
Outcome of surgical treatment for bone metastases caused by colorectal cancer
Introduction
Colorectal cancer (CRC) is a major reason for cancer death in the world today. CRC was calculated to be the second leading reason for cancer deaths in the world and the third most commonly diagnosed cancer, representing approximately 1.8 million new cases (10%) and 900,000 deaths/year (9%). The incidence is higher in men than in women (1). The incidence of bone metastases in CRC has been investigated in previous studies and ranged from 2.9% to 6.6% in unselected patients (2-6). Furthermore, studies on metastatic CRC patients have shown that the incidence of MBD was 6.9–10.4% (6-10) while bone metastases have been reported in as high as 23% of autopsy cases (11,12).
The outcome of surgical treatment of skeletal metastases due to CRC has not been investigated previously. The aim of this study is to describe the mode of clinical presentation of metastatic bone disease (MBD) requiring surgical treatment in CRC, report the outcome of surgery depending on the type of orthopedic reconstruction performed for the lesion and identify possible prognostic factors for patient survival. We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/jgo-21-108).
Methods
The study is a retrospective analysis of data from an institutional prospectively collected database. Inclusion criteria were surgery for impending or completed pathological fracture due to metastatic bone disease caused by colorectal cancer, between year 2000–2018. Data was retrieved from the database and patient files were checked to confirm the validity of the data. All patients were treated surgically at the Department of Orthopedics, Karolinska University Hospital, Stockholm, Sweden between the year 2000–2019.
A total of 46 patient files were studied compared to the inclusion criteria. Six patients were excluded because the origin of the tumor could not be verified and 4 because they never underwent orthopedic surgery for their bone metastases. In the final analysis, 36 patients (38 treated lesions) were included. Two male patients were treated for a second pathological fracture at a later occasion, accounting for a total number of 38 treated lesions. Data retrieved from the patient files were: gender, age of patient at first surgery, location of bone metastasis, blood hemoglobin and serum albumin level prior to surgery, reason for surgery, type of orthopedic reconstruction performed, adjuvant chemotherapy or radiation therapy prior and after surgery, local complications and secondary surgery as well as the status at last follow-up. Anemia was defined as a hemoglobin concentration below 13 g/dL in men and below 12 g/dL in women. Hypoalbuminemia was defined as serum albumin 34 g/L. Leukocytosis was defined as white blood cell count greater than 8.8/µL. Indications for surgery were severe pain, major neurological deficit or inability to weight bare. The neurological symptoms were classified according to the Frankel scoring system (2).
Statistical analysis
Statistical analysis was carried out in SPSS (version 25, SPSS Inc, Chicago, IL, USA). Comparisons between groups were done using the Pearson’s chi-square (χ2) test. Survival analysis was per Kaplan-Meier, comparisons between groups were done using the log-rank test. Multivariate analysis of possible prognostic factors was performed using the cox-proportional hazard test. All tests were double-sided, and a P value of ≤0.05 was considered significant.
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the regional ethics board of Stockholm (No.: 2012/272-31/4) and informed consent was waived due to the retrospective nature of the study.
Results
Patient characteristics and clinical presentation
The demographic characteristics and overall description of the cohort are presented in Table 1. The primary diagnosis was colon cancer for 19 patients and rectal cancer for 17 patients. Twenty-eight patients (78%) had surgery for the CRC before their skeletal event, which occurred at a median of 3.1 years (range, 0.2–12.5) after surgery for CRC. In 3 patients the disease debuted with a pathological fracture, and oncological treatment was administered after the orthopedic surgery. 8 patients did not have surgery for their CRC and were treated only with chemotherapy and/or radiation therapy. The interval between diagnosis and orthopedic surgery could be retrieved for 5 of these 8 patients and varied between 0.2 and 8.1 years (median 0.8 years).
