
Nutritional index for immune-checkpoint inhibitor in patients with metastatic gastro-esophageal junction/gastric cancer
Introduction
Malnutrition occurs in up to 80% of metastatic gastro-oesophageal junction (mGOJ)/gastric cancer (GC) patients (1,2). It is usually caused by the mechanical effect of the primary tumor that gives onset to symptoms such as dysphagia and early satiety, or by the reduction of food intake as part of the systemic cachexia (3-6). Nutritional support (oral, enteral or parenteral) is recommended in both the postoperative and advanced disease settings, given its favorable impact on prognosis, anticancer therapy compliance and quality of life (7).
The well-known Onodera’s Prognostic Nutritional Index (PNI), which takes into account serum albumin and total lymphocyte count, was previously studied to evaluate the nutritional status of GOJ/GC patients in both the post-operative and the metastatic settings and proved to be an independent prognostic factor (8-11).
Nivolumab and Pembrolizumab have recently been approved for the treatment of mGOJ/GC patients in several regions including Asia, Europe and Unites States, and approval has also been obtained for nivolumab as adjuvant treatment in resected oesophageal cancer patients (12-15). Nevertheless, analyses would be desirable to identify specific predictors of immune-checkpoint inhibitor (ICI) efficacy.
Tissue PD-1 combined positive score (CPS) is the most widely investigated marker for response and survival. However, there are challenges in terms of the ideal CPS cut off (i.e., >1%, >5% and >10%) to be adopted. In addition, different bioassays and different scoring systems [such as tumor positive score (TPS) vs. CPS] have been used which make it challenging to carry out cross-trial comparison.
The nutritional status and adipose tissue volume have both been reported to impact on immune response. Wang et al. have recently demonstrated, in a variety of tumors, that enlarged visceral adipose tissue might induce an immunosuppressive status in T lymphocytes via the over-expression of PD-1 on their surface. This could potentially predict increased efficacy of anti PD-1 agents (16-18).
The aim of the present study was to assess whether an ICI-specific nutritional index, inclusive also of a visceral adipose tissue marker such as waist-to-hip ratio (WHR), would be superior to classic nutritional indexes (such as Onodera’s PNI) in predicting survival in mGOJ/GC patients treated with second-line ICI.
We analyzed easily obtainable nutritional/inflammatory factors to render the index readily available. An ICI-specific NI would both help selecting patients more likely to benefit from immunotherapy and guide targeted nutritional interventions for patients with worse prognosis. We present the following article in accordance with the REMARK reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-217/rc).
Methods
Study design
Our study is a retrospective case-control study with an age- and gender-matched control cohort. The number of cases meeting the inclusion criteria during the study period determined the sample size. The primary objective was to correlate nutritional markers with overall survival (OS), defined as the time from the ICI start (or the second line chemotherapy start in the control group) to death from any cause. Patients who did not reach the death endpoint were censored at the last known follow-up date. Tissue PD-L1 expression status was not available for the majority of the patients and therefore not included in the analysis. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the Sarah Cannon Research Hospital and “Tor Vergata” University Hospital as part of protocol investigating the role of BMI in solid tumors (No. NCT03873064) and individual consent for this retrospective analysis was waived.
Patients
Eighty-nine patients with histologically confirmed GOJ/GC and measurable metastatic disease resistant to standard first-line fluoropyrimidine/platinum-based chemotherapy treated at the Sarah Cannon Research Institute (UK) or University College London Hospital between June 2014 and December 2018 were retrospectively reviewed. Only patients that were treated with second line ICI were included in the final analysis. Patients could receive either pembrolizumab, nivolumab or avelumab).
A same size gender- and age-matched control cohort of mGOJ/GC patients treated with standard paclitaxel- or fluoropyrimidine-based second-line chemotherapy was also analyzed by screening 109 patients treated between November 2014 and June 2019 at the “Tor Vergata” University Hospital of Rome, to confirm the ICI-specificity of the nutritional markers found significant in the ICI-treated cohort.
Specimen characteristics
Baseline blood samples were obtained from all patients prior to treatment start. Routine chemistry studies, including lymphocyte count (109/L), serum albumin (g/dL), serum proteins (PRO, g/dL), blood glucose (mmol/L), albumin/globulin ratio (AGR) were performed on fresh samples within one hour from blood withdrawal. Processed blood sample were aliquoted and stored at −80 ℃ for any eventual further re-evaluation. Storage conditions were carefully maintained and all aliquots were limited to one freeze-thaw cycle.
