
Pathology reporting of margin status in locally advanced pancreatic cancer: challenges and uncertainties
Introduction
Over the past decade, increasing attention has been paid to the clinical significance of the margin status in patients undergoing surgical resection for pancreatic ductal adenocarcinoma (PDAC). While a growing body of published evidence indicates that margin status is a prognostic factor in primary resected pancreatic cancer, some studies do not observe an association with patient outcome (1,2). This continued controversy likely reflects the fact that the pathology assessment of the margins still differs between centers, leading to considerable variation in the reported rates of resections with tumor-free margins (“R0”) (3).
As neoadjuvant therapy has become part of the standard treatment for pancreatic cancer, its impact on the assessment and clinical significance of traditional pathology-based parameters, including margin status, is to be revisited (4). This narrative review describes the challenges that are associated with margin assessment of surgical specimens following neoadjuvant treatment for locally advanced pancreatic cancer and discusses the uncertainties that surround the prognostic value of margin status in this particular patient group. Considering that the R0-rate is increasingly used as an endpoint in clinical trials that investigate the benefit of neoadjuvant treatment, the accuracy and prognostic value of the margin status have become highly relevant - but as yet little discussed - issues.
R0 following neoadjuvant treatment: is 1 mm clearance adequate?
Margin assessment is based on the measurement of the minimum distance of the tumor cells to the margins in order to evaluate the probability of microscopic residual disease (“R1”). Indeed, as the surgical bed cannot be examined to establish whether cancer cells were left in situ, the proximity of the tumor to the margins of the surgical specimen is used to predict the likelihood of what is commonly referred to as microscopic margin involvement.
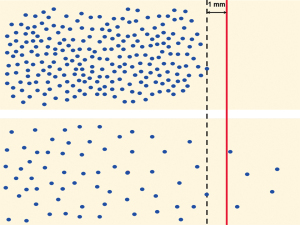
Central in this assessment is the minimum clearance, which is generally accepted to be 1 mm (5-7). It is important to note that the “1 mm rule” originated as a mere adoption from rectal cancer, for which meticulous studies had shown a significantly increased risk of local recurrence if clearance was <1 mm. Similar studies on pancreatic cancer revealed that clearance less than 1 mm is indeed associated with poorer patient outcome (8-12), but prognostic relevance was observed also for larger clearances, that is, 1.5 mm and 2 mm (10-12). Key in this respect is the fact that the minimum clearance, which allows to distinguish between microscopic margin involvement and a free margin, depends on the growth pattern of the cancer (Figure 1): the more dispersedly a cancer grows, the larger a clearance is required for the risk of residual microscopic disease to be negligible and the margin status to be reported as “R0”. Quantitative assessment of the growth pattern of rectal cancer and pancreatic cancer revealed that the former grows in a significantly more compact fashion, while the latter grows more dispersedly, that is, individual pancreatic cancer cells are separated by larger distances (13). This observation provides a rational explanation for the reported clinical relevance in pancreatic cancer of clearances of more than 1 mm.
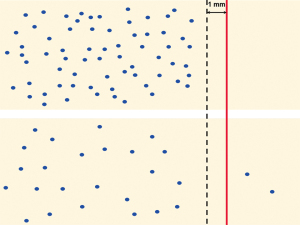
Because neoadjuvant treatment results in the loss of cancer cells, the distance between residual cancer cells increases in areas with cytotoxic effect (Figure 2). As a consequence, a distance of >1 mm does no longer reliably reflect the absence of residual microscopic disease in the surgical bed. In other words, following neoadjuvant treatment, R0-status based on 1 mm clearance can no longer be attributed the same oncological significance, because the underlying principles differ from those in treatment-naïve PDAC. Hence, the increased R0-rate following neoadjuvant treatment that has been reported for both borderline resectable and locally advanced pancreatic cancer (14-19) cannot be readily interpreted as a clear-cut increase in the rate of resections resulting in negative margins.
