
TRPV3 inhibits colorectal cancer cell proliferation and migration by regulating the MAPK signaling pathway
Introduction
Colorectal cancer (CRC), the most common malignancy of the gastrointestinal tract, is the third leading cause of cancer-related death worldwide, accounting for about 10% of global cancer cases (1), and the fourth most common cancer in China (2). Although great progress has been made in surgical treatment and adjuvant therapy for CRC, overall survival remains poor for patients with an advanced stage. Recently, lots of newly identified factors or regulators in different signaling pathways including cell death or tumor immune micro-environment have been investigated in CRC (1,2). However, limited factors have been used in clinical practice. Consequently, more prognostic markers with translational value are needed, and the underlying mechanisms of CRC progression should be explored.
The family of transient receptor potential vanilloid (TRPV) has been preliminarily found to play an important role in various cancers (3). To date, 6 subtypes of the TRPV family have been discovered. TRPV1–4 are heat sensitive ion channels, and TRPV5 and TRPV6 are highly selective calcium ion (Ca2+) channels that participate in the process of Ca2+ absorption and reabsorption (4). Previous studies have shown that calcium deficiency can lead to abnormal growth of colon epithelial cells and increase the risk of colon cancer (5-7), suggesting the important role of TRPVs in CRC. Jiang et al. confirmed that TRPV1 gain-of-function reprograms the immune microenvironment to facilitate colorectal tumorigenesis (8). In addition, inhibition of TRPV4 was found to suppress colon cancer development via activation of the phosphatase and tensin homolog (PTEN) pathway (9). TRPV3 is widely expressed and is involved in the formation of the skin barrier, wound healing, temperature perception, pruritus, pain, and other processes. However, no previous studies have been conducted to analyze the prognostic and oncogenic effect of TRPV3 in CRC progression.
In the present study, low expression of TRPV3 was found to be significantly associated with poor prognosis in CRC patients. Further experiments showed that TRPV3 stimulated cell proliferation and migration by provoking the mitogen-activated protein kinase (MAPK) signaling pathway. We present the following article in accordance with the MDAR reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-938/rc).
Methods
Source of patients
CRC patients in GSE39582 dataset from Gene Expression Omnibus (GEO) database were identified to test the prognostic value of TRPV3. In addition, a total of 100 patients were identified from Fujian Cancer Hospital to validate the correlation between protein expression level of TRPV3 and prognosis. Patients who met the following criteria were included: (I) pathologically diagnosed colorectal adenocarcinoma; (II) American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) stage I–IV; (III) CRC was the only primary tumor; (IV) patients with complete follow-up information. Patients with missing information were excluded. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was examined and approved by the ethics committee of Fujian Cancer Hospital (No. K2021-045-01). Written informed consent was obtained from the participants of this study.
Quantitative reverse transcription polymerase chain reaction (qRT-PCR)
NucleoZol (MACHEREY-NAGEL, Germany, 740404.200) was used for RNA extraction after tissue grinding, and SweScrip RT I First Strand cDNA Synthesis Kit (Servicebio Technology Co., Ltd., Wuhan, China; MPC2107001) was applied to reverse the RNA into complementary DNA (cDNA). A qPCR kit (Promega Corporation, Madison, WI, USA; Art. A6001) was then used for further experiments and analysis (Heal Force, Shanghai, China; Art. Cg-02/05).
Immunohistochemistry
The paraffin sections were dehydrated and rehydrated. Citrate buffer solution (Biyuntian Biotechnology Co., Ltd., Shanghai, China; No. ST368) was used for antigen repair. Endogenous peroxidase blocking agent (Biyuntian Biotechnology Co., Ltd.; No. P0100A) was incubated at room temperature for 10 minutes to eliminate endogenous peroxidase activity. The sections were incubated with TRPV3 primary antibody (Abcam, Shanghai, China; No. Ab231150) at 4 ℃ overnight. The next day, the sections were combined with secondary antibody and reacted with 3,3'-diaminobenzidine (DAB) chromogenic solution (Fuzhou Maixin Biotechnology Development Co., Ltd., Fuzhou, China; No. DAB-0031/1031) for 2 minutes. Double-distilled water (ddH2O) was used immediately to wash sections and terminate staining. Next, hematoxylin (Servicebio Technology Co., Ltd.; No. G1004-100 mL) was added to the sections, which were dyed for 3 minutes and then washed with ddH2O. Differentiation solution (Servicebio Technology Co., Ltd.; No. G1039-500 mL) was used for about 3 seconds, and the sections were then washed with ddH2O. Hematoxylin solution (Servicebio Technology Co., Ltd.; No. G1040-500 mL) was added to the sections until a blue color appeared (about 3 seconds), and the sections were then washed with ddH2O. Finally, the sections were dehydrated, sealed, and photographed under a microscope.
