
Clinical application of nano-carbon to improve the accuracy of lymph node staging in patients with advanced gastric cancer receiving neoadjuvant chemotherapy: a prospective randomized controlled trial
Introduction
Surgery remains an important method for treating advanced gastric cancer (GC), including complete tumor resection, enough cut edge, and regional lymphadenectomy. Among these, the number of dissected lymph nodes is an important index to test the quality of radical gastrectomy and is an independent risk factor affecting the prognosis of patients. Studies have shown that to more accurately evaluate the prognosis of advanced GC patients, the number of lymph nodes detected should be more than 30 (1-3).
Currently, neoadjuvant chemotherapy (NCT) has become the recommended treatment for advanced GC because it effectively increases the surgical radical degree and reduces the postoperative recurrence rate (4-7). However, tumor regression reaction after NCT can lead to a decrease in the number of lymph nodes and reduction in their volume. In turn, this may lead to a reduction in the number of lymph nodes detected and the migration of lymph node staging (N staging) after NCT, which affects the surgical quality, the evaluation of chemotherapy efficacy, and the judgment of prognosis (8). The studies on how to improve the N staging accuracy after neoadjuvant therapy for gastric cancer are rare.
Carbon nanoparticles with a diameter of 150 nm, after injected into submucosal tissue of the tumour, carbon nanoparticles can actively transported or passively transported to the local lymphatic vessels and lymph nodes are black stained, so lymph nodes can be easily identified and obtained, and once the lymph nodes are stained, they will not fade due to factors such as chemotherapy. As a result, the number of lymph nodes detected increased after GC surgery, and can reduce the deviation of postoperative pathological N staging. In previous studies on the application of carbon nanoparticles suspension injections (CNS) in NCT for advanced GC, CNS injections were carried out before or during the operation after the completion of NCT. However, reactions to chemotherapy could cause lymphoid tissue fibrosis, and regional lymph nodes were difficult to adequately stain within a limited time, affecting their detection rate (9). Therefore, how to effectively solve the problem of reduced lymph node detection caused by NCT and improving the accuracy of N staging has important clinical significance.
The purpose of this study was to investigate the clinical value of CNS in improving the accuracy of lymph node staging in patients with advanced GC by injecting CNS before and after NCT, and further analyzing the relevant data collected. We present the following article in accordance with the CONSORT reporting checklist (available at https://dx.doi.org/10.21037/jgo-21-457).
Methods
Patient selection criteria
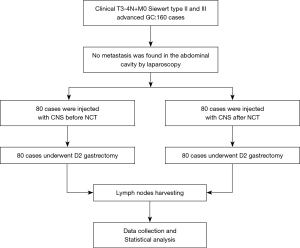
This study was designed as a randomized, parallel controlled clinical trial. A total of 160 patients with advanced GC who planned to receive NCT in the Fourth Hospital of Hebei Medical University from January 2020 to January 2021 were recruited. Using a random number table and allocation ratio is 1:1, patients were divided into an experimental group (n=80) and a control group (n=80). All patients corresponded to the following inclusion criteria: (I) 18–70 years old; (II) pathologically diagnosed as GC by gastroscopy and biopsy; and (III) abdominal computer tomography (CT) combined with laparoscopic exploration assessed the clinical stage as advanced T3-4N+M0. Patients were assessed before cancer treatment to confirm their ability to tolerate NCT and surgery by the treatment team.
The exclusion criteria included any of the following: (I) R0 resection could not be achieved, or the presence of distant metastatic lesions; (II) history of other malignant tumors and/or upper gastrointestinal surgery; (III) patients who had received previous chemotherapy before enrollment. Primary outcome measures were to calculate the total number of lymph nodes detected and the number of metastatic lymph nodes. The secondary endpoints: lymph node staining rate, lymph node metastasis rate, and the number of micro lymph nodes detected, which was aimed to reflect the degree of difficulty of picking up lymph nodes. Figure 1 shows the trial scheme. This study was approved by the Ethics Committee of the Fourth Hospital of Hebei Medical University, and informed consent was obtained from each patient. The ethics approval document number was 2018009, and the Chinese Clinical Trial Registration number was ChiCTR2100047407. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013).
Carbon nanoparticles
Carbon nanoparticles were applied in the form of injection of a standard suspension (1 mL; 50 mg), with a diameter of 150 nm. As the capillary endothelium contains 20–50 nm wide pores and capillary lymphatic cells contain pores 100–500 nm wide, nano-carbon does not enter the blood vessel but instead, rapidly enters the draining lymphatic vessels and lymph nodes. Consequently, the lymph nodes retain a large amount of ultra-fine carbon particles dyeing them black and facilitating their identification during surgery.
