were used to determine characteristics associated with receipt of CRT. All initially examined variables were considered for inclusion into models for stepwise selection (at the 0.05 level), except clinical T and N classification owing to the numerous patients with missing information. Survival analysis (performed with Kaplan-Meier methodology) evaluated overall survival (OS), defined as the interval between the date of diagnosis and the date of death or censored at last contact. Univariate and multivariate Cox proportional hazards modeling evaluated predictors of OS, performed with stepwise selection initially encompassing all available variables.
Results
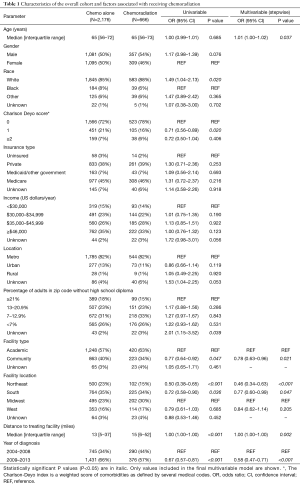
Figure 1 illustrates the patient selection diagram for this investigation. In total, 2,842 patients met study criteria (Table 1); 666 (23%) patients underwent CRT, and 2,176 (77%) received CT alone. Following univariable assessment, multivariable analysis revealed that patients receiving CRT were older, underwent therapy at academic centers, and lived farther from the treating facility (P<0.05 for all). There were also regional differences in CRT administration, with decreased use in the Northeast and South as compared to the Midwest; CRT was also delivered less in more recent time periods (2009–2013 vs. 2004–2008) (P<0.05 for all).
Full table
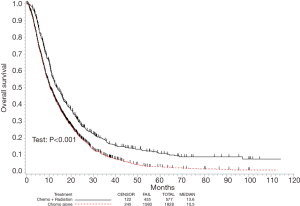
Kaplan-Meier estimates comparing OS in patients that underwent CT alone versus CRT are displayed in Figure 2. The median follow-up was 10 months (range, 0–114 months), the median OS in the respective cohorts was 10.5 [95% confidence interval (CI), 10.0–11.5] months and 13.6 (95% CI, 12.3–15.7) months (P<0.001).
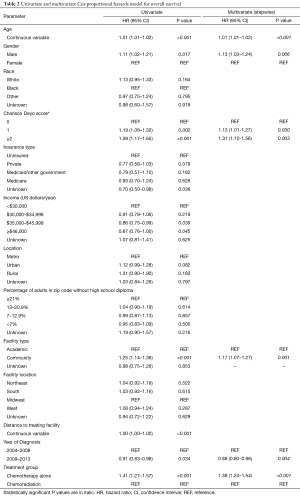
In all patients, there were several predictors of OS on univariate analysis (Table 2). Following multivariate analysis (Table 2), factors independently associated with decreased OS included advancing age, male gender, increased comorbidities, treatment at a community facility, and diagnosis in earlier years (P<0.05 for all). Of note, receipt of CT alone relative to CRT independently predicted for worse OS (hazard ratio 1.38; 95% CI, 1.24–1.54, P<0.001).
Full table
Discussion
Our investigation of a large, contemporary national database of this relatively rare neoplasm is the largest such analysis to date and demonstrates that the addition of RT to CT is independently associated with higher survival in unresected IC. These data directly support the currently accruing phase III trial of stage III-IV unresectable IC receiving gemcitabine/cisplatin with or without tumor-directed RT, whose primary endpoint is OS (15).
While randomized data are needed for this malignancy, it is clear that the retrospective data reported herein may carry selection biases similar to several aforementioned studies, including potentially performing more aggressive therapy in patients that are better able to tolerate multimodality therapy or with better risk features. However, it is also possible that those receiving CRT were a “higher-risk” population with poorer prognostic tumor features and yet still experienced a significantly higher OS (16,17). While we are not able to compare tumor size between cohorts owing to the lack of surgical resection, it is possible that local therapy may have been more often delivered to bulky disease at higher risk for future symptomatology, or from doubt that CT alone could sufficiently control disease progression. To this extent, a limitation of this study is the NCDB’s lack of information on tumor size, and that the T and N classifications were also missing in most patients, likely because this cohort consisted of non-operative patients. Additionally, because all patients received CT, it is unlikely that one group was more “unhealthy” than the other, since both cohorts were “fit” enough to receive CT.