Tumor regression around unresected structures: implicit assumptions
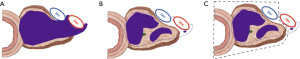
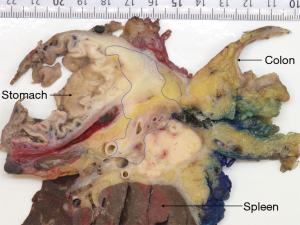
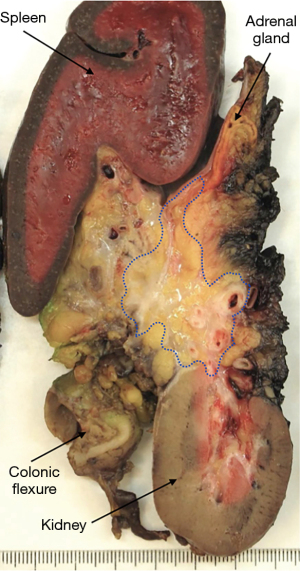
While imaging plays a central role in the assessment of the effect of neoadjuvant treatment, its accuracy to discriminate between tumor regression with fibrosis and fibrosis containing residual viable cancer cells is known to be limited (20). Nonetheless, in the absence of any better means to identify the presence and localization of residual cancer, the resectability of a tumor is determined based on radiological findings. This is especially relevant in the setting of locally advanced pancreatic cancer, where not all extrapancreatic tissues that were involved by tumor at baseline, can be resected. In most centers, resection of the superior mesenteric artery or celiac trunk will not be undertaken if imaging indicates tumor regression around these structures following neoadjuvant treatment. A similar approach may also be taken regarding tumor regression around other major anatomical structures. As a consequence, these structures are precluded from pathology examination, tacitly assumed to be free of tumor, and not given any further consideration when pathology examination of the surgical resection specimen reveals an “R0-resection” (Figure 3).
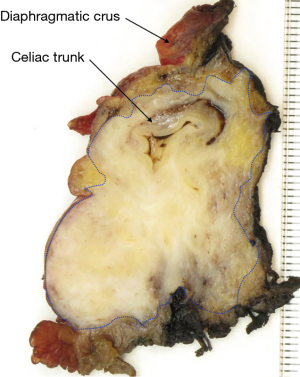
Specimen grossing: the challenges
Accurate margin assessment requires meticulous specimen grossing and microscopic examination. While the latter can be repeated by retrospective slide review in order to check and possibly correct microscopic findings, specimen grossing is usually a one-off procedure without the possibility of retrospective review and correction. Specimen grossing consists of the dissection, macroscopic examination, and sampling of a surgical resection specimen. It is well established that the quality of the grossing procedure for each of these steps affects the accuracy of margin assessment for standard pancreatoduodenectomy and distal pancreatectomy specimens (3,21). Despite the importance of meticulous, standardized specimen grossing, there is still significant divergence in practice that is likely responsible for the considerable variation in reported R0-rates (3).
Surgical resection of locally advanced pancreatic cancer that has responded favorably to neoadjuvant treatment often requires extended procedures, which result in large and complex specimens. Segments of major veins or arteries, stomach, large or small bowel may be resected as well as entire organs, such as the left adrenal gland or kidney, or a combination of these. Grossing of such specimens is often challenging and time-consuming, and unfortunately, none of the current protocols proposed by national pathology societies or professional bodies provide guidance on specimen dissection, tissue sampling, or margin assessment. While this has not been formally investigated, it is reasonable to assume that there is significant divergence in the grossing of these complex specimens. When it comes to the examination of the margins, multiple additional margins need to be considered in extended resection specimens, and both specimen dissection and tissue sampling may have to be tailored to the individual case.
A particularly crucial part of the grossing procedure is tissue sampling. First, sampling has to be relevant, that is, samples must be taken from the part of the specimen that represents a (threatened) transection or—more often—dissection margin. Second, sampling must be extensive, because (I) microscopic margin involvement is—by definition—invisible macroscopically, (II) the invasive front of pancreatic cancer is notoriously ill-defined and poorly discernible on naked-eye inspection, and (III) fibrosis induced by neoadjuvant treatment further effaces the distinction between cancer and noncancerous tissues.
High R0-rate in locally advanced pancreatic cancer: fact or fiction?
Data on the R0-rate for resected locally advanced pancreatic are difficult to retrieve from the literature, because studies often report on mixed patient cohorts, including primary resectable, borderline resectable, and locally advanced pancreatic cancer. The few results that are available from studies in which R0 was defined as 1 mm clearance, show a wide range—from 29.3% to 91% (Table 1)—not unlike the variation seen for R0-rates in treatment-naive primary and borderline resectable pancreatic cancer. However, the majority of studies on locally advanced pancreatic cancer report an R0-rate that is similar to or higher than that for primary resectable tumors. This is surprising in view of the extensive involvement of structures outside the standard surgical field that defines locally advanced disease.