Endoscopic injection of CNS
Nano-carbon was injected at a distance of 0.5–1 cm from the edge of the tumor into four sites: the submucosa in the cardia side, the pylorus side, the large curved side, and the small curved side, with 0.25 mL at each point (Figure 2A). This operation was performed by a specialist.

CNS injection timing selection
The experimental group received endoscopic injection of CNS within 24 hours prior to NCT, while the control group received this post NCT and within 24 hours before D2 radical resection. SOX [oxaliplatin: 130 mg/(body surface area, BSA): m2, first day + S-1: (BSA: <1.25 m2, 40 mg each time; ≥1.25 to <1.5 m2, 50 mg each time; ≥1.5 m2, 60 mg each time), 2 times a day, for 2 weeks] was chosen as the NCT regimen, each cycle taking three weeks, and each patient receiving four cycles before open or laparoscopic D2 radical surgery.
Intraoperative lymph node dissection range and postoperative pathology
After four cycles of NCT all patients were treated with open or laparoscopic D2 radical gastrectomy, and lymph node classification was based on the 15th edition of the Japanese Gastric Cancer Regulations (10). As carbon nanoparticles injected under the gastric mucosa can enter lymphatic vessels and lymph nodes, those draining from the tumor region were stained black allowing easy identification during surgery (Figure 2B and Figure 3). After the specimen was isolated, the nodes were examined by trained physicians, and their total number and staining rate were counted, while micro lymph nodes were measured with vernier calipers and counted (Figure 3). According to the postoperative pathological results, the lymph node metastasis rate and pathological N stage of each group were also counted.

Surgical complications
The incidence of postoperative complications, including pulmonary infection, incision infection, bleeding, anastomotic fistula, and intestinal obstruction, were compared in each group.
Statistical analysis
SPSS 22.0 statistical software was also used for data analysis. Measurement data were expressed as mean ± standard deviation, compared by t-test and analysis of variance. Enumeration data were expressed as percentage using the χ2 test, while the grade data were tested by nonparametric rank sum test. P<0.05 was regarded as statistically significance.
Results
Patient demographics and cancer characteristics
One hundred and sixty patients diagnosed with advanced GC who planned to receive NCT from January 2020 to January 2021 were recruited, of the 160 enrolled patients there were 54 males and 26 females in the experimental group, 51 males and 29 females in the control group. All enrolled patients received D2 radical resection. The database of this study was established based on the following information: age, gender, body mass index (BMI), tumor location, tumor longest diameter, clinical TNM stage, Luaren classification, tumor regression grade (TRG), differentiation degree, surgery range, total number of lymph nodes detected, the number of stained lymph nodes, the number of lymph node metastases, the number of stained lymph node metastases, the number of micro lymph nodes (diameter <2 mm) detected, and postoperative complications including pulmonary infection, wound infection, anastomotic leakage, and intestinal obstruction. There was no significant difference between the two groups in terms of age, sex, BMI, tumor size, tumor location, Luaren classification, clinical TNM staging, and differentiation degree (all P>0.05) (Table 1, Figure 1).
Table 1
| Item | Experimental group (n=80) | Control group (n=80) | t/χ2 | P |
|---|---|---|---|---|
| Age (year, ) | 59.61±8.93 | 60.78±9.34 | 0.32 | 0.578 |
| Gender, n (%) | ||||
| Male | 54 | 51 | 0.24 | 0.61 |
| Female | 26 | 29 | ||
| BMI (kg/m2, ) | 24.18±3.12 | 23.05±2.96 | 1.66 | 0.11 |
| Longest diameter of tumor (cm, ) | 3.76±1.44 | 4.13±1.52 | 1.12 | 0.27 |
| Tumor location, n | ||||
| U 1/3 | 24 | 22 | 0.46 | 0.80 |
| M 1/3 | 11 | 9 | ||
| L 1/3 | 45 | 49 | ||
| Differentiated degree, n | ||||
| Well differentiated | 11 | 13 | 0.78 | 0.68 |
| Moderately differentiated | 27 | 22 | ||
| Poorly differentiated | 42 | 45 | ||
| Luaren type, n | ||||
| Intestinal type | 26 | 28 | 0.31 | 0.86 |
| Diffuse type | 22 | 19 | ||
| Mixed type | 32 | 33 | ||
| cT stage, n | ||||
| T3 | 17 | 15 | 0.16 | 0.69 |
| T4a | 63 | 65 | ||
| cN stage, n | ||||
| N1 | 11 | 13 | 0.48 | 0.79 |
| N2 | 33 | 29 | ||
| N3 | 36 | 38 |
BMI, body mass index.
