
Utility and specificity of plasma heat shock protein 90 alpha, CEA, and CA199 as the diagnostic test in colorectal cancer liver metastasis
Introduction
Colorectal cancer (CRC) is one of the most common types of malignant tumor, with a global incidence of 10%, and is the 2nd highest cause of cancer-related mortality (1). As most CRC patients are asymptomatic at an early stage, CRC is under-treated. Distant metastasis is highly prevalent in CRC and is a major cause of CRC-related mortality (2,3). Previous research has reported that the liver is the most frequent anatomic site for metastasis from CRC, due to its proximity to the colorectum (4). Metastatic CRC (mCRC) patients have benefited from novel targeted and chemo-biologic therapies, but preventing the further progression of the disease depends on an even more detailed delineation of the molecular mCRC sub-types that are responsive to specific, targeted therapies (5). Thus, the timely screening of mCRC is important in the prognosis of CRC patients.
The main methods of diagnosing CRC, including imaging diagnosis, endoscopy, and pathology diagnosis, have been widely used in clinical settings (6). However, invasive examinations can both cause unwillingness in patients and complications. Additionally, preoperative chemotherapy or neo-adjuvant therapy may reduce CT scans’ sensitivity for monitoring metastasis in CRC patients (7). Thus, it is important to find better screening and prediction prognosis methods for mCRC patients.
The analysis of peripheral blood samples has been suggested as a sensitive and non-invasive method for biomarker determinations in screening cancer. Previous studies have indicated that carcinoembryonic antigen (CEA) and carbohydrate antigen199 (CA199) were significantly elevated in mCRC patients (8,9). In addition to the CEA and/or the CA199, the heat shock protein 90 alpha (Hsp90α) has also been reported to be linked to tumors. Hsp90, which includes the Hsp90α and Hsp90β isoforms, is exposed to the extracellular space by many kinds of cancer cells (10). Studies have reported that the overexpression of HSP90α is associated with tumor development, progression, invasiveness, metastatic potentials, and chemo-resistance in various types of cancers, such as CRC, liver cancer, and gastric cancer (11-13). Further, the levels of plasma Hsp90α have been shown to be associated with gender and disease progression, such as stage, and lymphatic and distant metastasis (14). A previous study has shown that Hsp90α and the combination of Hsp90α and CEA are optimal biomarkers for CRCs at an early stage (11). However, the diagnostic and prognostic utility of serum Hsp90α in mCRC, particularly liver metastasis, is still unclear. Thus, this study sought to investigate the plasma Hsp90α levels in patients with CRC and explore the clinical significance of Hsp90α and combinations of Hsp90α and CEA/CA199 and their diagnostic utility in mCRC. We present the following article in accordance with the STARD reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-797/rc).
Methods
Patients
We conducted a single-center retrospective observational study of patients with CRC. A total of 472 patients were admitted between 2020 January and 2020 December at The First Affiliated Hospital of University of Science and Technology of China (USTC) were enrolled in this study. To be eligible for inclusion in this study, subjects had to meet the follow inclusion criteria: (I) have CRC confirmed by clinical manifestations and a histopathological examination associated with an imaging diagnosis; (II) have an assessment of the state of distant metastasis; (III) have undergone no prior systemic treatments, including targeted therapy, immunotherapy, chemotherapy, and biological therapy; (IV) have had the tumor markers and Hsp90α in the peripheral blood evaluated before any treatment; (V) have available complete information of the clinical features; and (VI) have no other malignancies. The clinical data were obtained from the patients’ files, including age, sex, clinical stage, pathological differentiated degree, lymph node metastasis condition, neuro and vessel metastasis condition, liver metastasis condition, gene mutation, serum CEA, CA199 and Hsp90α level. All patients underwent tumour staging according to the American Joint Committee on Cancer Classification (8th edition). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of The First Affiliated Hospital of USTC (No. 2022-RE-304). The ethics committee of The First Affiliated Hospital of USTC waived the requirement for written informed consent since this was a retrospective study, it could not cause any adverse effects on included patients.