Scoring method: (I) staining intensity was classified as 0 (no staining), 1 (weak positive), 2 (moderate intensity), and 3 (strong positive). (II) Percentage scores were divided into 0 (0–5%), 1 (6–25%), 2 (26–50%), 3 (51–75%), and 4 (76–100%). The total score for each sample was the sum of the 2 scores: negative (<2 points), + (2–3 points), ++ (4–5 points), and +++ (6–7 points). Patients with fewer than 4 points were categorized as low expression.
Cell culture
Caco-2 and HCT8 cells were purchased from the National Cancer Institute (Bethesda, MD, USA). Caco-2 and HCT8 cells were taken from the liquid nitrogen tank and placed into a 37 ℃-water bath (Shanghai Jinghong Experimental Equipment Co., Ltd., Shanghai, China; No. Dk-8d) and quickly shaken and thawed. The cells were then placed in a cell culture dish (Corning Inc., Corning, NY, USA; No. 430167) with 8 mL Dulbecco’s Modified Eagle Medium (DMEM)-high glucose complete medium (Gibco, Waltham, MA, USA; No. 11965-092), shaken evenly, and placed in a 5% CO2 cell incubator (Sanyo Co., Ltd., Japan; No. VMCMMCO-5ACMMO) at 37 ℃.
Plasmid construction
The primer sequences of TRPV3 overexpression were as follows: upstream primer: 5'-CGGGATCCCGATGGTGCTGTGGGAGTCCCCG-3', downstream primer: 5'-CCCTCGAGGGCTAAAGTTTGGCCCTTGTGA-3'. The carrier was pcDNA3.1 and the restriction enzymes used were BamHI and XhoI.
Plasmid transfection
Once the cancer cells grew to 70% in the 6-well plate, the supernatant was discarded, the cells were washed with phosphate-buffered saline (PBS; HyClone, Logan, UT, USA; No. ABA212278), and 1.7 mL transfection solution (DMEM-high glucose without serum and double antibody) was added for later use. Two 1.5-mL Eppendorf tube (EP) tubes were used to contain 150 µL dye transfection solution each, with an appropriate concentration of plasmid added to tube A and an appropriate volume of LipofectamineTM 2000 (Invitrogen, Waltham, MA, USA; No. 11668027) to tube B. The mixtures were left to stand for 5 minutes, stirred gently, left for 20 minutes, and then evenly added to corresponding wells. After 5 hours, the medium was changed to DMEM-high glucose complete medium and cultured in the cell incubator for 19 hours for subsequent experiments.
Cell Counting Kit-8 (CCK-8) assay
After trypsin digestion of cells from each experimental group in the logarithmic growth phase, the cells were resuspended with complete medium, cell counting was performed, and the concentration of suspension was adjusted to guarantee consistency among groups. The diluted single-cell suspension was added to a 96-well plate with 3 repeat wells for each concentration.
Transwell assay
The cells were digested with trypsin and terminated with complete medium, pipetted and mixed evenly, centrifuged, and then the medium was discarded. Next, the cells were washed with PBS, and the cell density was adjusted to 1×105 with serum-free medium, after which 200 µL of the cell suspension was added into the transwell chamber and 600 µL medium containing 20% fetal bovine serum (FBS) was added into the lower chamber of the 24-well plate. After routine cell culture, the medium in the upper and lower chambers was removed, and 600 µL 4% paraformaldehyde was added to the lower chamber to fix the cells for 30 minutes. The paraformaldehyde was then removed and the cells that did not pass through the membrane were wiped off with cotton swabs. Crystal violet (600 µL 0.1%) was added to the lower chamber and the cells were stained for 10 minutes. The cells were then washed with PBS, and the migrating cells were observed and counted under a microscope.
Western blot
After the cell precipitation was collected, protein lysate containing phenylmethylsulfonyl fluoride (PMSF) was added, the mixture was placed on ice for 20 minutes, centrifuged at 12,000 rpm at 4 ℃ for 20 minutes, and the supernatant protein solution was transferred into an EP tube. A bicinchoninic acid (BCA) protein concentration determination kit (Beijing Solebo Technology Co., Ltd., Beijing, China; No. Pc0020-500) was utilized to detect the protein concentration of each group: the protein samples were diluted to the same concentration with radioimmunoprecipitation assay (RIPA) lysis solution, a 100-µL sample was removed, appropriate loading buffer was added, fully mixed, denaturated in a 98 ℃-water bath for 10 minutes, and stored at −20 ℃ after cooling on ice. Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) gel with appropriate concentration was used for electrophoresis, after which the protein was transferred to nitrocellulose film and sealed with 5% BSA for 2 hours. After the membranes were washed with tris-buffered saline with Tween (TBST), they were incubated with corresponding antibodies of pMKK4 (Abcam; No. Ab52958), pJNK1 (Abcam; No. Ab199380), pJNK2 (Abcam; No. Ab76125), PAP-1 (Abcam; No. Ab109004), P-MEK1 (Abcam; No. Ab96379), and β-actin (Abcam; No. Ab8226) at 4 ℃ overnight. The membranes were washed with TBST 3 times, and the corresponding secondary antibody was incubated at room temperature on a shaker for 2 hours. The membranes were then washed again 3 times with TBST, and an equal volume of liquid A and B from the Thermo Scientific enhanced chemiluminescence (ECL) kit was mixed and added to the membrane surface. After standing in the dark for 3 minutes, the luminescent liquid on the membrane was removed and placed on the transparent membrane. Gel imaging system (Shanghai Peiqing Technology Co., Ltd., Shanghai, China; No. JS-2012) was used to detect and collect membrane images.
