
Three-target treatment combined with surgery for BRAF V600E-mutant colon cancer with peritoneal metastasis: a case report
Introduction
The BRAF gene is a critical proto-oncogene in humans (1), with V600E the most common mutation of this gene. BRAF V600E mutation is present in a variety of tumors (2-4), and 3–15% of patients with advanced colorectal cancer (CRC) carry this mutation (5). The occurrence of CRC is associated with a high incidence of mutations in the mitogen-activated protein kinase (MAPK) pathway, and BRAF is a vital link in the MAPK pathway. BRAF mutation leads to MAPK pathway activation, thus driving the proliferation, growth, and differentiation of tumor cells and the promotion of tumor growth (6). Patients with metastatic CRC (mCRC) and BRAF V600E mutation are characterized by a low probability of long-term survival, a high incidence of lymph node and peritoneal metastases, and a poor prognosis (7).
Currently, BRAF inhibitors (such as vemurafenib) can achieve good results in patients with BRAF V600E-mutant melanoma, but not in CRC patients (8). Triple chemotherapy + bevacizumab is regarded as the level I recommendation for CRC in the Chinese Society of Clinical Oncology (CSCO) guideline, but a meta-analysis (9) showed the overall survival (OS) of patients with BRAF mutation was not significantly different between triple chemotherapy + bevacizumab and doublet chemotherapy + bevacizumab in the first-line treatment of mCRC (HR: 1.11, 95% CI: 0.75–1.73). Furthermore, regardless of the primary tumor location, triple chemotherapy had no advantage, but caused unbearable side effects. Therefore, first-line treatment for such populations in China, especially patients over 60 years old, is mainly doublets + bevacizumab.
The MAPK signaling pathway is one of the most crucial pathways in the epidermal growth factor receptor (EGFR) signaling pathway (10,11). In the transduction, RAS is located upstream while BRAF and MEK are located downstream. These 3 are all key protein kinases in pathway regulation, which can be activated through different molecular signals and transmit upstream signals to downstream response molecules through stage-by-stage phosphorylation. Finally, extracellular stimulation signals are transmitted into the nucleus, resulting in biological behaviors such as proliferation, differentiation, transformation, and apoptosis of cells (12,13). Since RAS is not mutated, upstream EGFR signaling continues to be transmitted downwards, so EGFR blockade should be attained from the source to control malignant cell behaviors. The above is the rationale for including cetuximab in targeted therapy regimens. Because BRAF located downstream has negative feedback regulation after its blockade, signaling will still be transmitted downstream through the bypass. This means the BRAF inhibitor alone loses its effect (14), and it is necessary to block downstream MEK at the same time. Therefore, three-target therapy (anti-EGFR, anti-BRAF, anti-MEK) is often required for patients with BRAF-mutant mCRC. Relevant studies (15,16) have shown that dual-target (anti-EGFR and anti-BRAF) or three-target (anti-EGFR, anti-BRAF, anti-MEK) blockade can significantly improve the antitumor activity.
Traditionally, it is believed that peritoneal metastasis is the end-stage lesion of CRC, which cannot be completely removed by surgery and has a very poor prognosis. Without effective treatment, the median survival is 5–7 months, and even shorter if combined with BRAF V600E mutation. We report a case of BRAF V600E mutated colon cancer combined with peritoneal metastasis treated with triple target drugs followed by aggressive surgery, and the postoperative targeted drugs maintained PFS for up to 28 months, which is a relatively successful case. We present the following article in accordance with the CARE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-748/rc).
Case presentation
All procedures performed in this study were approved by the institutional research ethics committee and were performed in accordance with the Declaration of Helsinki (as revised in 2013). The patient agreed to the publication of relevant clinical data and images, and signed an informed consent form. A copy of the written consent is available for review by the editorial office of this journal.
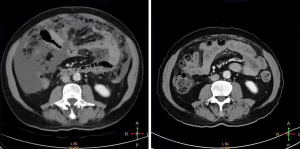
A 72-year-old male was admitted to our general surgery department on December 27, 2019. The patient mainly complained of persistent abdominal distension lasting for 1 month, accompanied by intermittent abdominal pain, dizziness, fatigue, low-grade fever, bloody stools, and other discomfort symptoms. Routine physical examination 3 days ago showed poorly differentiated adenocarcinoma of the ascending colon and multiple colorectal polyps on colonoscopy. Thoracoabdominal pelvic plain scan and enhanced computed tomography (CT) in our hospital showed space-occupying lesions of the proximal ascending colon with peripheral lymphadenopathy (Figure 1), which suggested the possibility of cancer (lesion length 2 cm). Furthermore, massive abdominopelvic effusion, irregular thickening of the parietal peritoneum, and soft tissue lesions on the greater omentum were observed, which suggested the possibility of implantation metastasis. No special treatment was given before the patient was referred to our hospital. He had a history of intermittent bloody stools for 1 year, once considered hemorrhoids bleeding.