Assessment of anthropometric nutritional indexes
Data on anthropometric nutritional markers at baseline (within one week before ICI start) were collected: height (in meters), body mass Index (BMI) [weight in kilograms divided by the square of height in meters (kg/m2)], Onodera’s PNI (i.e., serum albumin (g/dL) + 5 × total lymphocytes count (109/L) (19), sagittal diameter (using the L4-L5 baseline computed tomography (CT) cross-sectional image, distance from skin to skin through the center of the abdomen) and WHR (waist circumference using the L3 baseline CT cross-sectional image, perimeter at the L3 region divided by hip circumference).
All measurements were ascertained while blinded to the sample origin and to study endpoint.
Statistical analysis
All continuous variables were dichotomized by using the maximally selected rank statistics for overall survival (20) and differences in frequency of variable categories between the ICI and the control cohort were assessed by means of the chi-square test.
Univariate association of categorized variables and OS was examined using the Kaplan-Meier method with long rank test to assess for difference between subgroups, while univariate hazard ratios (HRs) with relevant 95% confidence interval (95% CI) were estimated by using the Cox proportional hazards regression.
Only variables found significant at the univariate analysis were included in a multivariable Cox-regression model to identify independent nutritional predictors of OS.
Significant variables were combined to create a NUTRItional Index for ICI (NUTRIICI) whose ICI-specificity was subsequently tested in the chemotherapy-treated control cohort.
All analyses were performed with the R software v. 4.0.2 and MedCalc software version 19.1.17. All tests were considered statistically significant for two tail P values <0.05.
Results
Baseline characteristics
A total 114 patients entered the study. Fifty-seven (14 females, 43 males) out of 89 screened patients were included in the ICI cohort. Median age 61 years (range, 29–85). Patients had lymph node metastasis (with or without other sites of metastasis) in 77% of cases, 23% of patients had non-lymph node metastasis, mainly in the liver and peritoneum. All the patients received previous treatment with platinum-based and fluoropyrimidine-based chemotherapy regimens, including regimens with anthracycline [32 (56.1%)]. Doublet or triplet first-line regimen had no impact on OS (P=0.38). All HER2 overexpressing patients [11 (19.3%)] received trastuzumab combined with platinum and fluoropyrimidine. Pembrolizumab, nivolumab and avelumab were the administered ICI for 26, 16 and 15 patients, respectively. OS endpoint was reached for 48 out of 57 patients. Median follow-up of the 9 patients censored at the end of the follow-up was 27 months (4 to 53 months). Median OS (mOS) of the entire cohort was 6.2 months (95% CI: 4.4–10.9 months), radiological response rate was 16%.
Variables were categorized according to the maximally selected rank statistics for overall survival as follow: height (<1.75 or ≥1.75 m), BMI (≥30 or <30), WHR (<1 or ≥1), sagittal diameter (<25 or ≥25 cm), lymphocytes (<1×109 or ≥1×109/L), blood sugar (<5 or ≥5 mmol/L), albumin (<30 or ≥30 g/dL), PRO (<60 or ≥60 g/dL), AGR (<1.5 or ≥1.5), Onodera’s PNI (≤33 or >33). Table 1 reports the frequency of the variable categories within the group.
Table 1
| Characteristic | Range or n (%) |
|---|---|
| Height (m) | |
| Range | 1.55–1.93 |
| <1.75 | 22 (39%) |
| ≥1.75 | 35 (61%) |
| BMI (kg/m2) | |
| Range | 15.8–36.4 |
| <30 | 50 (88%) |
| ≥30 | 7 (12%) |
| WHR | |
| Range | 0.74–1.15 |
| <1 | 51 (89%) |
| ≥1 | 6 (11%) |
| Sagittal diameter (cm) | |
| Range | 13.0–31.6 |
| <25 | 44 (77%) |
| ≥25 | 13 (23%) |
| Lymphocytes (×109/L) | |
| Range | 0.2–2.8 |
| <1 | 18 (32%) |
| ≥1 | 39 (68%) |
| Glucose (mmol/L) | |
| Range | 3.9–12.6 |
| <5 | 11 (19%) |
| ≥5 | 46 (81%) |
| Albumin (g/dL) | |
| Range | 23–48 |
| <35 | 12 (21%) |
| ≥35 | 45 (79%) |
| PRO (g/dL) | |
| Range | 47–82 |
| <60 | 12 (21%) |
| ≥60 | 45 (79%) |
| AGR | |
| Range | 0.8–3.6 |
| <1.5 | 23 (40%) |
| ≥1.5 | 34 (60%) |
| Onodera’s PNI | |
| Range | 29.5–57.1 |
| ≤33 | 10 (18%) |
| >33 | 47 (82%) |
GOJ/GC, gastro-oesophageal junction/gastric cancer; BMI, body mass index; WHR, waist-to-hip ratio; PRO, total serum proteins; AGR, albumin to globulin ratio; PNI, prognostic nutritional index.