Table 1
| Study | Study type | Number of patients with LAPC | R0-definition (minimum clearance) | R0-rate (%) | Difference in survival (R1 vs. R0) |
|---|---|---|---|---|---|
| Philip et al. 2020 (22) | PS | 16 | NS (according to national guidelines in European countries, USA, Canada) | 41.2 | NA |
| Wolfe et al. 2020 (23) | RS | 72& | NS | 73.6 | Significant in multi-variate analysis |
| Gemenetzis et al. 2019 (24) | RS | 415 | >1 mm | 88 | Significant in univariate analysis |
| Klaiber et al. 2019 (25) | PS, non-RCT | 190* | >1 mm | 29.3 | Significant in univariate analysis |
| Kourie et al. 2019 (26) | RS | 14* | NS | 100 | NA |
| Maggino et al. 2019 (27) | PS, non-RCT | 413* | >1 mm | 57.8 | Not significant |
| Napolitano et al. 2019 (28) | RS | 20 | Not explicitly stated, likely >1 mm | 62.5 | NA |
| Pouypoudat et al. 2019 (29) | RS | 13* | 0 and 1 mm | 92.3 (R0 =0 mm); 75.6 (R0 >1 mm) | NA |
| Lee et al. 2018 (30) | RS | 15 | Not explicitly stated, likely 0 mm | 73.3 | NA |
| Hackert et al. 2016 (31) | RS | 292 | NS | 32.9 | NA |
| Stein et al. 2016 (32) | PS | 6* | NS | 100 | NA |
| Khushman et al. 2015 (33) | RS | 12 | >1 mm | 83.3 | NA |
| Nanda et al. 2015 (34) | RS | 2* | NS | 83 | NA |
| Blazer et al. 2015 (35) | RS | 11 | >1 mm | 91 | NA |
| Sadot et al. 2015 (36) | RS | 31 | NS | 51.2 | NA |
| Faris et al. 2013 (37) | RS | 22 | NS | 23 | NA |
| Hosein et al. 2012 (38) | RS | 9 | >1 mm | 90 | NA |
*, study cohort includes also primary resectable, borderline resectable, or oligometastatic disease; only patients with locally advanced pancreatic cancer displayed. &, study cohort includes also borderline resectable, numbers are not disaggregated. LAPC, locally advanced pancreatic cancer; NA, not analysed; NS, not stated; PS, prospective study; RCT, randomized clinical trial; RS, retrospective study.
A possible explanation for the high(er) R0-rate is the beneficial effect of neoadjuvant therapy, because treatment-induced cancer cell death is likely to reduce the risk of tumor being present close to a margin. Indeed, a recent meta-analysis comparing upfront surgery with neoadjuvant treatment for primary and borderline resectable pancreatic cancer showed a significantly higher R0-rate for the neoadjuvant group (86.2% vs. 66.9%) (39).
However, these results need to be interpreted with caution. Of major concern is the varying, often only moderate quality of margin assessment in most studies (39) due to the lack of standardization of and/or information on key parts of the pathology examination procedure. It is important to note that in the vast majority of tumors, treatment-induced regression remains incomplete, such that viable cancer persists, often with a patchy and seemingly random distribution of cancer cells in the original tumor bed. As a consequence, the tissues at all surgical margins have to be embedded and microscopically examined in their entirety (Figures 4-6). Because resection of locally advanced pancreatic cancer often results in extended, multivisceral specimens with multiple additional dissection planes and transection margins (for example circumferential and transection margins of venous and arterial resections), the number of tissue blocks required to assess the overall margin status may be high, possibly unrealistic in the face of constraints that may be imposed in terms of budget and human resources. Yet, any deviation from (sub-)total sampling of the tissues at the margins bears the risk of underreporting microscopic margin involvement.
Considering that neoadjuvant therapy exerts a positive effect on the margin status through the reduction of cancer cells, the R-status could be expected to covary with the degree of tumor regression. Interestingly, only a single study has compared the R0-rate between subgroups with different grades of tumor regression (40). Unfortunately, however, due to the small size of the study cohort (n=32), conclusions cannot be drawn.
R0 in resected LAPC: prognostically relevant?
While the prognostic impact of the margin status in patients undergoing upfront surgery for primary and borderline resectable pancreatic cancer continues to be controversially debated, the limited data that have been published for locally advanced disease are outright conflicting (14,25,27,39,41). Obvious reasons for the lack of compelling evidence have been discussed above and relate to the lack of standardized and meticulous pathology assessment as well as to the small size and mixed composition of patient cohorts (Table 1).