Past medical history
The patient had no specific personal or family history. After previous cholecystectomy, he presented with hypertension for more than 3 years but blood pressure was controlled within normal limits. The patient has smoked for 50 years, with an average of 20 cigarettes a day, drank alcohol for 40 years, with an average of 100 g/day, abstained from alcohol for 1 year, and was allergic to penicillin without a history of food allergy.
Physical examination
The patient’s body temperature was 36.3 ℃, heart rate was 69 beats/min, respiratory rate was 18 breaths/min, blood pressure was 128/85 (133/88) mmHg, and blood oxygen saturation was 94%. His body surface area was 1.9 m2 and the Eastern Cooperative Oncology Group (ECOG) score was 1. Physical examination of the heart and lungs was unremarkable, but examination of the abdomen found distention, right abdomen pain when pressed, abdominal caking, and positive shifting dullness. Both lower limbs of the patient had mild edema. On digital rectal examination, fullness was felt above the dentate line, and the gloved finger was stained with blood.
Laboratory tests
Routine blood tests showed the following results: white blood cells 11.63×109/L, hemoglobin 143 g/L, and platelets 300×109/L. The test results of tumor markers were as follows: carbohydrate antigen 199 (CA199) >1,000 U/mL (reference value 0–27 U/mL) and carbohydrate antigen 125 (CA125) 461.6 U/mL (reference value 0–35 U/mL). Fecal occult blood test result was positive, and routine urine tests and blood biochemistry test results were within the normal range.
Imaging examination
Thoracoabdominal pelvic plain scan and enhanced CT showed space-occupying lesions of the proximal ascending colon with peripheral lymphadenopathy, which suggested the possibility of cancer (lesion length 2 cm). Furthermore, massive abdominopelvic effusion, irregular thickening of the parietal peritoneum, and soft tissue lesions on the greater omentum were observed, which suggested the possibility of implantation metastasis. Combined with the imaging and relevant laboratory tests, an initial diagnosis of ascending colon adenocarcinoma (T3NxM1c, stage IVC) with peritoneal and greater omental metastasis and abdominal effusion was considered.
Treatment
After admission, our multidisciplinary team (MDT) discussed and believed that the patient had stage IVC ascending colon adenocarcinoma without the chance of surgery. Therefore, abdominal paracentesis was recommended to remove ascites and reduce symptoms, and hyperthermic intraperitoneal chemoperfusion (HIPEC) was adopted. At the same time, genetic testing was also performed to provide a basis for targeted therapy and immunotherapy. On January 9, 2020, the patient received chemotherapy with oxaliplatin (200 mg, d1) + capecitabine (1.5 g, bid, d1–14). He received abdominal paracentesis first, followed by HIPEC. Chemotherapy drug use was discontinued 1 week after chemotherapy due to severe upper respiratory tract infection. Subsequently, cells with nuclear atypia were found in abdominal effusion samples by medical oncology examination, and HIPEC was performed once (lobaplatin 50 mg). On February 2, 2020, genetic testing showed BRAF V600E mutation and microsatellite stabilization (MSS). After the second MDT discussion, the patient was treated with first-line three-target therapy of dabrafenib + trametinib + cetuximab. After 6 weeks of medication, abdominal CT showed that the sizes of the tumor and metastases were significantly reduced compared with those at admission, and the abdominal effusion disappeared (Figure 2). The tumor marker CA199 decreased significantly, from undetectable to <400 U/mL, and CA125 returned to normal (Figure 3).
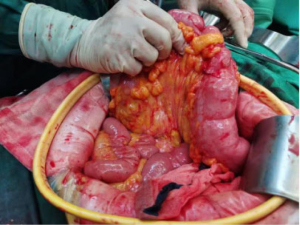
Based on the third MDT discussion, the patient underwent resection of the primary lesion and removal of greater omental metastases 3 weeks after medication discontinuation. At the time of surgery, there was already no metastatic nodule on the greater omentum (Figure 4). The three-target therapy was continued after the procedure was successfully completed and then changed to two-target therapy (cetuximab 300 mg/week + dabrafenib). Adhesive intestinal obstruction occurred after 4 months of treatment and the medication was stopped after surgical separation of adhesions. Subsequently, the patient was followed up and had normal tumor markers on consecutive reexamination. After 9 months of follow-up, CA199 was found to be significantly increased (492.4 U/mL), so three-target therapy was performed again. CA199 returned to normal after 4 months (Figure 3). The treatment regimen was further adjusted to two-target therapy (dabrafenib + cetuximab) based on the results of circulating tumor cells. Regular follow-up CA199 and abdominopelvic strengthening CT indicated stable disease, progression-free survival (PFS) 28 months. 2022-5-12 transverse colon cancer was detected by colonoscopy due to blood in stool, 5–17 PET-CT did not detect metastasis at other sites, 6–9 surgery was performed, postoperative pathology was considered poorly differentiated adenocarcinoma, partly mucinous adenocarcinoma with neuroendocrine differentiation, T4N2M0 BRAF V600E(+) (Figure 5). currently recovering from surgery and not on medication for the time being. Cetuximab-induced II–III rash occurred during the whole course of drug administration, which was relieved by local application of ointment containing glucocorticoids and topical herbal lotion, transient dabrafenib-induced fever with a temperature of 38.6°, which did not reappear after 2 days of discontinuation of the drug and was activated again, with occasional weakness and no bone marrow toxicity, with good overall safety.