Statistical analysis
All statistical analyses were performed using R software (version 3.5.0). All experiments were repeated biologically 3 times. Data is presented as mean ± standard deviation. To compare differences between groups, Wilcoxon rank-sum test was performed for data with skewed distribution, and Student’s t-test was conducted for data with normal distribution. Kaplan-Meier survival analysis was performed to compare the difference between survival curves. The X-tile program (https://x-tile.software.informer.com/), which identified the cutoff with the minimum P values from log-rank statistics, was used to sort patients from the GSE39582 dataset of the GEO into high- and low-TRPV3 expression groups. A P value less than 0.05 was considered significant.
Results
Low expression of TRPV3 indicates poor prognosis in CRC patients
To investigate the prognostic value of TRPV3 in CRC, we downloaded the largest microarray dataset (GSE39582) from the GEO database. This dataset consists of 557 CRC patients with transcriptome expression data and complete follow-up information. Based on the X-tile program (Figure 1A), patients were divided in low- and high-level groups (Figure 1B,1C), and patients with low expression of TRPV3 exhibited significantly poorer overall survival than patients with high TRPV3 expression (P=0.045; Figure 1C).
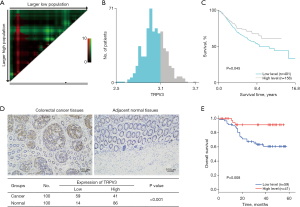
To further validate the value of TRPV3 at the protein level, we collected 100 paired CRC cancer and paracancer tissues to perform immunohistochemistry staining analysis. Among the 100 patients, 43 were male and 57 were female. The median age of these patients was 66. The results showed that TRPV3 was highly expressed in adjacent normal tissues (P<0.001; Figure 1D) and low expression of TRPV3 was significantly associated with poor overall survival (P=0.008; Figure 1E).
TRPV3 inhibits the proliferation and migration of CRC cells
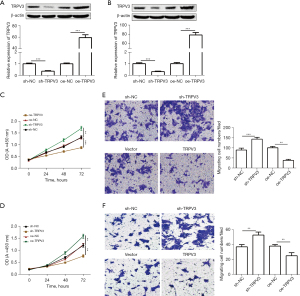
To explore the role of TRPV3 in CRC, we enhanced and silenced TRPV3 expression, respectively, in Caco-2 and HCT8 cells with an average constitutive expression of TRPV3. TRPV3 expression levels were validated by qRT-PCR and western blot (Figure 2A,2B). Next, CCK-8 assay was performed, with the results showing that knockdown of TRPV3 significantly enhanced cell proliferation ability, while its overexpression showed the opposite results (Figure 2C,2D). Further, the transwell assay showed that TRPV3 knockdown notably enhanced cell migration ability, while ectopic expression of TRPV3 markedly inhibited cell migration ability (Figure 2E,2F).
TRPV3 silence activated the MAPK signaling pathway
To identify the potential signaling pathway regulated by TRPV3, we performed GSEA analysis based on the GSE39582 dataset, and significant gene sets [false discovery rate (FDR) <5%] were visualized as an enrichment map (Figure 3A). The results showed that low expression of TRPV3 was accompanied by exceptional activation of several important cancer-related pathways, of which the MAPK signaling pathway was the most significant (Figure 3B).
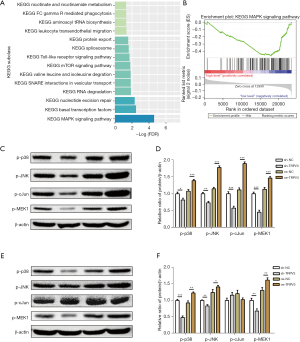
Therefore, we subsequently identified hub genes, including p-p38, p-JNK, p-cJun, and p-MEK1, in the MAPK pathways and confirmed their differential expression. Western blot assay was performed, and the results confirmed a downregulated phosphorylation state of p-p38, p-JNK, p-cJun, and p-MEK1 in the TRPV3-overexpression group compared to the control group (Figure 3C,3D). Conversely, the phosphorylation state of p38, JNK, c-Jun, and MEK1 were upregulated in the TRPV3-knockdown group compared to the control group (Figure 3E,3F).