Selected nutritional markers
All candidate nutritional markers were first evaluated in a univariate Cox regression analysis for overall survival (Table 2).
Table 2
| Characteristic | Univariate Cox regression analysis, HR (95% CI), P value | Multivariate Cox regression analysis, HR (95% CI), P value |
|---|---|---|
| Height (m) (<1.75/≥1.75) | 1.37 (0.76 to 2.47), 0.28 | |
| BMI (kg/m2) (<30/≥30) | 1.90 (0.91 to 3.98), 0.09 | |
| WHR (<1/≥1) | 3.54 (1.38 to 9.10), 0.009 | 3.75 (1.44 to 9.72), 0.007 |
| Sagittal diameter (cm) (<25/≥25) | 0.66 (0.34 to 1.26), 0.21 | |
| Lymphocytes (×109/L) (<1/≥1) | 0.70 (0.35 to 1.39), 0.31 | |
| Glucose (mmol/L) (<5/≥5) | 1.36 (0.68 to 2.72), 0.38 | |
| Albumin (g/dL) (<35/≥35) | 2.08 (1.07 to 4.05), 0.03 | 1.77 (0.53 to 5.89), 0.35 |
| PRO (g/dL) (<60/≥60) | 2.96 (1.43 to 6.12), 0.003 | 1.10 (0.34 to 3.61), 0.87 |
| AGR (<1.5/≥1.5) | 0.56 (0.30 to 1.04), 0.07 | |
| Onodera’s PNI (≤33/>33) | 5.28 (2.34 to 11.92), 0.0001 | 8.13 (1.39 to 47.48), 0.02 |
OS, overall survival; ICI, immune-checkpoint inhibitor; BMI, body mass index; WHR, waist-to-hip ratio; PRO, total serum proteins; AGR, albumin to globulin ratio; PNI, prognostic nutritional index; HR, hazard ratio.
Among all variables, WHR, albumin, PRO and PNI were found to be significantly associated with survival, with the following HR and P value, respectively: 3.54 (95% CI: 1.38 to 9.10), P=0.009; 2.08 (95% CI: 1.07 to 4.05), P=0.03; 2.96 (95% CI: 1.43 to 6.12), P=0.003; 5.28 (95% CI: 2.34 to 11.92), P=0.0001.
The four markers found to be significant at the univariate analysis were assessed in a multivariate Cox regression model. In the multivariate analysis only WHR and PNI retained the statistical significance as independent predictors for OS (HR 3.75, 95% CI: 1.44–9.72, P=0.007 and HR 8.13, 95% CI: 1.39–47.48, P=0.02, respectively) and were used to build the NUTRIICI (Table 2).
NUTRIICI and survival analysis
By combining WHR and PNI, we assessed a potential nutritional prognostic index predictive of survival for mGOJ/GC patients treated with second-line ICI (NUTRIICI).
Patients were divided into three groups: patients with both favorable factors, i.e., WHR ≥1 and PNI >33 (8 patients); patients with one unfavorable factor, either WHR <1 or PNI ≤33 (40 patients); patients with both unfavorable factors, i.e., PNI ≤33 and WHR <1 (9 patients). Distinct survival probabilities were obtained for these three patient subgroups with median OS of 18.0, 6.7 and 1.3 months, respectively, overall P value <0.0001 (Figure 1).