Discussion
Molecular targeted therapy is a treatment modality targeting already well-established oncogenic sites at the cellular and molecular level, showing advantages of minimal trauma, good selectivity, and good applicability (17). At present, molecular targeted drugs are widely used in cancer therapy. The ANCHOR CRC study (15) was a prospective study of cetuximab + encorafenib (BRAF inhibitor) + binimetinib (MEK inhibitor) in the first-line treatment of BRAF V600E-mutant mCRC. According to the results, nearly half of the patients responded (47.8%), the condition of most patients was well controlled (88%), PFS was 5.8 months, and OS was 17.2 months. The BEACON CRC study (16) was a favorable demonstration of second- and third-line multi-target combination therapy for BRAF V600E-mutant mCRC. In this phase III study, both three-target (encorafenib + binimetinib + cetuximab) and two-target therapy (encorafenib + cetuximab) improved OS, with an objective response rate (ORR) of 26.8% in the three-target group, 19.5% in the two-target group, and 1.8% in the control group. Although the improvement in OS was significant with both the three-target and two-target combinations, the dual-target regimen (encorafenib + cetuximab) had less toxicity and was thus approved by the U.S. Food and Drug Administration and recommended in the National Comprehensive Cancer Network (NCCN) guidelines. A phase III trial of cetuximab + irinotecan after fluoropyrimidine and oxaliplatin failure in patients with mCRC showed that cetuximab and irinotecan improved PFS and the response rate and led to better quality of life of patients compared with irinotecan alone (18). Bellyei et al. (19) reported a case of metastatic colon cancer following renal transplantation, in which the tumor was well controlled after chemotherapy and targeted therapy supplemented by stereotactic radiotherapy. In this case, we combined the concept of the ANCHOR CRC study with drugs marketed in China to creatively develop a treatment regimen of dabrafenib + trametinib + cetuximab. The therapeutic effect of these 3 targeted drugs was significant. After application, the peritoneal metastases disappeared and CA199 returned to the normal range, further verifying the efficacy and safety of molecular targeted therapy for patients with advanced colon cancer.
In our case, resection of the primary tumor was boldly performed after the disappearance of peritoneal metastases, which played a radical role. The postoperative tumor regression grade was 2, and targeted drugs were continued after surgery. The postoperative adjuvant therapy duration was adjusted according to circulating tumor cell results. At 9 months after medication discontinuation, the patient was found to have elevated levels of tumor markers and a small amount of ascites with a circulating tumor cell count of 7 on imaging. According to the test results, peritoneal metastasis was considered again, and we re-used the three-target therapy based on the relevant conclusions of the BEACON study. After 4 months of re-treatment, the circulating tumor cell count of 3 was examined and the treatment was adjusted to 2 targets. There is no similar study determining the duration of postoperative adjuvant targeted therapy for digestive tract tumors. However, in the ADURA study of lung cancer (20), the postoperative maintenance of osimertinib for 2–3 years had a disease-free survival and OS benefit to patients. Considering the poor prognosis of patients with BRAF V600E-mutant mCRC, we intend to learn from this model and maintain postoperative treatment for 2 years if the patient’s economic condition allows and side effects are tolerable.
Conclusions
In this successful case, a patient with BRAF V600E-mutant advanced colon cancer and peritoneal metastasis underwent surgery after first-line precise three-target therapy, and postoperatively, the three-target therapy was restarted in a timely manner according to changes in circulating tumor cells and tumor markers. PFS up to 28 months. This model provides new ideas for the treatment of such patients. Of course, we have a lot of thoughts in this case, such as the length of application of targeted drugs after tumor surgery. Targeted drugs will eventually become resistant, and what treatment options to choose after resistance? How to choose the first and second line drugs for BRAF V600E combined with MSI-H? This is the direction of our further research.
