
Abiraterone acetate for treatment of ectopic Cushing syndrome caused by ACTH-producing neuroendocrine tumor: a case report
Introduction
Neuroendocrine neoplasms (NENs) are a heterogeneous group of neoplasms that vary in presentation and prognosis. NENs are broadly divided into two groups, neuroendocrine tumors (NETs) and neuroendocrine carcinomas (NEC). NETs are well-differentiated tumors with the potential to metastasize and invade other tissues, the group subdivides into grades of low, intermediate, and high. NEC are poorly differentiated tumors that are highly malignant and aggressive in nature, histologically they may resemble small or large cell carcinomas. The revised WHO guidelines on classification of NENs considers the grade, mitotic count, and Ki-67, both of which carry prognostic relevance: NET, G1 with mitotic rate <2 mitoses/2 mm2, Ki-67 <3%; NET, G2 with mitotic rate of 2–20 mitoses/2 mm2, Ki-67 between 3–20%; NET, G3 with mitotic rate of >20 mitoses/2 mm2, Ki-67 >20% (1); NEC are poorly differentiated with mitotic rate of >20 mitoses/2 mm2 (1).
Pancreatic neuroendocrine tumors (PNETs) originate from the islets of Langerhans and are known to secrete various peptide hormones (2). Common functional NETs of the gastrointestinal system include carcinoid tumor, gastrinoma, insulinoma, glucagonoma, somatostatinoma, and vasoactive intestinal peptide tumor (VIPoma). The reported annual incidence of PNETs is approximately 1 per 100,000 (3) however certain subtypes of PNETs, such as adrenocorticotropin hormone (ACTH)-releasing NET are much less common.
ACTH-releasing NET of gastroenteropancreatic origin is a rare diagnosis that can cause uncontrolled release of ACTH leading to accelerated production and release of cortisol resulting in ectopic adrenocorticotropic hormone syndrome (EAS), a variant of Cushing syndrome (CS). These neoplasms are often found to have metastases at the time of diagnosis. These tumors are described to be aggressive and often resistant to traditional treatments. The main tenet of treatment for malignant EAS is to control symptoms of hypercortisolism as quickly and effectively as possible (4). When surgical resection is a practical option, it may allow for definitive management, however disease recurrence is not uncommon (5).
Abiraterone acetate (AA) is an inhibitor of 17α-hydroxylase and 17,20-lyase (CYP17) that blocks androgen synthesis and glucocorticoid production. Adrenal insufficiency is a common side-effect of AA, in fact a widely accepted practice is to prescribe concurrent prednisone with AA to avoid adrenal crisis (6). In EAS, the endogenous production of androgen synthesis can be blocked by AA and thereby mitigate the toxic effects of hypercortisolism. AA has the potential to allow for rapid quantitative reduction of cortisol, less than 10 days after initiating treatment (7). In the treatment of EAS for patients who are not fit for classic anti-neoplastic agents, AA has the potential to quickly control life-threatening symptoms. AA has been used in EAS secondary to primary adrenal malignancy, however this is the first case that reports on its use in EAS due to a NET. We present the following article in accordance with the CARE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-376/rc).
Case presentation
A 54-year-old female presented with progressively worsening altered mental status and weakness, her symptoms had been ongoing for several months. On presentation, the patient was alert and oriented to self, time, place, and situation, however she had mild decline in her baseline cognitive function. Physical exam was notable for elevated blood pressure, moon facies, weakness in bilateral lower extremities, reduced strength in bilateral hip flexors, inability to ambulate independently, sensation and reflexes were intact.
The patient had a past medical history of diabetes mellitus that recently become uncontrolled and a recent diagnosis of hypertension requiring anti-hypertensive therapy. The patient also had a past medical history of remote early-stage breast cancer, she underwent bilateral total mastectomy followed by adjuvant hormonal therapy. She had no relevant family history. The patient was a non-smoker and reported rare alcohol use.