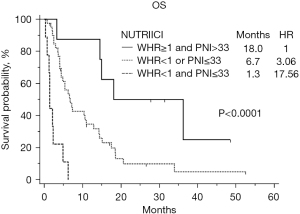
Taking as reference patients with both favorable factors, HR was 3.06 (95% CI: 1.17 to 7.98), P=0.02 and 17.56 (95% CI: 5.25 to 58.69), P<0.0001, for patients with one and two unfavorable factors, respectively.
Assessment of nutritional markers and NUTRIICI in the chemotherapy-treated control cohort
In the chemotherapy control cohort, 57 patients (14 females, 43 males) with mGOJ/GC were included. Median age was 61 years (range, 33–87) and 51 patients reached the OS endpoint. Median follow-up of censored patients was 12.3 months (4 to 18.2 months). Patients had lymph node metastasis (with or without other sites of metastasis) in 70% of cases, 30% of patients had non-lymph node metastasis, mainly in the liver and peritoneum. All the patients received previous treatment with platinum-based and fluoropyrimidine-based chemotherapy regimens, including regimens with anthracycline [7 (12%)]. No statistically significant difference was observed in OS between patients treated with doublet vs. triplet first-line regimens (P=0.06). Trastuzumab was administered in HER2 positive cases [11 (19,1%)]. The second-line regimen was paclitaxel-ramucirumab for 19 patients and fluoropirimidine-based doublet for 23 patients. Fifteen patients received other chemotherapy regimens. mOS in this cohort was 6.4 months (95% CI: 0.1 to 22.4 months), radiological response rate with second-line chemotherapy was 12%.
By applying the NUTRIICI in the control cohort, 8 patients had both favorable factors, 31 one unfavorable factor, and 18 both unfavorable factors. No differences were observed in terms of survival according to the three NUTRIICI subgroups in the chemotherapy cohort (P=0.57), thus suggesting that NUTRIICI was specific for patients treated with ICI (Figure 2).

Looking at the variable category frequencies within the chemotherapy cohort, we did not find any significant difference as compared to the ICI cohort, chi-square P values ranging from 0.06 to 0.57 (Table 3).
Table 3
| Characteristic | Range or n (%) | Difference with the ICI cohort (chi-square test), P value |
|---|---|---|
| Height (m) | 0.45 | |
| Range | 1.55–1.80 | |
| <1.75 | 26 (46%) | |
| ≥1.75 | 31 (54%) | |
| BMI (kg/m2) | 0.08 | |
| Range | 12.6–34.6 | |
| <30 | 55 (96%) | |
| ≥30 | 2 (4%) | |
| WHR | 0.41 | |
| Range | 0.70–1.60 | |
| <1 | 48 (84%) | |
| ≥1 | 9 (16%) | |
| Sagittal diameter (cm) | 0.23 | |
| Range | 14.5–28.7 | |
| <25 | 49 (86%) | |
| ≥25 | 8 (14%) | |
| Lymphocytes (×109/L) | 0.43 | |
| Range | 0.2–3.7 | |
| <1 | 22 (39%) | |
| ≥1 | 35 (61%) | |
| Glucose (mmol/L) | 0.09 | |
| Range | 3.5–10.6 | |
| <5 | 19 (33%) | |
| ≥5 | 38 (67%) | |
| Albumin (g/dL) | 0.20 | |
| Range | 20–44 | |
| <35 | 18 (32%) | |
| ≥35 | 39 (68%) | |
| PRO (g/dL) | 0.06 | |
| Range | 33–74 | |
| <60 | 21 (37%) | |
| ≥60 | 36 (63%) | |
| AGR | 0.57 | |
| Range | 0.4–3.6 | |
| <1.5 | 26 (46%) | |
| ≥1.5 | 31 (54%) | |
| Onodera’s PNI | 0.06 | |
| Range | 20.0–44.1 | |
| ≤33 | 19 (33%) | |
| >33 | 38 (67%) |
mGOJ/GC, metastatic gastro-oesophageal junction/gastric cancer; ICI, immune-checkpoint inhibitor; BMI, body mass index; WHR, waist-to-hip ratio; PRO, total serum proteins; AGR, albumin to globulin ratio; PNI, prognostic nutritional index.
At the univariate Cox regression analysis for OS in the chemotherapy cohort, only the total serum protein level was found to be significantly associated with survival: HR 2.23 (95% CI: 1.24 to 4.00), P=0.007, taking PRO >60 gr/dL as reference.