Patient outcome following neoadjuvant treatment and surgery for locally advanced pancreatic cancer is determined by a host of patient- and tumor-related factors, including chemosensitivity of the cancer cells, the size and site of residual cancer, lymph node metastasis, and perineural spread. Hence, singling out the prognostic value of the margin status is challenging and would require large study cohorts and fully standardized patient management and specimen examination. Moreover, local recurrence, which is the consequence of positive resection margins, may also result from perineural or lymphovascular tumor invasion and lymph node metastasis, that is, from modes of tumor propagation that are present, single or combined, in the majority of tumors.
Future studies are needed to improve our understanding of the prognostic impact of margin status in combination with other key determinants of disease recurrence, namely the response to treatment and characteristics of the residual cancer cell population. While joint initiatives to improve existing scoring systems of treatment effect are being undertaken (42), characterization of residual viable tumor—for example in terms of proliferative activity or expression of chemosensitivity markers—is left largely unexplored in the clinical setting. A concerted effort to both standardize and refine various aspects of the pathology assessment is essential to understand these complex tumor biological processes.
Conclusions
While R-status is much debated, now also for locally advanced pancreatic cancer, data are scarce and of varying, often limited quality, precluding comparison between studies and centers. As such, neither the incidence of R0-resections nor the impact of the margin status on outcome has been definitely established for patients with locally advanced disease. The number of clinical studies on this patient group seems to be incongruous with the lack of activity devoted to the harmonization and optimization of the methods that are essential to increasing our knowledge. By weakening the evidence generated by clinical studies, the continued lack of these basic prerequisites precludes true progress.
Acknowledgments
Funding: This work was supported by the Norwegian Cancer Society (CSV; grant number 212734-2019) and the KWF Dutch Cancer Society grant (UVA 2014-6803).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Elena Rangelova) for the series “Surgery for Locally Advanced Pancreatic Cancer” published in Journal of Gastrointestinal Oncology. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jgo-20-391). The series “Surgery for Locally Advanced Pancreatic Cancer” was commissioned by the editorial office without any funding or sponsorship. CSV reports that she has provided consultation services to FibroGen, Inc. The authors have no other conflicts of interest to declare.
Ethical Statement: Both authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Raut CP, Tseng JF, Sun CC, et al. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg 2007;246:52-60. [Crossref] [PubMed]
- Yamamoto T, Uchida Y, Terajima H. Clinical impact of margin status on survival and recurrence pattern after curative-intent surgery for pancreatic cancer. Asian J Surg 2019;42:93-9. [Crossref] [PubMed]
- Chandrasegaram MD, Goldstein D, Simes J, et al. Meta-analysis of radical resection rates and margin assessment in pancreatic cancer. Br J Surg 2015;102:1459-72. [Crossref] [PubMed]
- Schorn S, Demir IE, Reyes CM, et al. The impact of neoadjuvant therapy on the histopathological features of pancreatic ductal adenocarcinoma - A systematic review and meta-analysis. Cancer Treat Rev 2017;55:96-106. [Crossref] [PubMed]
- Tempero MA, Malafa MP, Chiorean EG, et al. Pancreatic adenocarcinoma, version 1.2019 featured updates to the NCCN guidelines. J Natl Compr Cancer Netw 2019;17:203-10. [Crossref]
- Burgart LJ, Shi C, Volkan AN, et al. Protocol for the Examination of Specimens From Patients With Carcinoma of the Pancreas. Available online: www.cap.org/cancerprotocols
