
Concurrent Epstein Barr virus-associated smooth muscle tumor and myeloid sarcoma of the liver and acute myeloid leukemia in a patient post kidney transplant: a case report and review of the literature
Introduction
Epstein-Barr virus-associated smooth muscle tumor (EBV-SMT) is a rare malignancy that arises in the setting of EBV infection and immunosuppression. The cell of origin is thought to be a myogenous venous wall cell (1). Three major subtypes of EBV-SMT are known: human immunodeficiency virus related (HIV-SMT), congenital immunodeficiency syndrome related, and post-transplant immunosuppression related (PT-SMT) (2,3). In patients with solid organ transplants, the estimated incidence of EBV-SMT is less than 1%, compared to a more common transplant related malignancy like the lymphoid malignancy post transplant lymphoproliferative disorder (PTLD) which may occur between 0.5–20% of patients depending on the transplanted graft (4).
The clinical behavior of EBV-SMT can range from indolent to aggressive, even in the presence of multifocal disease (2,5). Clinical features of EBV-SMT are nonspecific and depend on the location of the tumor (1). In post-transplant patients, the most commonly involved site of EBV-SMT is the native or transplanted liver (56%) whereas for HIV-SMT the most common is the central nervous system (34%) (1,3). The median survival of patients with single organ and multifocal PT-SMT is 16 and 20 months respectively, with intracranial PT-SMT having a median survival of 14 months (1). Although EBV-SMT most commonly presents in one organ, multifocal EBV-SMT may occur when multiple independent EBV infection events create distinct clones (3,6). Aside from immunosuppression, other risk factors for EBV-SMT have not been identified. There is no clear risk amongst gender, site of organ transplant, or immunosuppression. Most reported cases of post-transplant EBV-SMT are in kidney transplant patients (64%), although this may reflect the higher numbers of kidney transplants performed relative to other organ transplants (1,3,7). Patients with EBV-SMT are predominantly EBV seronegative at time of transplantation, suggesting primary EBV infection may have a role in subsequent development of EBV-SMT (2).
There is no consensus on EBV-SMT management, with treatment typically determined by institutional practices and expert opinion. Standard approaches include surgical resection and reduction in immunosuppressants (RIS) to promote immune response against the underlying EBV infection. In a retrospective study of 45 patients with PT-SMT, the median overall survival (OS) of patients who had surgery with or without RIS had a median OS of 108 months, whereas those who had RIS without surgery had a median OS of 28.5 months. However, the numerically superior OS of patients who underwent surgery did not reach statistical significance and may reflect selection bias of patients fit for surgery (1,8). No EBV-specific antiviral exists, although some centers have tried ganciclovir, valganciclovir, or famciclovir with unclear benefit (2). Radiation and systemic therapies have been used with modest utility. Systemic therapies reported in the literature have included common systemic chemotherapies such as doxorubicin, gemcitabine, vincristine, dactinomycin, cyclophosphamide, trabectedin, temozolomide, docetaxel (2,7,9-13). Checkpoint inhibitor immunotherapy is not ideal for study in post-transplant patients with EBV-SMT due to high risk of rejection, up to 39% in those with solid organ transplants (14). Cellular immunotherapy is currently under study for EBV-SMT. A refractory case of PT-SMT had stable disease for 3 months after receiving EBV specific adoptive cells transferred from an unrelated donor (15). Tabelecleucel, an off-the shelf allogeneic T-cell therapy and chimeric antigen receptor T-cells (CAR-T) are current currently under investigation in a wide variety of EBV related malignancies, including EBV-SMT (15,16).
Sirolimus, an mTOR/AkT inhibitor, has emerged as a promising treatment for EBV-SMT. Latent EBV infection causes EBV-SMT to produce LMP2A which subsequently activates the mTOR/Akt signaling pathway (17). Four of six reported cases of EBV-SMT were able to achieve a complete remission using sirolimus alone with a median follow-up of 3.8 years (1). No prospective clinical trials investigating sirolimus in this setting have been published to date (5,17).
To our knowledge, this is the first case report describing a patient who developed concurrent acute myeloid leukemia (AML) with myeloid sarcoma (AML/MS) and PT-SMT. We present the following case in accordance with the CARE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-21-700/rc) and review the current literature on EBV-SMT.