Table 1
| No | Percentage (%) | |
|---|---|---|
| Gender | ||
| Female | 19 | 53 |
| Male | 17 | 47 |
| Median age (years) | 66 | |
| Location (treated lesion) | ||
| Femur | 5 | 13 |
| Femur and pelvis | 2 | 5 |
| Pelvis | 4 | 11 |
| Humerus | 9 | 24 |
| Clavicle and acromion | 1 | 3 |
| Spine | 15 | 40 |
| Calcaneus | 1 | 3 |
| Tibia | 1 | 3 |
| Bone metastatic load (patients) | ||
| Single metastasis | 20 | 56 |
| Multiple metastases | 15 | 42 |
| Uncertain | 1 | 3 |
| Visceral metastatic load (patients) | ||
| Present | 33 | 92 |
| Absent | 2 | 6 |
| Uncertain | 1 | 3 |
| Status at last follow-up (patients) | ||
| Alive | 1 | 3 |
| Dead | 35 | 97 |
Only two patients did not have known visceral metastases at the time of surgery or follow-up. One of these patients had a known colorectal cancer before surgery but the generalization of the disease started with a skeletal event, the other had no cancer diagnosis before the skeletal event. Both patients underwent radiology (CT-scan/scintigraphy) in close context to the surgery which confirmed the absence of visceral metastases. For one patient it was unclear if he/she suffered from visceral metastases.
The treated lesions were predominantly located in the spine (Figure 1). Bone metastases of the spine presented with pain or signs of spinal cord compression. The neurological symptoms were classified according to the Frankel scoring system (13). At the time for surgery, 7 of the 15 patients presented as Frankel C and 7 with Frankel B. One patient had no neurological deficits (Frankel E), the main indication for surgery being intractable pain.
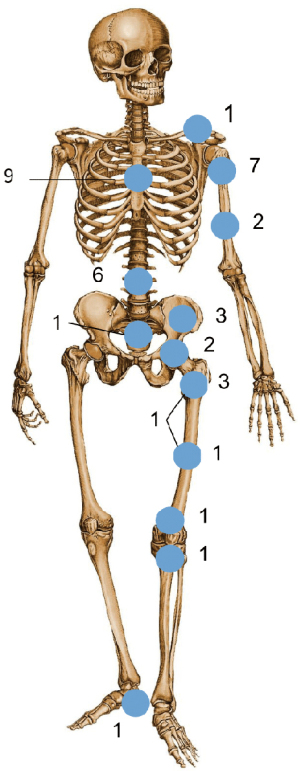
Regarding the fractures of the extremities, pelvis and clavicle, all patients presented with pain and/or inability to weight-bear. One patient with a pathological fracture of the tibia and one with pathological fracture in both the pelvis and femur also suffered from a severe contracture of the joint. Radiologically, lesions were mainly osteolytic and sometimes a soft-tissue component was obvious.
Oncological treatment and outcome
Most of the patients had documented treatment with both chemotherapy and radiation therapy. Only three did not receive cytostatic treatment, five did not receive radiation therapy, and one patient had missing data. Of the patients that did not receive chemotherapy, one patient had a Duke C rectal cancer and was treated with rectal amputation only. This patient developed skeletal metastases 2 years later and was scheduled for both cytostatic and radiation therapy but died before treatment began. The second patient was considered too ill to be treated with chemotherapy. The third patient presented with skeletal metastasis of unknown origin at the time of surgery. Preoperative CT scan suggested colorectal cancer and biopsy of the tumor confirmed the diagnosis. This patient was not given any cytostatic treatment within the follow-up period. All five patients not given radiation therapy were in poor overall condition, four were dead within 2 months of surgery and the 5th within 4 months.
Overall patient survival was poor, with only 5 of 36 patients surviving 1 year after surgery, and 2 at 2 years. Median patient survival was 3 months (95% CI: 0–7 months) and mean 6 months (95% CI: 4–9 months). The survival curve of the cohort is shown in Figure 2A. 25 out of 37 patients presented with anemia prior to first surgery. Analysis of possible factors associated with the oncologic outcome is presented in Table 2. In multivariate regression analysis the presence of solitary skeletal metastasis (P≤0.001) was the only factor associated with an improved overall survival. Even when the patient had known visceral metastases, the prognosis was better in case the bone metastasis was solitary (Figure 2B).