Discussion
Immune-checkpoint inhibitors have changed the prognosis and the treatment strategy for many cancer patients. However, there are still great challenges for clinicians to identify predictors of ICI efficacy, in particular as clinical or laboratory biomarkers.
Several real-world data of the prognostic factors in mGOJ/GC patients treated with ICI have been published in the last years. Sato et al. recently analyzed a large cohort of more than 200 Japanese heavily pretreated GC patients receiving Nivolumab (21). They found that C‐reactive protein (CRP) <0.5 mf/dL, immune‐related adverse events, albumin >3.5 g/dL, performance status 0, lymphocyte count >1,000/µL, and differentiated pathological type were independently associated with improved survival in multivariate analysis. Moreover, by dividing patients in 3 groups, according to the presence of 0 to 6 risk factors at baseline, different OS were obtained (P<0.001).
The nutritional status is one of the most important clinical aspects for GOJ/GC patients. It is regularly assessed during the treatment of these patients; nevertheless, little is known about the impact of the nutritional status on the efficacy of immunotherapy. Moreover, only a few small studies have evaluated nutritional indexes in standard chemotherapy-treated mGOJ/GC patients, with these indexes being mostly based on inflammation parameters (i.e., CRP), all of them in pan-Asian cohorts (21-24).
We analyzed 10 nutritional measures in a well selected set of mGOJ/GC patients treated with second-line ICI monotherapy and found that high Onodera’s PNI (>33) and WHR (>1) were the most significant nutritional predictors of better survival. These two variables were used to build the NUTRIICI.
Onodera’s PNI is a traditional ‘immune-nutritional’ parameter, initially used to predict prognosis in patients undergoing surgery for gastrointestinal cancer (19). It combines the serum albumin, a proxy of nutritional status and a marker of stem-cell differentiation and apoptosis (25-28), and the total lymphocyte count, that is known to be positively associated with response to chemotherapy and survival in mGOJ/GC (29,30). Hypoalbuminemia especially is commonly studied alone (21) or in combination with other lab values as neutrophil-to-lymphocyte ratio, CRP (31), serum lactate dehydrogenase (32) or total cholesterol level (33) as a negative predictor of treatment outcome in mGOJ/GC patients treated with anti-PD-1 agents. In our work, albumin level confirmed its prognostic value in univariate but not in multivariate analysis, while PNI retained significance value at P<0.05.
Namikawa et al., have previously analyzed the role of PNI in a small retrospective cohort of 27 Asian mGC patients treated with the anti-PD-1 agent nivolumab (34). They conducted a simple univariate analysis and found that higher PNI, together with lower neutrophil-lymphocyte ratio (NLR), higher Glasgow Prognostic Score (GPS) and occurrence of Immune-related adverse events (irAEs), were predictive of increased radiological response rate and longer survival (34). Median baseline PNI reported in this series was comparable to the that found in our cohort (32.1 vs. 33). PNI of 33 can thus be considered an adequate cut-off value in this setting.
Our results, besides underlining the importance of nutritional status and systemic inflammation as measured by PNI, also highlight the relationship between visceral adiposity and ICI efficacy.
We assessed for the first time in ICI-treated mGOJ/GC patients the anthropometric parameter WHR, which is a measure of abdominal obesity originally tested as prognostic marker in patients with heart failure and other cardiovascular diseases (35).
In patients with cancer, WHR has been previously evaluated in relation to the risk of developing gastric, breast, colorectal and prostate cancer, but data on its role as prognostic and predictive marker in patients with advanced disease are limited (36-40). In our study we found no significant prognostic role of WHR in the cohort of patients treated with chemotherapy (P=0.81), while demonstrating a significant association with survival in both the univariate and multivariate analysis in the ICI-treated cohort. To the best of our knowledge, this is first time that a visceral adiposity marker is found to be predictive of survival in ICI-treated patients. Previous studies have focused on the role of classic BMI as a parameter of obesity. In ICI-treated melanoma and kidney cancer patients a correlation between BMI and progression free survival and overall survival has been found in retrospective series (40-49), however BMI is considered inadequate to capture the overall complexity of obesity (50).
BMI is suboptimal as a measure of visceral and subcutaneous adipose tissue, since it does not take into account the whole-body composition made by fluids, muscle and lean masses. Contrary to WHR, BMI was not found to correlate with survival in our analysis (P=0.09), thus suggesting that markers that more precisely recapitulate the abundance of visceral adipose tissue have a superior performance in terms of survival prediction in ICI-treated patients.