- The Royal College of Pathologists. Dataset for histopathological reporting of carcinomas of the pancreas, ampulla of Vater and common bile duct. Available online: https://www.rcpath.org/uploads/assets/34910231-c106-4629-a2de9e9ae6f87ac1/G091-Dataset-for-histopathological-reporting-of-carcinomas-of-the-pancreas-ampulla-of-Vater-and-common-bile-duct.pdf
- Campbell F, Smith RA, Whelan P, et al. Classification of R1 resections for pancreatic cancer: The prognostic relevance of tumour involvement within 1 mm of a resection margin. Histopathology 2009;55:277-83. [Crossref] [PubMed]
- Chen JWC, Bhandari M, Astill DS, et al. Predicting patient survival after pancreaticoduodenectomy for malignancy: Histopathological criteria based on perineural infiltration and lymphovascular invasion. HPB (Oxford) 2010;12:101-8. [Crossref] [PubMed]
- Chang DK, Johns AL, Merrett ND, et al. Margin Clearance and Outcome in Resected Pancreatic Cancer. J Clin Oncol 2009;27:2855-62. [Crossref] [PubMed]
- Jamieson NB, Chan NIJ, Foulis AK, et al. The Prognostic Influence of Resection Margin Clearance Following Pancreaticoduodenectomy for Pancreatic Ductal Adenocarcinoma. J Gastrointest Surg 2013;17:511-21. [Crossref] [PubMed]
- Delpero JR, Jeune F, Bachellier P, et al. Prognostic Value of Resection Margin Involvement after Pancreaticoduodenectomy for Ductal Adenocarcinoma. Ann Surg 2017;266:787-96. [Crossref] [PubMed]
- Verbeke CS, Knapp J, Gladhaug IP. Tumour growth is more dispersed in pancreatic head cancers than in rectal cancer: implications for resection margin assessment. Histopathology 2011;59:1111-21. [Crossref] [PubMed]
- de Geus SWL, Kasumova GG, Sachs TE, et al. Neoadjuvant therapy affects margins and margins affect all: perioperative and survival outcomes in resected pancreatic adenocarcinoma. HPB (Oxford) 2018;20:573-81. [Crossref] [PubMed]
- Jang JY, Han Y, Lee H, et al. Oncological Benefits of Neoadjuvant Chemoradiation With Gemcitabine Versus Upfront Surgery in Patients With Borderline Resectable Pancreatic Cancer: A Prospective, Randomized, Open-label, Multicenter Phase 2/3 Trial. Ann Surg 2018;268:215-22. [Crossref] [PubMed]
- Van Tienhoven G, Versteijne E, Suker M, et al. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer (PREOPANC-1): A randomized, controlled, multicenter phase III trial. J Clin Oncol 2018;36:LBA4002 [Crossref]
- Sho M, Akahori T, Tanaka T, et al. Optimal indication of neoadjuvant chemoradiotherapy for pancreatic cancer. Langenbecks Arch Surg 2015;400:477-85. [Crossref] [PubMed]
- Lee JH, Kang CM, Bang SM, et al. The Role of Neoadjuvant Chemoradiation Therapy in Patients With Borderline Resectable Pancreatic Cancer With Isolated Venous Vascular Involvement. Medicine (Baltimore) 2015;94:e1233 [Crossref] [PubMed]
- Fujii T, Yamada S, Murotani K, et al. Inverse probability of treatment weighting analysis of upfront surgery versus neoadjuvant chemoradiotherapy followed by surgery for pancreatic adenocarcinoma with arterial abutment. Medicine (Baltimore) 2015;94:e1647 [Crossref] [PubMed]
- Katz MHG, Fleming JB, Bhosale P, et al. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer 2012;118:5749-56. [Crossref] [PubMed]
- Verbeke CS, Leitch D, Menon KV, et al. Redefining the R1 resection in pancreatic cancer. Br J Surg 2006;93:1232-7. [Crossref] [PubMed]
- Philip PA, Lacy J, Portales F, et al. Nab-paclitaxel plus gemcitabine in patients with locally advanced pancreatic cancer (LAPACT): A multicentre, open-label phase 2 study. Lancet Gastroenterol Hepatol 2020;5:285-94. [Crossref] [PubMed]
- Wolfe AR, Prabhakar D, Yildiz VO, et al. Neoadjuvant-modified FOLFIRINOX vs nab-paclitaxel plus gemcitabine for borderline resectable or locally advanced pancreatic cancer patients who achieved surgical resection. Cancer Med 2020;9:4711-23. [Crossref] [PubMed]
- Gemenetzis G, Groot VP, Blair AB, et al. Survival in Locally Advanced Pancreatic Cancer after Neoadjuvant Therapy and Surgical Resection. Ann Surg 2019;270:340-7. [Crossref] [PubMed]