Case presentation
A 15-year-old African-American female developed end stage renal disease (ESRD) due to idiopathic membranoproliferative glomerulonephritis (MPGN) type 1 and received a living donor kidney transplant from her mother. During transplant, she underwent induction with three doses of rabbit anti-thymocyte globulin. Post-transplant, she was started on mycophenolate mofetil, tacrolimus, steroids, and was documented as having poor medication compliance. Pre-transplant, her EBV serologies were all negative, and the donor’s EBV status was unknown. The patient then developed detectable EBV viral load (10,000 copies/mL) by whole blood polymerase chain reaction (PCR) later that year, which remained elevated up until 4 years later. Tacrolimus was continued but prednisone was stopped at the age of 18. At the age of 19, her creatinine was rising and kidney biopsy showed C3 glomerulopathy and glomerulonephritis without antibody mediated rejection and tubule-interstitial scarring with inflammation, which was interpreted as MPGN recurrence and evidence of chronic kidney rejection. As a result, she was restarted on prednisone and given one dose of rituximab; at the same time, her EBV DNA PCR level was 67,000 copies/mL. Her EBV PCR was undetectable 4 months later, but recurred 1 year after rituximab treatment. At the age of 20, 18 months after her first rituximab treatment, she developed of persistent class II donor specific antibodies and so she was given three doses of intravenous immunoglobulin (IVIG), another dose of rituximab and was started on everolimus. Up until this time, she had been on prednisone and tacrolimus with intermittent medication compliance; due to persistent leukopenia she was not a candidate for mycophenolate mofetil and valganciclovir was discontinued.
At the age of 23, the patient developed flu-like symptoms and reported 30 pound weight loss over one month. She was found to have hyperleukocytosis with 90% blasts. EBV viral load at that time was 18,300 copies/mL. Immunophenotyping by flow cytometry of both the peripheral blood and bone marrow revealed AML. CT of the abdomen and pelvis identified a 13 cm heterogeneous left hepatic lobe mass (Figure 1). Mass was biopsied and revealed myeloblasts consistent with MS (Figure 2A). Although it was not discovered at the time, a posthumous review of this liver biopsy revealed the presence of EBER positive spindle cells consistent with synchronous EBV-SMT (Figure 2B,2C). Relevant family history for myeloid neoplasms was negative. Transplant nephrology reduced the patient’s immunosuppression by discontinuing everolimus and lowering the dose of tacrolimus and prednisone. She then underwent induction chemotherapy with cytarabine and daunorubicin. On the 28th day post-induction, a bone marrow biopsy showed complete remission and no residual disease by flow cytometry.
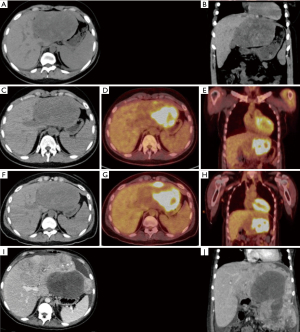
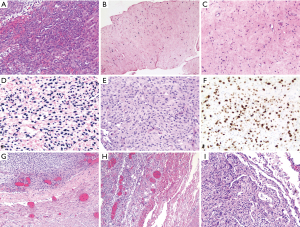
Despite completion of induction and consolidation therapy with high dose cytarabine, a positron emission tomography-computed tomography (PET-CT) showed the hepatic mass was unchanged in size (Figure 1C,1D). Subsequently, the patient underwent one cycle of fludarabine, etoposide, and cytarabine for suspected refractory MS. Post-treatment PET-CT showed that the hypodense portion of the mass remained stable in size, but there was an interval increase in fluorodeoxyglucose (FDG) avidity (Figure 1F-1H). A second liver biopsy showed no myeloblasts, but did show spindle cell proliferation (Figure 2D-2F). Assuming refractory MS, the patient was switched to decitabine and venetoclax (Dec-Ven). Several weeks after starting Dec-Ven, immunohistochemistry from the second liver biopsy revealed the tumor spindle cells were in fact EBV encoded RNA in-situ hybridization (EBER ISH) positive and the patient was diagnosed with EBV-SMT (Figure 2D-2F). The patient went on to receive a second cycle of Dec-Ven, coinciding with an undetectable EBV viral load.
A month after completion of the second cycle of Dec-Ven, the hepatic tumor increased to eight times its initial size (Figure 1I,1J). Due to poor nutrition and aggressive disease, the patient was not a surgical candidate for tumor resection. She was switched from tacrolimus to sirolimus with the palliative intent of treating EBV-SMT. Unfortunately, the patient developed acute respiratory distress syndrome from pneumonia. She then suffered a tension pneumothorax with chest tube placement complicated by pulmonary hemorrhage leading to cardiac arrest. A few days before her death, a repeat EBV DNA PCR yielded 118,000 copies/mL while on sirolimus. Autopsy revealed diffuse progression of multifocal mesenchymal tumors of the liver, bilateral lungs and pleura, diaphragm, right adnexa, bladder serosa, gallbladder serosa and peritoneum; no evidence of MS was found at autopsy. The tumor was hypocellular with bland looking spindle cells showing dense chromatin. In contrast with the second liver biopsy, the EBV-SMT was more cellular and pleomorphic with tumor cells, showing frequent oval to irregular shaped nuclei, vesicular chromatin, and occasional nucleoli (Figure 2D-2F). Finally, tumor samples from the autopsy showed widespread invasion including the lung and the bladder mesenchyme, but showed similar morphology as seen in the second liver biopsy (Figure 2G-2I).