Operation and lymph node detection between the two groups
In the experimental group 35 patients underwent total gastrectomy and 45 underwent distal gastrectomy, while in the control group 32 underwent total gastrectomy and 48 underwent distal gastrectomy. Analysis showed there was no significant difference between the two groups. Postoperative pathological TRG was also not significantly different between the two groups. A total of 3,197 lymph nodes were detected in the experimental group, including 384 metastatic lymph nodes, 1,424 stained lymph nodes, and 210 metastatic stained lymph nodes. The total number of lymph nodes in the control group was 2,565, including 244 metastatic lymph nodes, 796 stained lymph nodes, and 94 metastatic stained lymph nodes. The number of lymph nodes, the number of metastatic lymph nodes, the number of small lymph nodes, the rate of lymph node staining, and the rate of lymph node metastasis were all higher in the experimental group than in the control group (all P>0.05) (Table 2).
Table 2
| Item | Experimental group (n=80) | Control group (n=80) | F/χ2/Z | P |
|---|---|---|---|---|
| Resection range, n | ||||
| Total gastrectomy D2 | 35 | 32 | 0.23 | 0.63 |
| Distal gastrectomy D2 | 45 | 48 | ||
| Lymph nodes detected, | ||||
| Average total number of lymph nodes dissected | 39.96±13.06 | 32.06±9.13 | 5.79 | 0.00 |
| Number of lymph node metastasis | 4.80±5.97 | 3.05±4.09 | 4.68 | 0.03 |
| Number of micro lymph nodes | 8.53±3.18 | 6.26±2.21 | 3.71 | 0.00 |
| Number of stained lymph nodes | 17.80±8.68 | 9.95±4.33 | 11.57 | 0.00 |
| The number of metastases in black lymph nodes | 2.63±3.63 | 1.18±1.96 | 15.07 | 0.00 |
| Postoperative N stage, n | ||||
| N0 | 13 | 28 | -3.63 | 0.00 |
| N1 | 22 | 23 | ||
| N2 | 24 | 20 | ||
| N3a | 16 | 9 | ||
| N3b | 5 | 0 | ||
| Tumor regression grade (TRG), n | ||||
| 0 (no residual tumor cells in the tumor bed) | 4 | 2 | 1.16 | 0.76 |
| 1 (residual tumor cells < the tumor bed 10%) | 18 | 15 | ||
| 2 (residual tumor cells accounting for 10% to 50% of the tumor bed) | 32 | 35 | ||
| 3 (residual tumors of the > tumor bed 50%) | 26 | 28 |
Rate of lymph node staining compared between the two groups
The number of stained and unstained lymph nodes in the experimental group was 1,424 and 1,773, respectively, with a staining rate of 44.54% (1,424/3,197). In the control group, the number of stained and unstained lymph nodes was 796 and 1,769, respectively, with a staining rate of 31.03% (796/2,565). The staining rate of the experimental group was higher than that of the control group, and the difference was statistically significant (P<0.05) (Table 3).
Table 3
| Item | Experimental group | Control group | χ2 | P |
|---|---|---|---|---|
| Lymph nodes stained | ||||
| Yes | 1,424 | 796 | 109.65 | 0.000 |
| No | 1,773 | 1,769 | ||
| Lymph node metastasis | ||||
| Yes | 384 | 244 | 9.15 | 0.002 |
| No | 2,813 | 2,321 | ||
| Stained lymph node metastasis | ||||
| Yes | 210 | 94 | 3.73 | 0.053 |
| No | 1,214 | 702 | ||
Rate of lymph node metastasis between the two groups
The number of lymph node metastases in the experimental group was 384, and the number of lymph node metastases with black staining was 210. The lymph node metastasis rate was 12.01% (384/3,197) and the black staining lymph node metastasis rate was 14.75% (210/1,424). In the control group, the number of lymph node metastases was 244, the number of lymph node metastases with black staining was 94, the rate of lymph node metastasis was 9.51% (244/2,565), and the rate of lymph node metastasis stained was 11.81% (94/796). The lymph node metastasis rate of the experimental group was higher than that of the control group (P<0.05) (Table 3).
Postoperative pathological N staging of the two groups
In the experimental group 13 patients were staged as N0, 22 as N1, 24 as N2, 16 as N3a, and 5 as N3b, while the control group saw 28 as N0, 23 as N1, 20 as N2, 9 as N3a and 0 as N3b. More metastatic lymph nodes were detected in the experimental group than that in the control group, and postoperative N staging was more accurate in the experimental group, with significant difference between the two (P<0.05) (Table 2).