- Klaiber U, Schnaidt ES, Hinz U, et al. Prognostic Factors of Survival After Neoadjuvant Treatment and Resection for Initially Unresectable Pancreatic Cancer. Ann Surg 2021;273:154-62. [Crossref] [PubMed]
- Kourie H, Auclin E, Cunha AS, et al. Characteristic and outcomes of patients with pathologic complete response after preoperative treatment in borderline and locally advanced pancreatic adenocarcinoma: An AGEO multicentric retrospective cohort. Clin Res Hepatol Gastroenterol 2019;43:663-8. [Crossref] [PubMed]
- Maggino L, Malleo G, Marchegiani G, et al. Outcomes of Primary Chemotherapy for Borderline Resectable and Locally Advanced Pancreatic Ductal Adenocarcinoma. JAMA Surg 2019;154:932-42. [Crossref] [PubMed]
- Napolitano F, Formisano L, Giardino A, et al. Neoadjuvant treatment in locally advanced pancreatic cancer (LAPC) patients with FOLFIRINOX or gemcitabine nabpaclitaxel: A single-center experience and a literature review. Cancers (Basel) 2019;11:11. [Crossref] [PubMed]
- Pouypoudat C, Buscail E, Cossin S, et al. FOLFIRINOX-based neoadjuvant chemoradiotherapy for borderline and locally advanced pancreatic cancer: A pilot study from a tertiary centre. Dig Liver Dis 2019;51:1043-9. [Crossref] [PubMed]
- Lee J, Lee JC, Gromski MA, et al. Clinical outcomes of FOLFIRINOX in locally advanced pancreatic cancer: A single center experience. Medicine (Baltimore) 2018;97:e13592 [Crossref] [PubMed]
- Hackert T, Sachsenmaier M, Hinz U, et al. Locally advanced pancreatic cancer: Neoadjuvant therapy with folfirinox results in resectability in 60% of the patients. Ann Surg 2016;264:457-63. [Crossref] [PubMed]
- Stein SM, James ES, Deng Y, et al. Final analysis of a phase II study of modified FOLFIRINOX in locally advanced and metastatic pancreatic cancer. Br J Cancer 2016;114:737-43. [Crossref] [PubMed]
- Khushman M, Dempsey N, Cudris Maldonado J, et al. Full dose neoadjuvant FOLFIRINOX is associated with prolonged survival in patients with locally advanced pancreatic adenocarcinoma. Pancreatology 2015;15:667-73. [Crossref] [PubMed]
- Nanda RH, El-Rayes B, Maithel SK, et al. Neoadjuvant modified FOLFIRINOX and chemoradiation therapy for locally advanced pancreatic cancer improves resectability. J Surg Oncol 2015;111:1028-34. [Crossref] [PubMed]
- Blazer M, Wu C, Goldberg RM, et al. Neoadjuvant Modified (m) FOLFIRINOX for Locally Advanced Unresectable (LAPC) and Borderline Resectable (BRPC) Adenocarcinoma of the Pancreas. Ann Surg Oncol 2015;22:1153-9. [Crossref] [PubMed]
- Sadot E, Doussot A, O’Reilly EM, et al. FOLFIRINOX Induction Therapy for Stage 3 Pancreatic Adenocarcinoma. Ann Surg Oncol 2015;22:3512-21. [Crossref] [PubMed]
- Faris JE, Blaszkowsky LS, McDermott S, et al. FOLFIRINOX in Locally Advanced Pancreatic Cancer: The Massachusetts General Hospital Cancer Center Experience. Oncologist 2013;18:543-8. [Crossref] [PubMed]
- Hosein PJ, Macintyre J, Kawamura C, et al. A retrospective study of neoadjuvant FOLFIRINOX in unresectable or borderline-resectable locally advanced pancreatic adenocarcinoma. BMC Cancer 2012;12:199. [Crossref] [PubMed]
- Versteijne E, Vogel JA, Besselink MG, et al. Meta-analysis comparing upfront surgery with neoadjuvant treatment in patients with resectable or borderline resectable pancreatic cancer. Br J Surg 2018;105:946-58. [Crossref] [PubMed]
- Kim SS, Ko AH, Nakakura EK, et al. Comparison of Tumor Regression Grading of Residual Pancreatic Ductal Adenocarcinoma Following Neoadjuvant Chemotherapy Without Radiation. Am J Surg Pathol 2019;43:334-40. [Crossref] [PubMed]
- Mokdad AA, Minter RM, Zhu H, et al. Neoadjuvant therapy followed by resection versus upfront resection for resectable pancreatic cancer: A propensity score matched analysis. J Clin Oncol 2017;35:515-22. [Crossref] [PubMed]
- Janssen B V, Tutucu F, van Roessel S, et al. Amsterdam International Consensus Meeting: Tumor response scoring in the pathology assessment of resected pancreatic cancer after neoadjuvant therapy. Mod Pathol 2021;34:4-12. [Crossref] [PubMed]