Another element that further adds credence to these findings is the study design. Although the NCDB records RT dose information, we intentionally opted not to utilize it as an inclusion/exclusion criterion. Placing a dose threshold may have artificially inflated survival for the CRT cohort, since only the “healthiest” patients would tolerate full-dose RT. Despite evaluating all RT patients, including those with suboptimal dose and/or tolerance, the CRT cohort still experienced statistically higher OS. Other reasons for not evaluating RT dose included existing studies utilizing a wide variety of doses (5-12), the NCCN’s lack of a single-best recommended dose for unresected tumors (1), incomplete NCDB dose reporting in many cases, and the overall uncommonness of this malignancy such that further limiting patient numbers would not have allowed adequate sample sizes for comparative analysis.
An interesting area of ongoing investigation of RT for unresected IC is the impact of RT modality. Although conventionally-fractionated RT has been historically utilized, advances in radiation oncology have involved the application of stereotactic body RT (SBRT) (5,8,9,12) and proton beam therapy (PBT) (10,18,19). Both allow for high conformality; SBRT offers the ability to deliver ablative doses in far fewer treatments than conventional fractionation. This is highly important for the practical utility of a local therapy modality; secondary analyses of prospective data have supported high doses per fraction with improved local control and OS (11). Additionally, PBT is being actively investigated for numerous gastrointestinal neoplasms and may allow the maintenance of favorable dosimetric profiles despite large irradiated volumes (20). However, because the NCDB largely has unknown/missing codes for RT modality, this work cannot address this issue further.
Lastly, a recent report from Korea demonstrated that delivering combined-modality therapy can allow for tumor downsizing in a small proportion of patients, which can then facilitate the ability to undergo surgical resection (20). Because patients receiving surgery were excluded from this work, we also cannot speak to this notion, but it is certainly conceivable that well-selected patients may benefit from RT so as to allow for resection, making the potential magnitude of benefit for RT even greater than what is reported in this analysis. However, predicting tumor response is more challenging, and hence patient selection for combined-modality treatment must be more completely addressed in future work.
As observed herein, the independent association between treatment at an academic center and OS has far-reaching implications on patient counseling and management by both oncologists and referring providers. Potential causes of this finding are not limited to greater multimodality coordination, streamlined and thorough diagnostic processes, technical expertise, ancillary support staff for close clinical monitoring, and the availability of salvage therapies or clinical trials. Nevertheless, this finding may impact any case of unresected IC and could warrant revisions in patterns of patient education.
Although the NCDB provides a unique platform with which to study this important clinical question, this investigation is not without shortcomings, as described elsewhere (21-43). First, NCDB studies are inherently retrospective, with selection biases and lack of several endpoints such as locoregional control or cancer-specific survival. Second, although we excluded palliative care patients (based on the NCDB variable), definitions of this variable are subject to interpretation and bias. Third, as mentioned extensively above, the NCDB does not keep track of several other factors, including CT details, performance/functional status, RT field design/techniques/volumes. Furthermore, information on T/N-classification and tumor size is largely missing and was hence not able to be analyzed. Additionally, the NCDB does not allow for an assessment of subsequent lines of treatment (e.g., re-irradiation, further systemic and/or targeted therapy), which could also influence OS.
Conclusions
This is the largest study to date evaluating CRT versus CT alone for unresectable IC. Administration of CRT independently predicted for improved survival. However, causation is not implied, and an ongoing phase III study will provide definitive evidence regarding this issue.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: As all patient information in the NCDB database is de-identified, this study was exempt from institutional review board evaluation.
References
- National Comprehensive Cancer Network. Hepatobiliary cancers. Version 3.2017. Available online: https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf, accessed October 1, 2017.