Acknowledgments
Funding: The study was supported by Shanxi Provincial Health Commission (No. 2019022).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-748/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-748/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were approved by the institutional research ethics committee and were performed in accordance with the Declaration of Helsinki (as revised in 2013). The patient agreed to the publication of relevant clinical data and images, and signed an informed consent form. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012;487:330-7. [Crossref] [PubMed]
- Gorayski P, Dzienis M, Foote M, et al. Radiotherapy utilization in BRAF mutation-tested metastatic melanoma in the targeted therapy era. Asia Pac J Clin Oncol 2017;13:e117-23. [Crossref] [PubMed]
- Al Hashmi M, Sastry KS, Silcock L, et al. Differential responsiveness to BRAF inhibitors of melanoma cell lines BRAF V600E-mutated. J Transl Med 2020;18:192. [Crossref] [PubMed]
- Eurboonyanun K, Lahoud RM, Kordbacheh H, et al. Imaging predictors of BRAF mutation in colorectal cancer. Abdom Radiol (NY) 2020;45:2336-44. [Crossref] [PubMed]
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature 2002;417:949-54. [Crossref] [PubMed]
- Yamauchi M, Morikawa T, Kuchiba A, et al. Assessment of colorectal cancer molecular features along bowel subsites challenges the conception of distinct dichotomy of proximal versus distal colorectum. Gut 2012;61:847-54. [Crossref] [PubMed]
- Margonis GA, Buettner S, Andreatos N, et al. Association of BRAF Mutations With Survival and Recurrence in Surgically Treated Patients With Metastatic Colorectal Liver Cancer. JAMA Surg 2018;153:e180996. [Crossref] [PubMed]
- Corcoran RB, Ebi H, Turke AB, et al. EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov 2012;2:227-35. [Crossref] [PubMed]
- Cremolini C, Antoniotti C, Stein A, et al. Individual Patient Data Meta-Analysis of FOLFOXIRI Plus Bevacizumab Versus Doublets Plus Bevacizumab as Initial Therapy of Unresectable Metastatic Colorectal Cancer. J Clin Oncol 2020; Epub ahead of print. [Crossref] [PubMed]
- Wei J, Liu R, Hu X, et al. MAPK signaling pathway-targeted marine compounds in cancer therapy. J Cancer Res Clin Oncol 2021;147:3-22. [Crossref] [PubMed]
- Blagden SP. Targeting MAPK in recurrent, low-grade serous ovarian cancer. Lancet 2022;399:499-501. [Crossref] [PubMed]
- Centuori SM, Gomes CJ, Trujillo J, et al. Deoxycholic acid mediates non-canonical EGFR-MAPK activation through the induction of calcium signaling in colon cancer cells. Biochim Biophys Acta 2016;1861:663-70. [Crossref] [PubMed]
- Centuori SM, Martinez JD. Differential regulation of EGFR-MAPK signaling by deoxycholic acid (DCA) and ursodeoxycholic acid (UDCA) in colon cancer. Dig Dis Sci 2014;59:2367-80. [Crossref] [PubMed]
- Patil DT, Ma S, Konishi M, et al. Utility of BRAF V600E mutation-specific immunohistochemistry in detecting BRAF V600E-mutated gastrointestinal stromal tumors. Am J Clin Pathol 2015;144:782-9. [Crossref] [PubMed]
- Grothey A, Tabernero J, Taieb J, et al. ANCHOR CRC: a single-arm, phase 2 study of encorafenib, binimetinib plus cetuximab in previously untreated BRAF V600E-mutant metastatic colorectal cancer. Ann Oncol 2020;31:S242-3. [Crossref]
- Tabernero J, Grothey A, Van Cutsem E, et al. Encorafenib Plus Cetuximab as a New Standard of Care for Previously Treated BRAF V600E-Mutant Metastatic Colorectal Cancer: Updated Survival Results and Subgroup Analyses from the BEACON Study. J Clin Oncol 2021;39:273-84. [Crossref] [PubMed]
- Yang Z, Chen S, Ying H, et al. Targeting syndecan-1: new opportunities in cancer therapy. Am J Physiol Cell Physiol 2022;323:C29-45. [Crossref] [PubMed]
- Sobrero AF, Maurel J, Fehrenbacher L, et al. EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol 2008;26:2311-9. [Crossref] [PubMed]
- Bellyei S, Boronkai Á, Pozsgai E, et al. Effective chemotherapy and targeted therapy supplemented with stereotactic radiotherapy of a patient with metastatic colon cancer following renal transplantation: a case report. J Med Case Rep 2021;15:125. [Crossref] [PubMed]
- Wu YL, Tsuboi M, He J, et al. Osimertinib in Resected EGFR-Mutated Non-Small-Cell Lung Cancer. N Engl J Med 2020;383:1711-23. [Crossref] [PubMed]