Table 2
| Category [number of patients available for analysis] | Percentage (%) | Mean survival in months [95% CI] | P value |
|
|---|---|---|---|---|
| Gender | Male [17] | 47 | 8 [3–13] | 0.941 |
| Female [19] | 53 | 7 [4–10] | ||
| Age | 66 years or more [18] | 50 | 6 [3–10] | 0.340 |
| Less than 66 years [18] | 50 | 8 [5–12] | ||
| Bone metastases | Solitary [20] | 57 | 11 [7–15] | 0.001 |
| Multiple [15] | 43 | 3 [1–4] | ||
| Hemoglobin | Normal [25] | 71 | 11 [4–18] | 0.080 |
| Anemia [10] | 29 | 6 [3–9] | ||
| Albumin | Normal [12] | 40 | 10 [4–15] | 0.204 |
| Hypoalbuminemia [18] | 60 | 6 [3–9] | ||
| While Blood Cells | Normal [12] | 34 | 9 [3–15] | 0.429 |
| Leukocytosis [23] | 66 | 6 [4–8] |
Surgical treatment and outcome
Of the 38 lesions treated surgically 16 were treated with osteosynthesis, 13 lesions were treated with debulking/resection of the tumor with or without filling of the cavity with bone cement and 9 were treated with a joint replacement (hip, knee or shoulder arthroplasty). Follow-up was not standardized, but most patients had a follow-up appointment at 6–12 weeks postoperatively, and then as needed, either via the treating oncologist or orthopedic surgeon.
We next reviewed the local complications as well as the re-operation risk due to implant failure. The complications are presented in Table 3. In total, 11 patients (30%) suffered complications after surgery, 2 systemic and 9 local. The local complication rate was most considerable in the group treated for metastases in the humerus (3/9 patients) and pelvis (2/4 patients). The rate of revision surgery due to implant failure was 7% at 6 months and 49% at 1 year after index surgery (Figure 3).
Table 3
| Complications | Type [number of patients] | Percentage (%) | Location of metastases [number of patients] | Number of surgeries | Type of surgery [number of patient] |
|---|---|---|---|---|---|
| General Complications | Pneumonia [1] | 3 | Spine [1] | ||
| Pressure ulcer [1] | 3 | Femur [1] | |||
| Local Complications | Failure of Osteosynthesis [3] | 8 | Humerus [2] | 1 | Conversion to prosthesis |
| Regrowth [5] | 14 | Humerus [1]; calcaneus [1]; |
4 | Amputation [2]; laminectomy [1]; laminectomy with posterior stabilization [1] | |
| Deep infection [1] | 3 | Pelvis [1] | 1 | Surgical debridement. Drainage [1] | |
| Superficial infection [2] | 6 | Spine [1] Pelvis [1] | 0 | ||
| Nerve damage [1] | 3 | Pelvis [1] | 0 | ||
| Dural rift [1] | 3 | Spine [1] | 0 | ||
| Failure of surgical material [1] | 3 | Pelvis [1] | 1 | Removal of surgical drainage [1] |

Of the 9 patients treated for lesions in the humerus, 3 patients suffered complications and 2 underwent revision surgery. One patient with shoulder prosthesis suffered from tumor regrowth and underwent amputation. Two patients treated with osteosynthesis suffered from implant failure, where one led to revision surgery. The patient in need of revision surgery originally had a fracture in humeral diaphysis treated with plate and screws and was treated with conversion to elbow prosthesis after implant failure. The other patient, primarily treated with an intramedullary nail, suffered from a secondary dislocation of the fracture due to non-healing, but was treated conservatively because of the seriousness of his general condition.
Out of the four patients treated for metastases in the pelvis, two suffered from complications and one underwent revision surgery. One patient initially operated with a prosthesis had to undergo 2 revision surgeries, one for a technical error and one for deep infection. The other patient suffered from nerve damage, superficial infection and later tumor regrowth but never underwent revision surgery.