Measurements of serum markers
All the laboratory markers were analyzed in the laboratory at The First Affiliated Hospital of USTC. The circulating levels of CEA and CA199 were analyzed with electrochemiluminescence immunoassays (ECLIAs) using the Cobas 8000 e602 modular analyzer series (Hoffmann-La Roche AG, Basel, Switzerland). Hps90α was analyzed with the enzyme-linked immunoassay (ELISA) kit for Hsp90α protein (Yantai Protgen Biotechnology Development Co., Ltd., Yantai, China). All the operations were conducted in accordance with the manufacturer’s instructions.
Gene mutation
Paraffin-embedded sections were used to extract deoxyribonucleic acid, and real-time fluorescent quantitative polymerase chain reaction technology was employed for amplification detection. Next-generation sequencing (NGS) was used to analyze 17 mutations in the Kirsten rat sarcoma 2 viral oncogene homolog (KRAS) gene (exons 2, 3, and 4), 13 mutations in the neuroblastoma rat sarcoma viral oncogene homolog (NRAS) gene (exons 2, 3, and 4), and 1 mutation in the v-raf murine sarcoma viral oncogene homolog B1 (BRAF) gene (exon 15: V600 E) (15).
Statistical analysis
Patients’ general characteristics, clinical parameters, and levels of serum markers are presented as the mean [standard deviation (SD)] or number (%) as appropriate. Student’s t-tests and chi-square tests were used to compare differences in means and proportions between CRC patients with and without liver metastasis. Odds ratios (ORs) were calculated for the associations between plasma levels of CEA, CA199, and Hsp90α, and the presence of liver metastasis in CRC was computed using logistic regressions with adjustments for age, sex, and stage. Linear regression was used to analyze associations between the plasma levels of CEA, CA199, mutant genes, and Hsp90α. Receiver operating characteristic curves were used to estimate and compare the utility of serum makers in identifying liver metastasis. Additionally, we compared the diagnostic utility of the models, including the Hsp90α plus 1 of the other serum markers (i.e., CEA and CA199), and a combination of the 3 serum makers. For the combinations for biomarker, the subtype of diagnostic test was in series. A 2-tailed P value <0.05 was considered statistically significant. All the analyses were performed using Stata V.17.0 (StataCorp LP). Uncertain or missing data were not included in the analyses.
Results
This study included 472 CRC patients (35% female) with a mean age 59 years (range, 21–88 years). Of them, 49% were aged ≥60 years, 98% had a poorly to moderately pathological differentiated degree. Of the CRC patients, 37% had liver metastasis. The levels of CEA, CA199, and Hsp90α were 62 ng/mL, 106 U/mL, and 81 ng/mL, respectively (Table 1).
Table 1
| Characteristics | CRC (N=472) |
|---|---|
| Age (years) | 59 [12] |
| Age ≥60 years, n [%] | 233 [49] |
| Sex (female), n [%] | 167 [35] |
| Stage (N=466), n [%] | |
| I | 4 [1] |
| II | 73 [16] |
| III | 116 [25] |
| IV | 273 [58] |
| Differentiated degree (N=320), n [%] | |
| Poorly | 87 [27] |
| Moderately | 227 [71] |
| Highly | 6 [2] |
| Lymph node metastasis, n (present %) | 218 [46] |
| Neuro vessel metastasis, n (present %) | 165 [35] |
| Liver metastasis, n (present %) | 174 [37] |
| Gene mutation | |
| KRAS (N=220), n (present %) | 96 [44] |
| NRAS (N=220), n (present %) | 6 [3] |
| BRAF (N=213), n (present %) | 10 [5] |
| CEA (N=439) (ng/mL) | 62 [171] |
| CA199 (N=434) (U/mL) | 106 [262] |
| Heat shock protein 90 alpha (N=455) (ng/mL) | 81 [61] |
Values are presented as the mean [standard deviation] or percentage. CRC, colorectal cancer; KRAS, Kirsten rat sarcoma 2 viral oncogene homolog; NRAS, neuroblastoma RAS viral oncogene homolog; BRAF, v-raf murine sarcoma viral oncogene homolog B1; CEA, carcinoembryonic antigen; CA199, carbohydrate antigen199.