Ethical statement
All procedures performed in the were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the mother of the patient post-humously for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
This case illustrates an aggressive therapy resistant phenotype of EBV-SMT. Although complete remission was achieved for her concurrent AML/MS, the synchronous EBV-SMT was resistant to a number of different cytotoxic leukemia regimens.
Notably after initial doxorubicin and cytarabine based induction, high dose cytarabine consolidation therapy, and fludarabine, etoposide, and cytarabine salvage therapy, there was no significant change in the size of the hepatic mass. Doxorubicin has been previously reported in use against multifocal EBV-SMT with poor activity; in one case report its use as a single agent only achieved a 5–10% reduction in size of their metastatic lesions (2,9). Theoretically doxorubicin, and to lesser extent etoposide, could have the potential to induce EBV viral reactivation, and cause growth of multifocal EBV-SMT tumors due to independent infection events (6). A study showed the potential of these drugs to induce EBV viral replication in EBV positive lymphoma cells via increasing BZLF1 mRNA levels, which encodes the viral immediate-early lytic protein Zta (18). EBV viral levels did not increase in our patient after these chemotherapeutic agents, nor did the tumor progress. After completion of fludarabine, etoposide, and cytarabine, a portion of the patient’s mass had an interval increase in FDG avidity. The biopsy at this time showed new pleomorphic changes in the EBV-SMT compared to the first liver biopsy, including a higher nuclear-cytoplasmic (N:C) ratio, large irregular nuclear contour, and dispersed chromatin, suggesting the poor effect of the above agents in this patient’s EBV-SMT.
Subsequently, the patient received two cycles of the BCL2 inhibitor venetoclax and the hypomethylating agent decitabine. Decitabine causes genome wide hypomethylation, resulting in activation of previously silenced genes (19). EBV related malignancies display one of three latency patterns, which result from epigenetic regulation of various promoters on its episome (5). EBV-SMT is known to have a EBV latency type III pattern due to activation of the Cp or Wp promoters, resulting in expression of all latent EBV genes (5). Unlike other malignancies that express a type III pattern, the major viral proto-oncogene, LMP1, is rarely detectable in EBV-SMT, possibly due to low LMP1 expression (1,5). LMP1 mimics CD40 signaling, activates NFkB, and has a major role of oncogenesis among EBV related malignancies. However, its low levels or absence in EBV-SMT suggests the reliance upon alternative mechanisms of oncogenesis (1). The hypomethylating agent decitabine in xenograft mice epigenetically increases expression of the proto-oncogene LMP1 and other immunogenic EBV antigens by activating EBV viral promoters. Decitabine is currently under study as a method to express these latency type III immunogenic antigens with the goal of sensitizing immunologically silent EBV+ lymphomas to EBV specific immunotherapies, such the off the shelf allogeneic T-cell therapy, tabelecleucel (19). In addition to latent gene activation, decitabine can also induce the lytic phase of EBV by activating the immediate-early gene BZLF1, resulting in EBV viral replication (19). Although immediately after two cycles of Dec-Ven the patient’s EBV viral copies were not elevated, the patient’s hepatic tumor progressed markedly a month later (Figure 1I,1J). Just before her death, the patient was discovered to have high EBV viral copies in the blood. It is possible that decitabine may have contributed to increased EBV viral replication via activation of BZLF1 (19). This may have led to multiple independent infection events and development of distant, multiple clonally distinct tumors, as is known to happen in EBV-SMT (6). Secondly, although the proto-oncogene LMP1 is rarely detectable in EBV-SMT, decitabine could have activated transcription of this proto-oncogene, resulting in further aggressive growth (19). The interaction of hypomethylating agents with EBV-SMT may require further study.
In summary, our report suggests that the chemotherapeutic agents utilized for AML may have poor efficacy against EBV-SMT.