In the group treated for spinal metastases, four patients out of 15 suffered from complications. One from superficial infection and two from regrowth. Two patients underwent revision surgery because of regrowth of the tumor. The patients in need of revision surgery were treated with extended laminectomy and debulking of the tumor, in one case combined with posterior stabilization.
One patient was treated for a pathological fracture in the calcaneus with debulking and cement. This patient suffered from tumor regrowth and was later treated with below-knee amputation.
Conclusions
Bone metastases should be suspected in patients with colorectal cancer presenting with signs and symptoms of an impending or complete pathological fracture such as pain, inability to weight-bear, or signs of spinal cord compression. When present, it is associated with poor prognosis (14). In our study, with patients with metastatic lesions needing surgical treatment, the mean survival was 6 month and only 10% survived one year. This indicates a worse prognosis for patients in this group compared to the group with CRC and bone metastases not needing surgical intervention. According to our experience, patients with pathological fractures of the long bones (femur, humerus, tibia) cannot be managed non-surgically. Patients with solitary spinal metastases and major neurological deficits of acute onset also benefit from surgery.
Various prognostic factors have been proposed for patients with CRC. Even though most patients had a dismal prognosis, there were some long-time survivors, which is in accordance with previous case-reports (15,16). In our comparatively small cohort of CRC patients with MBD needing surgical treatment, patients with localized MBD (solitary bone metastases) had a better prognosis than the ones with generalized MBD, irrespective of the extent of visceral involvement of the disease. Solitary bone metastasis is also shown to be a good prognostic factor for other cancers such as breast (17), lung cancer (18) and renal cancer (19).
In our study there were more patients with treated lesions from colon cancer than from rectal cancer. This differs from the findings in the previously described studies where they described that the risk of developing bone metastases seems to be higher the more distal the CRC is (6,14,20). However, our cohort was selected for cases admitted for surgical treatment and does not cover the whole spectrum of MBD in patients with CRC.
We observed a high local complication rate and the re-operation rate due to failure of the initial operation was not negligible. Compared to previous studies of pathological fractures in femur and humerus (21-23), all local failures in our study were in the humerus and none in the femur, suggesting different treatment considerations for these locations in metastatic CRC (24). Awareness for local complications should be especially high for the patients with pathological fractures in the humerus and the pelvis, and a standardized follow-up looking for both local and systemic complications is indicated. The complications can arrive at any time after surgery (mean 4 months). The complications in need of revision surgery (such as regrowth) seems to appear later than average (mean 8 months). Patients with solitary bone metastases have longer survival and the complication rate is considerable with almost 50% revision rate for patients surviving more than 1 year. We thus advocate careful consideration of surgical methods and estimation of patient survival when planning surgical treatment.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/jgo-21-108
Data Sharing Statement: Available at https://dx.doi.org/10.21037/jgo-21-108
Peer Review File: Available at https://dx.doi.org/10.21037/jgo-21-108
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jgo-21-108). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the regional ethics board of Stockholm (NO:2012/272-31/4) and informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Portales F, Thézenas S, Samalin E, et al. Bone metastases in gastrointestinal cancer. Clin Exp Metastasis 2015;32:7-14. [Crossref] [PubMed]