Table 2 shows the comparisons of the plasma levels of CEA, CA199, and Hsp90α between the CRC patients with and without liver metastasis. A higher proportion of CRC patients with liver metastasis had a IV stage than CRC patients with no liver metastasis. CRC patients with liver metastasis had higher plasma levels of CEA, CA199, and Hsp90α than CRC patients with non-liver metastasis.
Table 2
| Characteristics | Liver | |
|---|---|---|
| Metastasis (N=174) | Non-metastasis (N=298) | |
| Age (years) | 59 [11] | 60 [11] |
| Age group (≥60 years) (%) | 50 | 50 |
| Sex (female) (%) | 33 | 37 |
| Stage (I/II/III/IV) (%) | 0/0/0/100* | 1/25/40/34* |
| Differentiated degree (poorly/moderately/highly) (%) | 24/72/4 | 29/70/1 |
| KRAS (present %) | 41 | 47 |
| NRAS (present %) | 2 | 4 |
| BRAF (present %) | 7 | 2 |
| CEA (ng/mL) | 142.27 [257.01]* | 15.08 [44.30]* |
| CA199 (U/mL) | 216.30 [382.50]* | 40.87 [110.30]* |
| Hsp90α (ng/mL) | 91.79 [68.81]* | 75.03 [54.45]* |
Values are presented as the mean [standard deviation] or percentage. * indicates a statistically significant result. KRAS, Kirsten rat sarcoma 2 viral oncogene homolog; BRAF, v-raf murine sarcoma viral oncogene homolog B1; CA199, carbohydrate antigen199; CEA, carcinoembryonic antigen; Hsp90α, heat shock protein 90 alpha; NRAS, neuroblastoma RAS viral oncogene homolog.
Table 3 sets out the ORs for the plasma levels of CEA, CA199, and Hsp90α for liver metastasis. Higher plasma levels for CEA, CA199, and Hsp90α were associated with a higher prevalence of liver metastasis. The strongest association with liver metastasis was observed for the plasma level of CEA. These associations remained significant after adjustments for age, sex, and stage. Additionally, the plasma levels of CEA and CA199 were associated with the plasma level of Hsp90α (see Table 4).
Table 3
| Variable | Univariable | Multivariable | |||
|---|---|---|---|---|---|
| OR (95% CI) (N=439) | P value | OR (95% CI)* (N=439) | P value | ||
| CEA | 7.82 (3.75, 16.31)# | <0.001# | 2.72 (1.49, 4.98)# | 0.001# | |
| CA199 | 2.98 (1.93, 4.60)# | <0.001# | 1.60 (1.14, 2.25)# | 0.006# | |
| Hsp90α | 1.31 (1.08, 1.58)# | 0.005# | 1.36 (1.03, 1.80)# | 0.03# | |
The odds ratio (OR) is shown for 1 standard deviation increment in each plasma biomarker. *, adjustment for age, sex, and stage; #, indicates a statistically significant result. CA199, carbohydrate antigen199; CRC, colorectal cancer; CEA, carcinoembryonic antigen; CI, confidence interval; Hsp90α, heat shock protein 90 alpha.
Table 4
| Variable | Univariable | Multivariable | |||
|---|---|---|---|---|---|
| β (95% CI) (N=423) | P value | β (95% CI)† (N=422) | P value | ||
| CEA | 0.15 (0.05, 0.25)* | 0.004* | 0.16 (0.06, 0.26)* | 0.002* | |
| CA199 | 0.11 (0.008, 0.20)* | 0.04* | 0.10 (0.005, 0.20)* | 0.04* | |
| KRAS | –0.10 (–0.37, 0.17) | 0.45 | –0.11 (–0.38, 0.16) | 0.42 | |
| NRAS | –0.28 (–1.09, 0.53) | 0.49 | –0.24 (–1.05, 0.57) | 0.56 | |
| BRAF | –0.20 (–0.84, 0.44) | 0.53 | –0.22 –0.86, 0.41) | 0.49 | |
†, adjustment for age and sex; *, statistically significant. CI, confidence interval; Hsp90α, heat shock protein 90 alpha; CEA, carcinoembryonic antigen; CA199, carbohydrate antigen199; KRAS, Kirsten rat sarcoma 2 viral oncogene homolog; NRAS, neuroblastoma RAS viral oncogene homolog; BRAF, v-raf murine sarcoma viral oncogene homolog B1.