Acknowledgments
We acknowledge the contribution of physician and dear friend Dr. Muhammad Omair Kamal, who cared for this patient before his passing.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-21-700/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-21-700/coif). HC receives honoraria from BMS and Pfizer for Advisory Board Meeting and receives research grant from NIH NCI Diversity Supplement Grant and the Bristol Myers Squibb Foundation for the Robert A. Winn Diversity in Clinical Trials Award Program. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jonigk D, Laenger F, Maegel L, et al. Molecular and clinicopathological analysis of Epstein-Barr virus-associated posttransplant smooth muscle tumors. Am J Transplant 2012;12:1908-17. [Crossref] [PubMed]
- Stubbins RJ, Alami Laroussi N, Peters AC, et al. Epstein-Barr virus associated smooth muscle tumors in solid organ transplant recipients: Incidence over 31 years at a single institution and review of the literature. Transpl Infect Dis 2019;21:e13010. [Crossref] [PubMed]
- Hussein K, Rath B, Ludewig B, et al. Clinico-pathological characteristics of different types of immunodeficiency-associated smooth muscle tumours. Eur J Cancer 2014;50:2417-24. [Crossref] [PubMed]
- Dierickx D, Habermann TM. Post-Transplantation Lymphoproliferative Disorders in Adults. N Engl J Med 2018;378:549-62. [Crossref] [PubMed]
- Ong KW, Teo M, Lee V, et al. Expression of EBV latent antigens, mammalian target of rapamycin, and tumor suppression genes in EBV-positive smooth muscle tumors: clinical and therapeutic implications. Clin Cancer Res 2009;15:5350-8. [Crossref] [PubMed]
- Deyrup AT, Lee VK, Hill CE, et al. Epstein-Barr virus-associated smooth muscle tumors are distinctive mesenchymal tumors reflecting multiple infection events: a clinicopathologic and molecular analysis of 29 tumors from 19 patients. Am J Surg Pathol 2006;30:75-82. [Crossref] [PubMed]
- Hirama T, Tikkanen J, Pal P, et al. Epstein-Barr virus-associated smooth muscle tumors after lung transplantation. Transpl Infect Dis 2019;21:e13068. [Crossref] [PubMed]
- El Hennawy HM, Habhab W, Almutawa A, et al. Long-term follow-up of post renal transplantation Epstein-Barr virus-associated smooth muscle tumors: Report of two cases and review of the literature. Transpl Infect Dis 2018;20:e12841. [Crossref] [PubMed]
- Moore Dalal K, Antonescu CR, Dematteo RP, et al. EBV-Associated Smooth Muscle Neoplasms: Solid Tumors Arising in the Presence of Immunosuppression and Autoimmune Diseases. Sarcoma 2008;2008:859407. [Crossref] [PubMed]
- Conrad A, Brunet AS, Hervieu V, et al. Epstein-Barr virus-associated smooth muscle tumors in a composite tissue allograft and a pediatric liver transplant recipient. Transpl Infect Dis 2013;15:E182-6. [Crossref] [PubMed]
- Kingma DW, Shad A, Tsokos M, et al. Epstein-Barr virus (EBV)-associated smooth-muscle tumor arising in a post-transplant patient treated successfully for two PT-EBV-associated large-cell lymphomas. Case report. Am J Surg Pathol 1996;20:1511-9. [Crossref] [PubMed]
- Boudjemaa S, Boman F, Guigonis V, et al. Brain involvement in multicentric Epstein-Barr virus-associated smooth muscle tumours in a child after kidney transplantation. Virchows Arch 2004;444:387-91. [Crossref] [PubMed]
- Lee ES, Locker J, Nalesnik M, et al. The association of Epstein-Barr virus with smooth-muscle tumors occurring after organ transplantation. N Engl J Med 1995;332:19-25. [Crossref] [PubMed]
- d’Izarny-Gargas T, Durrbach A, Zaidan M. Efficacy and tolerance of immune checkpoint inhibitors in transplant patients with cancer: A systematic review. Am J Transplant 2020;20:2457-65. [Crossref] [PubMed]
- Hansen BT, Bacher P, Eiz-Vesper B, et al. Adoptive Cell Transfer of Allogeneic Epstein-Barr Virus-Specific T Lymphocytes for Treatment of Refractory EBV-Associated Posttransplant Smooth Muscle Tumors: A Case Report. Front Immunol 2021;12:727814. [Crossref] [PubMed]
- Münz C, Redirecting T. Cells against Epstein-Barr Virus Infection and Associated Oncogenesis. Cells 2020;9:1400. [Crossref] [PubMed]
- Tan CS, Loh HL, Foo MW, et al. Epstein-Barr virus-associated smooth muscle tumors after kidney transplantation: treatment and outcomes in a single center. Clin Transplant 2013;27:E462-8. [Crossref] [PubMed]
- Lima RT, Seca H, Brás S, et al. Treatment of Akata EBV-positive cells with doxorubicin causes more EBV reactivation than treatment with etoposide. Chemotherapy 2011;57:195-203. [Crossref] [PubMed]
- Dalton T, Doubrovina E, Pankov D, et al. Epigenetic reprogramming sensitizes immunologically silent EBV+ lymphomas to virus-directed immunotherapy. Blood 2020;135:1870-81. [Crossref] [PubMed]