Laboratory studies were obtained (Table 1) with findings of profound hypokalemia and alkalemia. Aldosterone and aldosterone/renin ratio were normal, direct renin was decreased. Serum random cortisol was elevated as well as serum ACTH. Dexamethasone suppression testing was abnormal, 8 mg of dexamethasone did not suppress AM cortisol (84.6 mcg/dL). Serum chromogranin level was elevated.
Table 1
| Serum laboratory testing | Patient result | Reference range |
|---|---|---|
| Potassium | 1.5 mEq/L | 3.5–5.3 mEq/L |
| Bicarbonate | 39 mEq/L | 22–28 mEq/L |
| Cortisol, random | 185 mcg/dL | 10–20 mcg/dL |
| Adrenocorticotropic hormone | 1,186 pg/mL | 46 pg/mL |
| Aldosterone | 4.7 ng/dL | ≤23.2 ng/dL (supine) |
| Renin | <2.1 pg/mL | 3.1–57.1 pg/mL |
| Aldosterone/renin ratio | 2.2 | 0.1–3.7 |
| Chromogranin | 205 pg/mL | <160 pg/mL |
CT imaging of the chest, abdomen, pelvis revealed bilateral pulmonary lesions, intraabdominal lymphadenopathy, bilateral diffuse adrenal gland thickening, multiple hepatic metastases, and a 2.5 cm lesion at the pancreatic tail. The pancreatic lesion was also visualized on magnetic resonance imaging (MRI) of the abdomen, suggestive of the primary tumor. Bone scan was negative for metastatic lesions. Pituitary MRI was negative for a pituitary mass.
The patient underwent electromyogram of the bilateral lower extremities, results were abnormal, suggestive of severe proximal myopathy. She underwent muscle biopsy which was reported as severe generalized myofiber atrophy.
Biopsy of a liver lesion was reported to be metastatic NET, G1 per recent WHO criteria. The histology of the NET was further described as well differentiated NET, grade-1, with a Ki-67 proliferative index <3%, the mitotic rate was not reported. The neoplastic cells were positive for synaptophysin, chromogranin, caudal-type homeobox 2 (CDX2), and negative for calretinin, inhibin, S100, cytokeratin-7 (CK7), GATA binding protein 3 (GATA-3), and thyroid transcription factor-1 (TTF-1). Germline genetic lining testing was not completed.
The patient’s clinical presentation and imaging findings were initially concerning for recurrent and metastatic breast cancer. This differential diagnosis was ruled out after liver tissue biopsy was reported. Regarding the patient’s motor weakness, Guillain-Barre syndrome was considered, however, electromyography findings and muscle biopsy ruled this out. Muscle biopsy findings were negative for evidence of inflammation or vasculitis. Other autoimmune myositis conditions were ruled out with negative serum markers and muscle biopsy findings.
Overall, the clinical presentation and workup was consistent with hypercortisolemia. Given the liver tissue biopsy findings of NET, muscle biopsy findings and elevated serum cortisol and ACTH levels, the patient’s weakness was ascertained to be secondary to glucocorticoid-induced myopathy.
The patient was diagnosed with metastatic ACTH-produce NET. An extensive multi-disciplinary approach was utilized, involving internal medicine, endocrinology, neurology, physiatry and oncology, to facilitate in the management of this patient. The primary tumor was suspected to be within the pancreas based on the MRI abdomen findings. Although the patient’s tumor burden was not high, malignant hypercortisolism is difficult to control and is a poor prognostic feature. Invasive diagnostic procedures were avoided due to the patient’s tenuous clinical status. The patient was started on octreotide acetate at 100 mg twice daily with minimal improvement. She was later started on ketoconazole at 400 mg twice daily and eventually titrated up to 600 mg three times daily. The patient received a total of 38 days of oral ketoconazole, the clinical reasoning of continuing ketoconazole was to reduce the risk of worsening hypercortisolism, although it did not particularly lower the serum cortisol levels. While there was a slight decrease in ACTH and cortisol levels, there was no meaningful change in motor strength appreciated. After thorough consideration of all other medical interventions, AA was started at a dose of 500 mg twice daily. Effects of serum cortisol reduction were immediate, serum cortisol levels started to downtrend by day 3 of treatment, cortisol levels completely normalized with 10 days of starting AA (Figure 1).