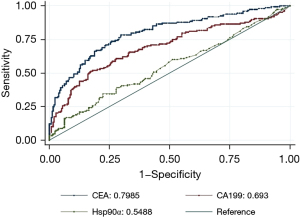
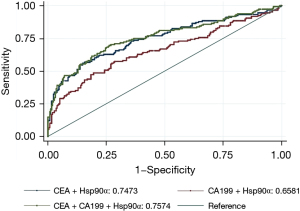
Figure 1 shows the AUCs for the plasma levels of CEA, CA199, and Hsp90α in identifying CRC patients with liver metastasis. In this study, thresholds of AUC for CEA, CA199, Hsp90α were 9.590 ng/mL, 35.880 U/mL, and 93.500 ng/mL, respectively. When used alone, CEA had the largest AUC among the 3 plasma markers. After combining Hsp90α with CEA and/or CA199, the AUC for the combination of Hsp90α, CEA, and CA199 was the largest among the combination models. However, adding the Hsp90α to the other plasma markers did not improve AUCs compared to the AUCs for the plasma markers alone (see Figure 2).
Discussion
This study found that the plasma levels of Hsp90α, CEA, and CA199 were positively associated with a higher risk of liver metastasis in CRC, which suggests that these plasma levels have value of in diagnosing CRC liver metastasis (CRLM). The level of CEA has the largest utility in diagnosing CRLMs. In addition, the Hsp90α level was associated with the CEA and CA199 levels. To our knowledge, this study is the first to investigate the relationship between the Hsp90α and CRLM. The results clearly showed that levels of Hsp90α, CEA and CA199 can predict CRLM. However, adding plasma Hsp90α to CEA/CA199 did not improve the diagnostic value for CRLMs.
In this study, the results showed that the plasma Hsp90α levels were significantly higher in CRC patients. Similar results were obtained in other gastrointestinal tumors, such as hepatocellular carcinoma (12) and gastric cancer (13). As a member of the Hsp90 family, Hsp90α is a vital chaperone protein conserved across all organisms (16). Hsp90α is a molecular chaperone that regulates the late-stage maturation, activation, and stability of many client proteins (17). These proteins play an essential role in cellular processes and regulatory pathways, such as apoptosis, cell cycle control, cell signaling, cell viability, protein folding, and degradation, as they interact with client proteins and co-chaperones (16). Further, it has been shown that Hsp90α is a vital promoter of oncogene dependence and that increased Hsp90α expression promotes the survival of cancer cells in adverse physiological and stress conditions (18). Thus, Hsp90α plays a key role in the pathogenesis and progression of cancers.
We also found that the plasma levels of CEA and CA199 were associated with the plasma level of Hsp90α. However, gene mutation was not associated with the plasma level of Hsp90α. Moreover, the CRC patients with liver metastasis had higher plasma levels of CEA, CA199, and Hsp90α than the non-liver metastasis CRC patients. CEA is the most important tumor marker for patients with CRLM. In patients with resectable CRLM, a preoperative CEA level >200 ng/mL may indicate a higher risk of recurrence and a poorer prognosis than a lower CEA level (19). Another tumor marker that is useful in the diagnosis of liver metastases is CA199. However, the role of CA199 in CRC patients is still controversial (20,21).
Liu et al. (20) evaluated 691 patients with CRC liver metastases, and found no statistical difference in the lymph node stage between the low-level preoperative CA199 group and the high-level preoperative CA199. Zhou et al. (22) confirmed that patients with a high CA199 level presented a significantly higher risk of total postoperative metastasis, lung metastasis, and abdominopelvic metastasis than those with a low level of CA199. However, they did not observe the same phenomena in liver metastasis. This is likely due to the data source. These studies only targeted specific categories of CRC patients. Conversely, our study enrolled patients who underwent various surgical treatments, patients within and beyond oligometastasis, and patients with or without recurrence.