- Bonnheim DC, Petrelli NJ, Herrera L, et al. Osseous metastases from colorectal carcinoma. Am J Surg 1986;151:457-9. [Crossref] [PubMed]
- Zhenghong Zihua Zhu. Retrospective study of predictors of bone metastasis in colorectal cancer patients. J Bone Oncol 2017;9:25-8. [Crossref] [PubMed]
- Kanthan R, Loewy J, Kanthan SC. Skeletal metastases in colorectal carcinomas: a Saskatchewan profile. Dis Colon Rectum 1999;42:1592-7. [Crossref] [PubMed]
- Riihimäki M, Hemminki A, Sundquist J, et al. Patterns of metastasis in colon and rectal cancer. Sci Rep 2016;6:29765. [Crossref] [PubMed]
- Santini D, Tampellini M, Vincenzi B, et al. Natural history of bone metastasis in colorectal cancer: final results of a large Italian bone metastases study. Ann Oncol 2012;23:2072-7. [Crossref] [PubMed]
- Patanaphan V, Salazar OM. Colorectal cancer: metastatic patterns and prognosis. South Med J 1993;86:38-41. [Crossref] [PubMed]
- Besbeas S, Stearns MW Jr. Osseous metastases from carcinomas of the colon and rectum. Dis Colon Rectum 1978;21:266-8. [Crossref] [PubMed]
- Sundermeyer ML, Meropol NJ, Rogatko A, et al. Changing patterns of bone and brain metastases in patients with colorectal cancer. Clin Colorectal Cancer 2005;5:108-13. [Crossref] [PubMed]
- Hugen N, van de Velde CJH, de Wilt JHW, et al. Metastatic pattern in colorectal cancer is strongly influenced by histological subtype. Ann Oncol 2014;25:651-7. [Crossref] [PubMed]
- Katoh M, Unakami M, Hara M, et al. Bone metastasis from colorectal cancer in autopsy cases. J Gastroenterol 1995;30:615-8. [Crossref] [PubMed]
- van Middendorp JJ, Goss B, Urquhart S, et al. Diagnosis and prognosis of traumatic spinal cord injury. Global Spine J 2011;1:1-8. [Crossref] [PubMed]
- Christensen TD, Jensen SG, Larsen FO, et al. Systematic review: Incidence, risk factors, survival and treatment of bone metastases from colorectal cancer. J Bone Oncol 2018;13:97-105. [Crossref] [PubMed]
- Cassar N, Cresswell AB, Moran B. Oligometastatic colorectal cancer: is single-site bony colorectal metastasis a treatable condition? Int J Colorectal Dis 2017;32:1229-31. [Crossref] [PubMed]
- Choi SJ, Kim JH, Lee MR, et al. Long-term disease-free survival after surgical resection for multiple bone metastases from rectal cancer. World J Clin Oncol 2011;2:326-8. [Crossref] [PubMed]
- Harries M, Taylor A, Holmberg L, et al. Incidence of bone metastases and survival after a diagnosis of bone metastases in breast cancer patients. Cancer Epidemiol 2014;38:427-34. [Crossref] [PubMed]
- Zhang L, Gong Z. Clinical Characteristics and Prognostic Factors in Bone Metastases from Lung Cancer. Med Sci Monit 2017;23:4087-94. [Crossref] [PubMed]
- Ruatta F, Derosa L, Escudier B, et al. Prognosis of renal cell carcinoma with bone metastases: Experience from a large cancer centre. Eur J Cancer 2019;107:79-85. [Crossref] [PubMed]
- Chiang JM, Hsieh PS, Chen JS, et al. Rectal cancer level significantly affects rates and patterns of distant metastases among rectal cancer patients post curative-intent surgery without neoadjuvant therapy. World J Surg Oncol 2014;12:197. [Crossref] [PubMed]
- Forsberg JA, Wedin R, Bauer H. Which implant is best after failed treatment for pathologic femur fractures? Clin Orthop Relat Res 2013;471:735-40. [Crossref] [PubMed]
- Steensma M, Boland PJ, Morris CD, et al. Endoprosthetic treatment is more durable for pathologic proximal femur fractures. Clin Orthop Relat Res 2012;470:920-6. [Crossref] [PubMed]
- Wedin R, Hansen BH, Laitinen M, et al. Complications and survival after surgical treatment of 214 metastatic lesions of the humerus. J Shoulder Elbow Surg 2012;21:1049-55. [Crossref] [PubMed]
- De Divitiis C, Nasti G, Montano M, et al. Prognostic and predictive response factors in colorectal cancer patients: between hope and reality. World J Gastroenterol 2014;20:15049-59. [Crossref] [PubMed]