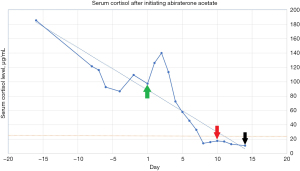
The patient had complete resolution of the hypercortisolemia within days of starting AA, however cortisol-induced myopathy remained stable in the initial days of treatment. Although AA did allow for rapid reduction in cortisol levels, there was concern for durability of the response and whether it would be a feasible long-term treatment once the patient was discharged from the hospital. The patient underwent bilateral adrenal artery embolization ten days after starting AA. The rationale of this subsequent therapy was to augment the effects of AA in terminating cortisol production and maintaining the response. Since the patient underwent definitive intervention for hypercortisolism, AA was discontinued after 14 days of treatment. She was discharged to a rehabilitation facility in stable condition for advanced nursing care and physical therapy. However, one week after her discharge the patient was admitted to the medical intensive care unit for management of septic shock secondary to pneumonia, she ultimately succumbed. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
PNETs are a rare and challenging diagnosis to make and to treat. The disease has often metastasized at the time of diagnosis, consequently eliminating the possibility of definitive or debulking surgery. The presentation is variable and nonspecific, the activity of the disease often correlates with the site of cellular origin and neuropeptide secretion. Metastatic NETs originating from the midgut (jejunal, ileal, cecal, appendiceal) are often indolent, however NETs originating from the foregut (bronchial, stomach, duodenum), hindgut (distal colon, rectal) behave more aggressively (7).
The goal for treatment of any metastatic NET is to prevent disease progression and control effects of the secreted peptide. Treatment of EAS secondary to PNET is directed at controlling and preventing further cortisol production. The recommended for first-line therapy is somatostatin analogs (SSAs), octreotide or lanreotide, to control NET-related symptoms and stop tumor growth (8). SSA inhibitor effects are mediated through SSTR subtypes 2 (inhibit hormone secretion), 3 and 5 (both modulate tumor growth suppression and apoptosis). SSAs primarily bind to SSTR-2, primarily associated with temporizing hormonal and modest downstream SSTR effects of apoptosis and cell growth with variable interaction with SSTR 3 and 5, though low rates of durable radiologic response are reported (9,10).
Prior studies of the use of SSAs in patient with NETs have shown a benefit in progression-free survival as well as symptom control follow up long term analysis showed sustained benefit and benefit in survival (11,12). A separate study found no difference in long term survival in patients treated with octreotide compared to those treated with placebo (13). Although these landmark trials are relevant when broadly discussing NETs, analysis studying specific groups subdivided by the peptide-secretion (i.e., serotonin, ACTH, insulin) were not discussed in either trial, therefore it is unclear if these results vary in less-common tumor tissue types that may not have been widely represented larger studies. Broadly speaking, SSAs are effective in 50% of patients with metastatic NET in controlling symptoms and mitigating progression of disease. With regards to ACTH-releasing PNETs, in vitro studies identifying somatostatin receptor profiles in ACTH-producing NET cells suggests that there may not be a strong response to SSAs due to the difference in somatostatin receptor profile (14). While SSAs have been reported to suppress ACTH to undetectable levels (15), there have been other reports that did not report any effect (16-18). Further research is needed to determine the effectiveness of SSAs in ACTH-producing PNETs.
Due to its obscurity, ACTH-releasing NETs have not been extensively studied. SSAs are used in the treatment of metastatic NETs, however these agents may not provide potent disease control if needed. National guidelines recommend implementing treatment with chemotherapy agents such as everolimus, temozolomide and capecitabine in patients who are symptomatic despite SSAs. Metyrapone, an inhibitor of cortisol production, and mitotane, an adrenolytic agent, are treatment options for adrenocortical carcinoma (19). Both were considered for this patient but not available on inpatient formulary. Furthermore, it was unclear how effective these agents would be in this case especially since the patient required rapid response. Liver-directed therapies were considered, such as transarterial chemoembolization (TACE) and transarterial radioembolization (TARE), however there was question of benefit in only treating the metastases if the primary tumor was left untreated and adrenal glands were intact. AA was proposed given its known side effect profile and potential for rapid disease control.