- Wo JY, Dawson LA, Zhu AX, et al. An emerging role for radiation therapy in the treatment of hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Surg Oncol Clin N Am 2014;23:353-68. [Crossref] [PubMed]
- Sahai P, Kumar S. External radiotherapy and brachytherapy in the management of extrahepatic and intrahepatic cholangiocarcinoma: available evidence. Br J Radiol 2017;90:20170061. [Crossref] [PubMed]
- Macdonald OK, Crane CH. Palliative and postoperative radiotherapy in biliary tract cancer. Surg Oncol Clin N Am 2002;11:941-54. [Crossref] [PubMed]
- Tse RV, Hawkins M, Lockwood G, et al. Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol 2008;26:657-64. [Crossref] [PubMed]
- Shinohara ET, Mitra N, Guo M, et al. Radiation therapy is associated with improved survival in the adjuvant and definitive treatment of intrahepatic cholangiocarcinoma. Int J Radiat Oncol Biol Phys 2008;72:1495-501. [Crossref] [PubMed]
- Chen YX, Zeng ZC, Tang ZY, et al. Determining the role of external beam radiotherapy in unresectable intrahepatic cholangiocarcinoma: a retrospective analysis of 84 patients. BMC Cancer 2010;10:492. [Crossref] [PubMed]
- Barney BM, Olivier KR, Miller RC, et al. Clinical outcomes and toxicity using stereotactic body radiotherapy (SBRT) for advanced cholangiocarcinoma. Radiat Oncol 2012;7:67. [Crossref] [PubMed]
- Mahadevan A, Dagoglu N, Mancias J, et al. Stereotactic body radiotherapy (SBRT) for intrahepatic and hilar cholangiocarcinoma. J Cancer 2015;6:1099-104. [Crossref] [PubMed]
- Hong TS, Wo JY, Yeap BY, et al. Multi-institutional phase II study of highdose hypofractionated proton beam therapy in patients with localized, unresectable hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol 2016;34:460-8. [Crossref] [PubMed]
- Tao R, Krishnan S, Bhosale PR, et al. Ablative radiotherapy doses lead to a substantial prolongation of survival in patients with inoperable intrahepatic cholangiocarcinoma: a retrospective dose response analysis. J Clin Oncol 2016;34:219-26. [Crossref] [PubMed]
- Sandler KA, Veruttipong D, Agopian VG, et al. Stereotactic body radiotherapy (SBRT) for locally advanced extrahepatic and intrahepatic cholangiocarcinoma. Adv Radiat Oncol 2016;1:237-43. [Crossref] [PubMed]
- Bilimoria KY, Stewart AK, Winchester DP, et al. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol 2008;15:683-90. [Crossref] [PubMed]
- Glimelius B, Hoffman K, Sjoden PO, et al. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol 1996;7:593-600. [Crossref] [PubMed]
- Clinicaltrials.gov. Gemcitabine hydrochloride and cisplatin with or without radiation therapy in treating patients with localized liver cancer that cannot be removed by surgery. Available online: https://clinicaltrials.gov/ct2/show/NCT02200042, accessed October 1, 2017.
- Haque W, Verma V, Fakhreddine M, et al. Addition of chemotherapy to definitive radiotherapy for IB1 and IIA1 cervical cancer: Analysis of the National Cancer Data Base. Gynecol Oncol 2017;144:28-33. [Crossref] [PubMed]
- Verma V, McMillan MT, Grover S, et al. Stereotactic body radiation therapy and the influence of chemotherapy on overall survival for large (≥5 centimeter) non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2017;97:146-54. [Crossref] [PubMed]
- Verma V, Lin SH, Simone CB 2nd, et al. Clinical outcomes and toxicities of proton radiotherapy for gastrointestinal neoplasms: a systematic review. J Gastrointest Oncol 2016;7:644-64. [Crossref] [PubMed]
- Haque W, Verma V, Butler EB, et al. Utilization of neoadjuvant intensity-modulated radiation therapy and proton beam therapy for esophageal cancer in the United States. J Gastrointest Oncol 2018;9:282-94. [PubMed]
- Cho Y, Kim TH, Seong J. Improved oncologic outcome with chemoradiotherapy followed by surgery in unresectable intrahepatic cholangiocarcinoma. Strahlenther Onkol 2017;193:620-9. [Crossref] [PubMed]
- Bott MJ, Patel AP, Verma V, et al. Patterns of care in hilar node-positive (N1) non-small cell lung cancer: A missed treatment opportunity? J Thorac Cardiovasc Surg 2016;151:1549-1558.e2. [Crossref] [PubMed]
- Stahl JM, Corso CD, Verma V, et al. Trends in stereotactic body radiation therapy for stage I small cell lung cancer. Lung Cancer 2017;103:11-6. [Crossref] [PubMed]