The combined analysis of Onodera’s PNI and WHR was used to build a new prognostic nutritional tool (NUTRIICI), that was demonstrated to be ICI-specific in mGOJ/GC (P=0.57 in the chemotherapy cohort) and with very high statistical significance (P<0.0001) (23,51).
Nonetheless, we acknowledge a number of limitations in our study. Above all the limitations is the retrospective design of the study. Moreover, data on concomitant medications or other medical conditions, that are known to possibly influence both the inflammation and the nutritional status, were not part of the analysis. Furthermore, the analysis was conducted on a relatively small sample size with unknown PD-L1 status. PD-L1 is a widely recognized tissue marker in ICI-treated patients, its integration in the analysis would have clarified the role of NUTRIICI in relation to established tissue immune variables. Finally, WHR was manually derived from available CT scans by gating waist and hip circumferences. An automatic measurement of WHR would be desirable to minimize operator-dependent errors in the analytic process.
In conclusion, NUTRIICI is the first ICI-specific nutritional index for mGOJ/GC patients. NUTRIICI has confirmed the strength of Onodera’s PNI as a prognostic marker but also underlined the importance of WHR as a measure of visceral adipose tissue volume. A prospective validation of NUTRIICI is strongly warranted.
Acknowledgments
The present study was carried out within the PhD Programme on Experimental System and Medicine (XXXV cycle) at the University of Rome Tor Vergata. The abstract has been presented at AIOM meeting, Rome, IT, October 30 - November 1, 2020.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-217/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-217/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-217/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the Sarah Cannon Research Hospital and “Tor Vergata” University Hospital as part of protocol investigating the role of BMI in solid tumors (No. NCT03873064) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Deans DA, Tan BH, Wigmore SJ, et al. The influence of systemic inflammation, dietary intake and stage of disease on rate of weight loss in patients with gastro-oesophageal cancer. Br J Cancer 2009;100:63-9. [Crossref] [PubMed]
- Ravasco P, Monteiro-Grillo I, Vidal PM, et al. Cancer: disease and nutrition are key determinants of patients' quality of life. Support Care Cancer 2004;12:246-52. [Crossref] [PubMed]
- Suzuki H, Asakawa A, Amitani H, et al. Cancer cachexia--pathophysiology and management. J Gastroenterol 2013;48:574-94. [Crossref] [PubMed]
- Tong H, Isenring E, Yates P. The prevalence of nutrition impact symptoms and their relationship to quality of life and clinical outcomes in medical oncology patients. Support Care Cancer 2009;17:83-90. [Crossref] [PubMed]
- Shahmoradi N, Kandiah M, Peng LS. Impact of nutritional status on the quality of life of advanced cancer patients in hospice home care. Asian Pac J Cancer Prev 2009;10:1003-09. [PubMed]
- Van Cutsem E, Arends J. The causes and consequences of cancer-associated malnutrition. Eur J Oncol Nurs 2005;9:S51-63. [Crossref] [PubMed]
- Arends J. Struggling with nutrition in patients with advanced cancer: nutrition and nourishment-focusing on metabolism and supportive care. Ann Oncol 2018;29:ii27-34. [Crossref]
- Liu X, Qiu H, Kong P, et al. Gastric cancer, nutritional status, and outcome. Onco Targets Ther 2017;10:2107-14. [Crossref] [PubMed]
- Liu X, Wu Z, Lin E, et al. Systemic prognostic score and nomogram based on inflammatory, nutritional and tumor markers predict cancer-specific survival in stage II-III gastric cancer patients with adjuvant chemotherapy. Clin Nutr 2019;38:1853-60. [Crossref] [PubMed]
- Oh SE, Choi MG, Seo JM, et al. Prognostic significance of perioperative nutritional parameters in patients with gastric cancer. Clin Nutr 2019;38:870-6. [Crossref] [PubMed]