Incidence of postoperative complications
There were five cases of postoperative pulmonary infection and two of anastomotic fistula in the experimental group, and no bleeding, incision infection, intestinal obstruction or other complications. In the control group there was one case of postoperative bleeding, six of pulmonary infection, one of incision infection, one of anastomotic fistula, and no intestinal obstruction and other complications. No statistically significant differences in complications between the two groups was seen (χ2=0.28, P>0.05).
Discussion
Several clinical trials in recent years have demonstrated NCT can improve the radical cure rate and 5-year survival rate after surgery of GC including the MAGIC and JCOG 0405 trials (5-7). While this has resulted in NCT for GC being written into the NCCN guidelines, as Lordick (11) contends, NCT may also make tumor tissue and regional lymph nodes shrink, making their detection more difficult during operation and affecting the accuracy of N staging. Although NCCN guidelines emphasize the accuracy of N staging and require at least 16 lymph nodes to be detected in each radical resection of GC specimens (12), studies have confirmed that in advanced GC, the number of potentially positive lymph nodes can only be increased when more than 30 lymph nodes are submitted for examination, thereby reducing the N staging offset and ensuring the accuracy of N staging (2,3).
Lymph node detection methods include macroscopic examination, finger palpation, and lymph node tracer methods (13,14). CNS is a suspension made of polyvinylpyrrolidone and normal saline, wrapped in smooth carbon particles, with an average diameter of 150 nm. As the intercellular space of capillary endothelial cells is 20–50 nm, and that of capillary lymphatic cells is 100–500 nm, CNS only enters lymphatic capillaries; 1.0 mL of CNS was drawn into a 1ml syringe and injected into the submucosa of the stomach at four points around the tumor 2 cm away on average, each point was injected with 0.25 mL. After the injection, each injection point was lightly pressed with dry gauze for 10 seconds to prevent the tracer extravasation from affecting the staining effect. From here it can rapidly enter lymphatic vessels to be retained in lymph nodes through macrophage phagocytosis, and can be retained in both for periods of 3–4 months providing an excellent tracer effect (15). In addition, CNS has no biological activity, is non-toxic, and will not cause adverse reactions (16). However, as NCT can cause lymphatic contraction and fibrosis in surrounding tumor tissue, CNS injection after NCT often affects the effective detection of intraoperative lymph nodes due to the decreased degree of lymphatic staining (17,18). Labeling CNS before NCT can theoretically provide a long enough dispersion time and avoid the influence of lymph node tracer tracing caused by NCT on lymph node contraction and fibrosis in surrounding tumor tissues (15,17,18). Therefore, in patients with advanced GC, providing NCT before injection facilitates the diffusion of CNS within lymph nodes, and can, theoretically, improve their staining, reduce the effect of NCT, and increase the detection of smaller and greater numbers of nodes to improve the precision of postoperative N staging. In this study, compared with the application of CNS before NCT, the staining rate and metastasis rate of lymph nodes detected were significantly increased, and the injection of carbon nanoparticles before NCT improved the number of lymph nodes detected without increasing postoperative complications. Among the black-stained lymph nodes detected, the number of black-stained lymph nodes in the experimental group was higher than that in the control group, but there was no statistical difference. The reason may be related to the limited number of enrolled cases. With the increase of the number of enrolled cases, there may be significant statistical difference. There were significant differences in postoperative pathological N staging between the two groups, indicating the application of CNS before NCT could significantly increase the number of lymph nodes and metastasis detected in a safe and effective manner, and render the postoperative pathological lymph node staging more accurate, indicating the use of carbon nanoparticles was safe and effective. At present, domestic and foreign scholars define lymph nodes with diameter less than 2 mm as micro lymph nodes, and some studies have found that the metastasis rate of these nodes can exceed 17% of the total number of positive nodes (19). This study showed that the detection rate of small lymph nodes in the experimental group was significantly higher than in the control group, indicating CNS could increase the number of micro lymph nodes detected, and the tracer effect of CNS injection before NCT was better.
In conclusion, the injection of CNS before NCT can increase the total number of lymph nodes and number of metastatic lymph nodes detected, improve the accuracy of N staging after NCT for GC, and create a more accurate estimation of the postoperative pathological staging and prognosis of patients. Moreover, it can improve follow-up treatment and evaluate the prognosis of patients. However, long-term follow-up and observation are required to verify the results and improve the survival of patients.
Acknowledgments
Funding: This study was funded by the Hebei Medical Science Research project, No. 20211338; Hebei Medical Application Tracking project, No. 2703006; Hebei Health Commission County-level Public Hospital Appropriate Health Technology Promotion project, No. 2019024; and Hebei Government-funded Clinical Medicine Outstanding Talent Training project, No. 2019012.