A previous study showed that the level of plasma Hsp90α was associated with disease progression, including stage, lymphatic metastasis, and distant metastasis, in CRC patients (14). However the relationship between Hsp90α and liver metastasis of CRC had not been previously investigated, despite CRC being the most common primary cancer that metastasizes to the liver (23).
Against this background, we focused on the value of plasma Hsp90α and CRLMs. When used as a tumor marker in the auxiliary diagnosis of CRLMs, the AUC of Hsp90α was 0.5488, and the AUCs of CEA and CA199 were 0.7985 and 0.693, respectively. The results indicated that plasma Hsp90α could be used as an auxiliary diagnostic agent for CRLM, but its specificity was imperfect. After combining Hsp90α with CEA and/or CA199, the AUC for the combination of Hsp90α, CEA, and CA199 was the largest among the combination models. However, adding Hsp90α to the other plasma markers did not improve the AUCs compared to the AUC for the plasma markers alone. Wei et al. (14) demonstrated that the AUCs of CEA, CA199, and Hsp90α, and the panel for identifying the presence of distant metastasis in CRC patients were 0.776, 0.755, 0.690, and 0.819, respectively. We speculate that (I) plasma Hsp90α is actually secreted in ELISA-negative patients; and (II) the recognition ability of the antibody in the ELISA kit is compromised by the heterogeneity of the secreted Hsp90α (24). Basic research using western blotting and parallel reaction monitoring (PRM)-based quantitative proteomics has shown that partial false ELISA-negative patients secret high levels of plasma Hsp90α (24).
As far as we know, the current study included a larger number of participants than previous studies. As a result, it is possible that it has better statistical power. However, there are certain limitations to the current study that should be noted. First, this was a retrospective and single-center study, which resulted in selection bias. Second, in comparison to the CRC subjects, the number of control subjects was small, which could be one of the reasons for the discrepancy in results compared to those of earlier research. Third, the long-term prognosis of patients and the factors affecting survival analysis should be investigated in future. Finally, the plasma level of Hsp90α was only assessed by ELISAs, which may have led to false negatives. Thus, our results need to be further confirmed in large-scale prospective cohort studies.
In conclusion, the level of plasma Hsp90α was elevated in CRC and associated with liver metastasis in CRC. Thus, Hsp90α is a potential biomarker for liver metastasis in CRC. CEA has the largest utility in diagnosing liver metastasis in CRC. Additionally, adding plasma Hsp90α to CEA/CA199 did not improve the utility of these biomarkers.
Acknowledgments
Funding: This work was supported by the Anhui Provincial Natural Science Foundation of China (No. 2008085MH299), the Key Research and Development Program of Anhui Province, China (No. 2022e07020012), the Fundamental Research Funds for the Central Universities (No. WK9110000086), and the Postdoctoral Research Fund of Anhui Province in 2019 (No. 2019B371).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-797/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-797/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-797/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of The First Affiliated Hospital of USTC (No. 2022-RE-304). The ethics committee of The First Affiliated Hospital of USTC waived the requirement for written informed consent since this was a retrospective study, it could not cause any adverse effects on included patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Angelsen JH, Horn A, Sorbye H, et al. Population-based study on resection rates and survival in patients with colorectal liver metastasis in Norway. Br J Surg 2017;104:580-9. [Crossref] [PubMed]