- Nie R, Yuan S, Chen S, et al. Prognostic nutritional index is an independent prognostic factor for gastric cancer patients with peritoneal dissemination. Chin J Cancer Res 2016;28:570-8. [Crossref] [PubMed]
- Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;390:2461-71. [Crossref] [PubMed]
- Fuchs CS, Doi T, Jang RW, et al. Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol 2018;4:e180013. Correction in JAMA Oncol 2019;5:579. [Crossref] [PubMed]
- Joshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer. CA Cancer J Clin 2021;71:264-79. [Crossref] [PubMed]
- Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 2021;398:27-40. [Crossref] [PubMed]
- Wang Z, Aguilar EG, Luna JI, et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat Med 2019;25:141-51. [Crossref] [PubMed]
- Lysaght J. The 'obesity paradox' in action with cancer immunotherapy. Nat Rev Endocrinol 2019;15:132-3. [Crossref] [PubMed]
- Murphy WJ, Longo DL. The Surprisingly Positive Association Between Obesity and Cancer Immunotherapy Efficacy. JAMA 2019;321:1247-8. [Crossref] [PubMed]
- Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi 1984;85:1001-5. [PubMed]
- Schulgen G, Lausen B, Olsen JH, et al. Outcome-oriented cutpoints in analysis of quantitative exposures. Am J Epidemiol 1994;140:172-84. Correction in Am J Epidemiol 1995;141:792. [Crossref] [PubMed]
- Sato S, Oshima Y, Matsumoto Y, et al. The new prognostic score for unresectable or recurrent gastric cancer treated with nivolumab: A multi-institutional cohort study. Ann Gastroenterol Surg 2021;5:794-803. [Crossref] [PubMed]
- Shoji F, Takeoka H, Kozuma Y, et al. Pretreatment prognostic nutritional index as a novel biomarker in non-small cell lung cancer patients treated with immune checkpoint inhibitors. Lung Cancer 2019;136:45-51. [Crossref] [PubMed]
- Ohba T, Takamori S, Toyozawa R, et al. Prognostic impact of the Controlling Nutritional Status score in patients with non-small cell lung cancer treated with pembrolizumab. J Thorac Dis 2019;11:3757-68. [Crossref] [PubMed]
- Coss CC, Clinton SK, Phelps MA. Cachectic Cancer Patients: Immune to Checkpoint Inhibitor Therapy? Clin Cancer Res 2018;24:5787-9. [Crossref] [PubMed]
- McMillan DC, Watson WS, O'Gorman P, et al. Albumin concentrations are primarily determined by the body cell mass and the systemic inflammatory response in cancer patients with weight loss. Nutr Cancer 2001;39:210-3. [Crossref] [PubMed]
- Seaton K. Albumin concentration controls cancer. J Natl Med Assoc 2001;93:490-3. [PubMed]
- Yamanaka H, Nishi M, Kanemaki T, et al. Preoperative nutritional assessment to predict postoperative complication in gastric cancer patients. JPEN J Parenter Enteral Nutr 1989;13:286-91. [Crossref] [PubMed]
- China L, Freemantle N, Forrest E, et al. A Randomized Trial of Albumin Infusions in Hospitalized Patients with Cirrhosis. N Engl J Med 2021;384:808-17. [Crossref] [PubMed]
- Bruckner HW, Lavin PT, Plaxe SC, et al. Absolute granulocyte, lymphocyte, and moncyte counts. Useful determinants of prognosis for patients with metastatic cancer of the stomach. JAMA 1982;247:1004-6. [Crossref] [PubMed]
- Gooden MJ, de Bock GH, Leffers N, et al. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer 2011;105:93-103. [Crossref] [PubMed]
- Formica V, Morelli C, Patrikidou A, et al. Gastric Inflammatory Prognostic Index (GIPI) in Patients with Metastatic Gastro-Esophageal Junction/Gastric Cancer Treated with PD-1/PD-L1 Immune Checkpoint Inhibitors. Target Oncol 2020;15:327-36. [Crossref] [PubMed]
- Nakazawa N, Sohda M, Yamaguchi A, et al. An Elevated Serum Lactate Dehydrogenase-to-albumin Ratio Is a Useful Poor Prognostic Predictor of Nivolumab in Patients With Gastric Cancer. Anticancer Res 2021;41:3925-31. [Crossref] [PubMed]