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at https://dx.doi.org/10.21037/jgo-21-457
Trial Protocol: Available at https://dx.doi.org/10.21037/jgo-21-457
Data Sharing Statement: Available at https://dx.doi.org/10.21037/jgo-21-457
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jgo-21-457). All authors reported that this study was funded by the Hebei Medical Science Research project, No. 20211338; Hebei Medical Application Tracking project, No. 2703006; Hebei Health Commission County-level Public Hospital Appropriate Health Technology Promotion project, No. 2019024; and Hebei Government-funded Clinical Medicine Outstanding Talent Training project, No. 2019012. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of the Fourth Hospital of Hebei Medical University, and informed consent was obtained from each patient. The ethics approval document number was 2018009, and the Chinese Clinical Trial Registration number was ChiCTR2100047407.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ajani JA, Bentrem DJ, Besh S, et al. Gastric cancer, version 2.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw 2013;11:531-46. [Crossref] [PubMed]
- Sano T, Coit DG, Kim HH, et al. Proposal of a new stage grouping of gastric cancer for TNM classification: International Gastric Cancer Association staging project. Gastric Cancer 2017;20:217-25. [Crossref] [PubMed]
- Deng J, Liu J, Wang W, et al. Validation of clinical significance of examined lymph node count for accurate prognostic evaluation of gastric cancer for the eighth edition of the American Joint Committee on Cancer(AJCC) TNM staging system. Chinese Journal of Cancer Research 2018;30:477-91.
- Ajani JA, D'Amico TA, Almhanna K, et al. Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2016;14:1286-312. [Crossref] [PubMed]
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 2011;29:1715-21. [Crossref] [PubMed]
- Tsuburaya A, Mizusawa J, Tanaka Y, et al. Neoadjuvant chemotherapy with S-1 and cisplatin followed by D2 gastrectomy with para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis. Br J Surg 2014;101:653-60. [Crossref] [PubMed]
- Wu ZM, Teng RY, Shen JG, et al. Reduced lymph node harvest after neoadjuvant chemotherapy in gastric cancer. J Int Med Res 2011;39:2086-95. [Crossref] [PubMed]
- Hsieh FJ, Wang YC, Hsu JT, et al. Clinicopathological features and prognostic factors of gastric cancer patients aged 40 years or younger. J Surg Oncol 2012;105:304-9. [Crossref] [PubMed]
- The Japanese Society for Cancer of the Stomach. Gastric cancer treatment protocol. Tokyo: Gold Original Publishing Corporation, 2017.
- Lordick F, Ott K, Sendler A. Gastric cancer and adenocarcinoma of the esophagogastric junction: principles of neoadjuvant therapy. Chirurg 2011;82:968-73. [Crossref] [PubMed]
- In H, Solsky I, Palis B, et al. Validation of the 8th Edition of the AJCC TNM Staging System for Gastric Cancer using the National Cancer Database. Ann Surg Oncol 2017;24:3683-91.
- Aoyama T, Fujikawa H, Cho H, et al. A methylene blue-assisted technique for harvesting lymph nodes after radical surgery for gastric cancer: a prospective, randomized, controlled study. Am J Surg Pathol 2015;39:266-73. [Crossref] [PubMed]
- Li Z, Ao S, Bu Z, et al. Clinical study of harvesting lymph nodes with carbon nanoparticles in advanced gastric cancer: a prospective randomized trial. World J Surg Oncol 2016;14:88. [Crossref] [PubMed]
- Lucci A, Turner RR, Morton DL. Carbon dye as an adjunct to isosulfan blue dye for sentinel lymph node dissection. Surgery 1999;126:48-53. [Crossref] [PubMed]
- Magrez A, Kasas S, Salicio V, et al. Cellular toxicity of carbon-based nanomaterials. Nano Lett 2006;6:1121-5. [Crossref] [PubMed]
- Yokota T, Saito T, Narushima Y, et al. Lymph-node staining with activated carbon CH40: a new method for axillary lymph-node dissection in breast cancer. Can J Surg 2000;43:191-6. [PubMed]
- Schwarz RE, Smith DD. Clinical impact of lymphadenectomy extent in resectable gastric cancer of advanced stage. Ann Surg Oncol 2007;14:317-28. [Crossref] [PubMed]
- Baxter NN, Virnig DJ, Rothenberger DA, et al. Lymph node evaluation in colorectal cancer patients: a population-based study. J Natl Cancer Inst 2005;97:219-25. [Crossref] [PubMed]
(English Language Editor: B. Draper)