To our best knowledge, the use of AA in the management of EAS secondary to an ACTH-producing tumor has yet to been described. The pharmacologic properties and mechanism of action taken by AA allows for direct inhibition of 17α-hydroxylase/17,20-lyase and inhibit downstream cortisol production. Exploitation of this pathway allows for rapid cessation of cortisol production in instances of life-threatening EAS (20). AA has been widely used in metastatic prostate cancer and its side effect profile is well understood. In vitro studies have shown the cytotoxic effects of AA on adrenocortical carcinoma cells and steroidogenesis (21). An Italian case report showed the potential use of AA to treat EAS secondary to adrenocortical carcinoma that had reported with positive results of disease control. Though the use of AA has yet to be described in the setting of a NET, its physiologic mechanism would still allow for cortisol reduction in Cushing’s syndrome, regardless of the primary malignancy.
The side effect profile for AA is not negligible but typically tolerable. AA can cause mineralocorticoid excess symptoms such as hypertension, hypertriglyceridemia, edema, symptoms that must be actively monitored and medically managed.
Despite serum cortisol normalization in treating EAS, clinical recovery is variable across patient populations. Long-term effects of EAS despite definitive treatment include cognitive dysfunction, psychiatric disorders, chronic fatigue, cardiac dysfunction, and adrenal insufficiency. EAS induced myopathy may persist for months and even years, time is needed for muscle fibers to regenerate after prolonged muscle wasting in the setting of hypercortisolism. A German study that reported on the recovery of CS induced myopathy found that grip strength worsened after cortisol levels decreased, hypothesizing that this could be do due to time needed for other anabolic hormones, such as growth hormone and sex hormones, to recover after prolonged suppression in florid CS (22). For our patient, the recovery of motor strength did not worsen after cortisol levels decrease and in fact, she was noted to have made some progress in terms of strength prior to hospital discharge. Her death was ultimately due to several factors including prolonged hospitalization, exposure to hospital-acquired infections and poor pulmonary hygiene due to reduced mobility, resulting in hospital acquired pneumonia. Due to the abrupt nature of her death, it is premature to determine if the patient would have fared well in terms of an improvement in her motor strength over time.
Conclusions
The use of AA in this context still requires further exploration, including durability of response and reversibility of EAS effects once serum cortisol levels have been reduced. Further studies and evidence are needed to substantiate this use, identify optimal length of treatment, durability of responses and reveal any possible adverse effects in this specific patient population. In conclusion, this case demonstrates that effective utility of AA to treat EAS in an ACTH-secreting PNET.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-376/rc
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-376/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-376/coif). RL has received consulting honoraria from Xcenda and Aptitude health in the past 36 months. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rindi G, Klimstra DS, Abedi-Ardekani B, et al. A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod Pathol 2018;31:1770-86. [Crossref] [PubMed]
- Strosberg JR, Cheema A, Weber J, et al. Prognostic validity of a novel American Joint Committee on Cancer Staging Classification for pancreatic neuroendocrine tumors. J Clin Oncol 2011;29:3044-9. [Crossref] [PubMed]