- Chen L, Sun H, Zhao R, et al. Controlling Nutritional Status (CONUT) Predicts Survival in Gastric Cancer Patients With Immune Checkpoint Inhibitor (PD-1/PD-L1) Outcomes. Front Pharmacol 2022;13:836958. [Crossref] [PubMed]
- Namikawa T, Yokota K, Tanioka N, et al. Systemic inflammatory response and nutritional biomarkers as predictors of nivolumab efficacy for gastric cancer. Surg Today 2020;50:1486-95. [Crossref] [PubMed]
- Streng KW, Voors AA, Hillege HL, et al. Waist-to-hip ratio and mortality in heart failure. Eur J Heart Fail 2018;20:1269-77. [Crossref] [PubMed]
- Sanikini H, Muller DC, Sophiea M, et al. Anthropometric and reproductive factors and risk of esophageal and gastric cancer by subtype and subsite: Results from the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Int J Cancer 2020;146:929-42. [Crossref] [PubMed]
- Du X, Hidayat K, Shi BM. Abdominal obesity and gastroesophageal cancer risk: systematic review and meta-analysis of prospective studies. Biosci Rep 2017;37:BSR20160474. [Crossref] [PubMed]
- Huang Z, Willett WC, Colditz GA, et al. Waist circumference, waist:hip ratio, and risk of breast cancer in the Nurses' Health Study. Am J Epidemiol 1999;150:1316-24. [Crossref] [PubMed]
- Boehm K, Sun M, Larcher A, et al. Waist circumference, waist-hip ratio, body mass index, and prostate cancer risk: results from the North-American case-control study Prostate Cancer & Environment Study. Urol Oncol 2015;33:494.e1-7. [Crossref] [PubMed]
- Dong Y, Zhou J, Zhu Y, et al. Abdominal obesity and colorectal cancer risk: systematic review and meta-analysis of prospective studies. Biosci Rep 2017;37:BSR20170945. [Crossref] [PubMed]
- Cortellini A, Bersanelli M, Buti S, et al. A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: when overweight becomes favorable. J Immunother Cancer 2019;7:57. [Crossref] [PubMed]
- McQuade JL, Daniel CR, Hess KR, et al. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol 2018;19:310-22. [Crossref] [PubMed]
- De Giorgi U, Procopio G, Giannarelli D, et al. Association of Systemic Inflammation Index and Body Mass Index with Survival in Patients with Renal Cell Cancer Treated with Nivolumab. Clin Cancer Res 2019;25:3839-46. [Crossref] [PubMed]
- Richtig G, Hoeller C, Wolf M, et al. Body mass index may predict the response to ipilimumab in metastatic melanoma: An observational multi-centre study. PLoS One 2018;13:e0204729. [Crossref] [PubMed]
- Naik GS, Waikar SS, Johnson AEW, et al. Complex inter-relationship of body mass index, gender and serum creatinine on survival: exploring the obesity paradox in melanoma patients treated with checkpoint inhibition. J Immunother Cancer 2019;7:89. [Crossref] [PubMed]
- Donnelly DA, Bajaj S, Zhong J, et al. The relationship between obesity and immunotherapy: It's complicated. J Clin Oncol 2019;37:9562. [Crossref]
- Renfro LA, Loupakis F, Adams RA, et al. Body Mass Index Is Prognostic in Metastatic Colorectal Cancer: Pooled Analysis of Patients From First-Line Clinical Trials in the ARCAD Database. J Clin Oncol 2016;34:144-50. [Crossref] [PubMed]
- Lalani AA, Xie W, Flippot R, et al. Impact of body mass index (BMI) on treatment outcomes to immune checkpoint blockade (ICB) in metastatic renal cell carcinoma (mRCC). J Clin Oncol 2019;37:566. [Crossref]
- Rogado J, Romero-Laorden N, Sanchez-Torres JM, et al. Effect of excess weight and immune-related adverse events on the efficacy of cancer immunotherapy with anti-PD-1 antibodies. Oncoimmunology 2020;9:1751548. [Crossref] [PubMed]
- Lee CS, Devoe CE, Zhu X, et al. Pretreatment nutritional status and response to checkpoint inhibitors in lung cancer. Lung Cancer Manag 2020;9:LMT31. [Crossref] [PubMed]
- Elghiaty A, Kim J, Jang WS, et al. Preoperative controlling nutritional status (CONUT) score as a novel immune-nutritional predictor of survival in non-metastatic clear cell renal cell carcinoma of ≤ 7 cm on preoperative imaging. J Cancer Res Clin Oncol 2019;145:957-65. [Crossref] [PubMed]