- Haque W, Verma V, Butler EB, et al. Patterns of care and outcomes of multi-agent versus single-agent chemotherapy as part of multimodal management of low grade glioma. J Neurooncol 2017;133:369-75. [Crossref] [PubMed]
- Haque W, Verma V, Butler EB, et al. National practice patterns and outcomes for T4b urothelial cancer of the bladder. Clin Genitourin Cancer 2017. [Epub ahead of print]. [PubMed]
- Moreno AC, Verma V, Hofstetter WL, et al. Patterns of care and treatment outcomes of elderly patients with stage I esophageal cancer: analysis of the National Cancer Data Base. J Thorac Oncol 2017;12:1152-60. [Crossref] [PubMed]
- McMillan MT, Ojerholm E, Verma V, et al. Radiation Treatment Time and Overall Survival in Locally Advanced Non-small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2017;98:1142-52. [Crossref] [PubMed]
- Verma V, Ryckman JM, Simone CB 2nd, et al. Patterns of care and outcomes with the addition of chemotherapy to radiation therapy for stage I nasopharyngeal cancer. Acta Oncol 2018;57:257-61. [Crossref] [PubMed]
- Verma V, Ahern CA, Berlind CG, et al. National Cancer Data Base Report on Pneumonectomy Versus Lung-Sparing Surgery for Malignant Pleural Mesothelioma. J Thorac Oncol 2017;12:1704-14. [Crossref] [PubMed]
- Haque W, Verma V, Butler EB, et al. Definitive chemoradiation at high volume facilities is associated with improved survival in glioblastoma. J Neurooncol 2017;135:173-81. [Crossref] [PubMed]
- Haque W, Verma V, Butler EB, et al. Radical cystectomy versus chemoradiation for muscle-invasive bladder cancer: impact of treatment facility and sociodemographics. Anticancer Res 2017;37:5603-8. [PubMed]
- Haque W, Verma V, Butler EB, et al. Radiation dose in neoadjuvant chemoradiation therapy for esophageal cancer: patterns of care and outcomes from the National Cancer Data Base. J Gastrointest Oncol 2018;9:80-9. [PubMed]
- Haque W, Verma V, Butler EB, et al. Addition of chemotherapy to hypofractionated radiotherapy for glioblastoma: practice patterns, outcomes, and predictors of survival. J Neurooncol 2018;136:307-15. [Crossref] [PubMed]
- Verma V, Allen PK, Simone CB 2nd, et al. Association of Treatment at High-Volume Facilities With Survival in Patients Receiving Chemoradiotherapy for Nasopharyngeal Cancer. JAMA Otolaryngol Head Neck Surg 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Verma V, Allen PK, Simone CB 2nd, et al. Addition of definitive radiotherapy to chemotherapy in patients with newly diagnosed metastatic nasopharyngeal cancer. J Natl Compr Canc Netw 2017;15:1383-91. [Crossref] [PubMed]
- Haque W, Verma V, Butler EB, et al. Chemotherapy Versus Chemoradiation for Node-Positive Bladder Cancer: Practice Patterns and Outcomes from the National Cancer Data Base. Bladder Cancer 2017;3:283-91. [Crossref] [PubMed]
- Haque W, Verma V, Bernicker E, et al. Management of pathologic node-positive disease following initial surgery for clinical T1-2 N0 esophageal cancer: patterns of care and outcomes from the national cancer data base. Acta Oncol 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Haque W, Lewis GD, Verma V, et al. The role of adjuvant chemotherapy in locally advanced bladder cancer. Acta Oncol 2018;57:509-15. [Crossref] [PubMed]
- Verma V, Surkar SM, Brooks ED, et al. Chemoradiotherapy Versus Chemotherapy Alone for Unresected Nonmetastatic Gallbladder Cancer: National Practice Patterns and Outcomes. J Natl Compr Canc Netw 2018;16:59-65. [Crossref] [PubMed]
- Verma V, Simone CB 2nd, Lin C. Human papillomavirus and nasopharyngeal cancer. Head Neck 2018;40:696-706. [Crossref] [PubMed]
- Haque W, Verma V, Naik N, et al. Metaplastic Breast Cancer: Practice Patterns, Outcomes, and the Role of Radiotherapy. Ann Surg Oncol 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Ryckman JM, Kusi Appiah A, Simone CB 2nd, et al. Treatment approaches for nasopharyngeal adenoid cystic carcinoma. Acta Oncol 2018.1-7. [Epub ahead of print]. [Crossref] [PubMed]
- Haque W, Verma V, Butler EB, et al. Patterns of Care and Outcomes of Hypofractionated Chemoradiation Versus Conventionally Fractionated Chemoradiation for Glioblastoma in the Elderly Population. Am J Clin Oncol 2018;41:167-72. [PubMed]
- Haque W, Verma V, Butler EB, et al. Omission of radiotherapy in elderly women with early stage metaplastic breast cancer. Breast 2018;38:154-9. [Crossref] [PubMed]