- Zhou J, Zhao R, Wen F, et al. Economic evaluation study (CHEER-compliant): Cost-effectiveness analysis of RAS screening for treatment of metastatic colorectal cancer based on the CALGB 80405 trial. Medicine (Baltimore) 2016;95:e3762. [Crossref] [PubMed]
- Wang J, Li S, Liu Y, et al. Metastatic patterns and survival outcomes in patients with stage IV colon cancer: A population-based analysis. Cancer Med 2020;9:361-73. [Crossref] [PubMed]
- Gmeiner WH. Recent Advances in Our Knowledge of mCRC Tumor Biology and Genetics: A Focus on Targeted Therapy Development. Onco Targets Ther 2021;14:2121-30. [Crossref] [PubMed]
- Liang F, Wang S, Zhang K, et al. Development of artificial intelligence technology in diagnosis, treatment, and prognosis of colorectal cancer. World J Gastrointest Oncol 2022;14:124-52. [Crossref] [PubMed]
- Rojas Llimpe FL, Di Fabio F, Ercolani G, et al. Imaging in resectable colorectal liver metastasis patients with or without preoperative chemotherapy: results of the PROMETEO-01 study. Br J Cancer 2014;111:667-73. [Crossref] [PubMed]
- Loosen SH, Roderburg C, Alizai PH, et al. Comparative Analysis of Circulating Biomarkers for Patients Undergoing Resection of Colorectal Liver Metastases. Diagnostics (Basel) 2021;11:1999. [Crossref] [PubMed]
- Hamfjord J, Guren TK, Glimelius B, et al. Clinicopathological factors associated with tumour-specific mutation detection in plasma of patients with RAS-mutated or BRAF-mutated metastatic colorectal cancer. Int J Cancer 2021;149:1385-97. [Crossref] [PubMed]
- Eustace BK, Sakurai T, Stewart JK, et al. Functional proteomic screens reveal an essential extracellular role for hsp90 alpha in cancer cell invasiveness. Nat Cell Biol 2004;6:507-14. [Crossref] [PubMed]
- Kasanga M, Liu L, Xue L, et al. Plasma heat shock protein 90-alpha have an advantage in diagnosis of colorectal cancer at early stage. Biomark Med 2018;12:881-90. [Crossref] [PubMed]
- Wei W, Liu M, Ning S, et al. Diagnostic value of plasma HSP90α levels for detection of hepatocellular carcinoma. BMC Cancer 2020;20:6. [Crossref] [PubMed]
- Liang XQ, Li KZ, Li Z, et al. Diagnostic and prognostic value of plasma heat shock protein 90alpha in gastric cancer. Int Immunopharmacol 2021;90:107145. [Crossref] [PubMed]
- Wei W, Zhou J, Chen L, et al. Plasma Levels of Heat Shock Protein 90 Alpha Associated With Colorectal Cancer Development. Front Mol Biosci 2021;8:684836. [Crossref] [PubMed]
- He P, Zou Y, Qiu J, et al. Pretreatment 18F-FDG PET/CT Imaging Predicts the KRAS/NRAS/BRAF Gene Mutational Status in Colorectal Cancer. J Oncol 2021;2021:6687291. [Crossref] [PubMed]
- Birbo B, Madu EE, Madu CO, et al. Role of HSP90 in Cancer. Int J Mol Sci 2021;22:10317. [Crossref] [PubMed]
- Pearl LH, Prodromou C, Workman P. The Hsp90 molecular chaperone: an open and shut case for treatment. Biochem J 2008;410:439-53. [Crossref] [PubMed]
- Jafari A, Rezaei-Tavirani M, Farhadihosseinabadi B, et al. HSP90 and Co-chaperones: Impact on Tumor Progression and Prospects for Molecular-Targeted Cancer Therapy. Cancer Invest 2020;38:310-28. [Crossref] [PubMed]
- Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999;230:309-18; discussion 318-21. [Crossref] [PubMed]
- Liu JM, Wang YY, Liu W, et al. Preoperative CA19-9: a competitive predictor of recurrence in patients with colorectal cancer liver metastases after hepatectomy. Int J Colorectal Dis 2021;36:767-78. [Crossref] [PubMed]
- Morita S, Nomura T, Fukushima Y, et al. Does serum CA19-9 play a practical role in the management of patients with colorectal cancer? Dis Colon Rectum 2004;47:227-32. [Crossref] [PubMed]
- Zhou W, Yang F, Peng J, et al. High pretreatment serum CA19-9 level predicts a poor prognosis for patients with stage III colon cancer after curative resection and adjuvant chemotherapy. J Cancer 2019;10:3810-8. [Crossref] [PubMed]
- Tsilimigras DI, Brodt P, Clavien PA, et al. Liver metastases. Nat Rev Dis Primers 2021;7:27. [Crossref] [PubMed]
- Liu W, Li J, Zhang P, et al. A novel pan-cancer biomarker plasma heat shock protein 90alpha and its diagnosis determinants in clinic. Cancer Sci 2019;110:2941-59. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