- Oberg K, Eriksson B. Endocrine tumours of the pancreas. Best Pract Res Clin Gastroenterol 2005;19:753-81. [Crossref] [PubMed]
- Fazel P, Ganesa P, Mennel RG, et al. The ectopic adrenocorticotropic hormone syndrome in carcinoid tumors. Proc (Bayl Univ Med Cent) 2008;21:140-3. [Crossref] [PubMed]
- Wang W, Miao R, Zhang L, et al. An Uncommon Case of Ectopic Adrenocorticotropic Hormone Syndrome from a Pancreatic Neuroendocrine Tumor. Cureus 2019;11:e4076. [Crossref] [PubMed]
- Sonpavde G, Attard G, Bellmunt J, et al. The role of abiraterone acetate in the management of prostate cancer: a critical analysis of the literature. Eur Urol 2011;60:270-8. [Crossref] [PubMed]
- Claps M, Lazzari B, Grisanti S, et al. Management of severe Cushing syndrome induced by adrenocortical carcinoma with abiraterone acetate: a case report. AACE Clinical Case Reports 2016;2:e337-41. [Crossref]
- Kondo T, Matsuyama R, Ashihara H, et al. A case of ectopic adrenocorticotropic hormone-producing pancreatic neuroendocrine tumor with multiple liver metastases. Endocr J 2010;57:229-36. [Crossref] [PubMed]
- Neuroendocrine and Adrenal Tumors. National Comprehensive Cancer Network. 2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/neuroendocrine.pdf
- Rogoza O, Megnis K, Kudrjavceva M, et al. Role of Somatostatin Signalling in Neuroendocrine Tumours. Int J Mol Sci 2022;23:1447. [Crossref] [PubMed]
- Caplin ME, Pavel M, Ćwikła JB, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med 2014;371:224-33. [Crossref] [PubMed]
- Caplin ME, Pavel M, Phan AT, et al. Lanreotide autogel/depot in advanced enteropancreatic neuroendocrine tumours: final results of the CLARINET open-label extension study. Endocrine 2021;71:502-13. [Crossref] [PubMed]
- Rinke A, Wittenberg M, Schade-Brittinger C, et al. Placebo-Controlled, Double-Blind, Prospective, Randomized Study on the Effect of Octreotide LAR in the Control of Tumor Growth in Patients with Metastatic Neuroendocrine Midgut Tumors (PROMID): Results of Long-Term Survival. Neuroendocrinology 2017;104:26-32. [Crossref] [PubMed]
- de Bruin C, Feelders RA, Lamberts SW, et al. Somatostatin and dopamine receptors as targets for medical treatment of Cushing’s Syndrome. Rev Endocr Metab Disord 2009;10:91-102. [Crossref] [PubMed]
- Patel C, Matson M. The role of interventional venous sampling in ocalizing neuroendocrine tumours. Curr Opin Endocrinol Diabetes Obes 2011;18:269-77. [Crossref] [PubMed]
- Pedroncelli AM. Medical treatment of Cushing’s disease: somatostatin analogues and pasireotide. Neuroendocrinology 2010;92:120-4. [Crossref] [PubMed]
- Hofland LJ, van der Hoek J, Feelders R, et al. The multi-ligand somatostatin analogue SOM230 inhibits ACTH secretion by cultured human corticotroph adenomas via somatostatin receptor type 5. Eur J Endocrinol 2005;152:645-54. [Crossref] [PubMed]
- de Herder WW, Kwekkeboom DJ, Valkema R, et al. Neuroendocrine tumors and somatostatin: imaging techniques. J Endocrinol Invest 2005;28:132-6. [PubMed]
- Baudry C, Coste J, Bou Khalil R, et al. Efficiency and tolerance of mitotane in Cushing’s disease in 76 patients from a single center. Eur J Endocrinol 2012;167:473-81. [Crossref] [PubMed]
- Attard G, Reid AH, Yap TA, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol 2008;26:4563-71. [Crossref] [PubMed]
- Fiorentini C, Fragni M, Perego P, et al. Antisecretive and Antitumor Activity of Abiraterone Acetate in Human Adrenocortical Cancer: A Preclinical Study. J Clin Endocrinol Metab 2016;101:4594-602. [Crossref] [PubMed]
- Berr CM, Stieg MR, Deutschbein T, et al. Persistence of myopathy in Cushing’s syndrome: evaluation of the German Cushing’s Registry. Eur J Endocrinol 2017;176:737-46. [Crossref] [PubMed]