The MAPK pathway is essential for promoting the effect of TRPV3 attenuation on cell proliferation and migration
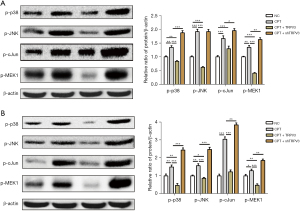
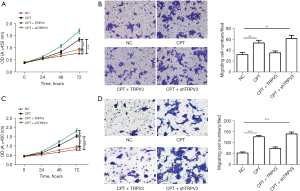
To confirm the fundamental role of the MAPK pathway in mediating the promoting effect of TRPV3 on cell proliferation and migration, we then treated cells with an EPAC-selective agonist (8-pCPT-2-O-Me-cAMP, 100 µM), a MAPK activator, to rescue p-p38, p-JNK, and p-cJun expression. The results showed that upon addition of the activator, p-p38, p-JNK, p-MEK1, and p-cJun were upregulated (Figure 4A,4B). To further verify the essential role of the MAPK pathway in the TRPV3 knockdown-induced oncogenic effect, we investigated cell proliferation and migration activity when exposed to 8-pCPT-2-O-Me-cAMP. The results showed that TRPV3 overexpression partially eliminated the enhancing effect on cell proliferation (Figure 5A,5B) and migration (Figure 5C,5D) caused by 8-pCPT-2-O-Me-camp treatment, while TRPV3 attenuation increased the effect further, indicating that MAPK signaling pathway activity was responsible for TRPV3-mediated growth and migration in CRC.
Discussion
In recent years, CRC has become a common malignant tumor, especially in China. The latest estimates show that there are more than 376,000 new cases of CRC in China every year, and 191,000 people die from it (10). Therefore, it is of great importance to explore the occurrence and development mechanisms of CRC for its prevention and treatment. A previous study has shown that TRPV3 expression is low in CRC (10), indicating that TRPV3 has a potential regulatory role in the progression of CRC. However, no previous studies have reported the prognostic and oncogenic role of TRPV3 in CRC. Consequently, we evaluated TRPV3 expression in cancer tissues using a public database and our cancer center to investigate its prognostic value, with the results showing that low expression of TRPV3 correlated to poor survival. TRPV3 expression was then enhanced and silenced, respectively, in CRC cells and it was revealed that TRPV3 attenuation promoted cell proliferation and migration. Further mechanism experiments showed that the MAPK signaling pathway was markedly activated in patients with low expression of TRPV3, and the activation of MAPK was responsible for the promoting effect on cell growth and TRPV3-expression deficiency.
In order to sense and convert physical cues into biological and molecular responses, all tissues possess specific cellular machineries (11,12). Hence, basing on the external stimuli, mechanosensation mediated by cell structure and functions will regulate important cellular biological process and thus maintaining homeostasis of cells and tissues. Changes in Ca2+-homeostasis participates in each step of metastatic process and directs the initiation of epithelial-mesenchymal transition (EMT) and invasion (13,14). Plasma membrane-embedded calcium channels is one of the most critical regulator of Ca2+-homeostasis. Transient receptor potential (TRP) family proteins have been identified as one of the most essential part of Ca2+-channels. There proteins will trigger activation of specific intracellular cascades through subtle changes in ion influx by responding to a variety of external stimulus (15,16). According to sequence homology, the TRP superfamily can be categorized into 7 subfamilies including the TRPV, TRPA, TRPC, TRPM, TRPML, TRPN, and TRPP families (17). Once stimulated, these channels will transform the membrane potential or intracellular Ca2+ concentration to influence specific cellular events. Therefore, deregulation of TRPV also plays a crucial role in tumor development and progression (18-22).
Studies have reported that overexpression of TRPV3 in human lung cancer cells can promote tumor progression. The inhibition of TRPV3 can block the cell cycle at the first growth/synthesis (G1/S) boundary and inhibit lung cancer cell proliferation and cyclin D1 expression (23). In addition, it has been reported that TRPV3 could promote the activation of the EGFR/ERK signaling pathway to stimulate skin cell proliferation (24). In atopic dermatitis, inhibition of TRPV3 attenuated skin lesions and dermatitis in mice, proving that TRPV3 could induce the expression of inflammatory factors (25). Experimental evidence has demonstrated that TRPV3 has regulatory roles in cell proliferation and inflammation, and so we collected CRC tissue samples for further analysis. The results showed that TRPV3 expression in CRC was markedly reduced compared with adjacent normal tissues. Via overexpression and knockdown of TRPV3 in CRC cells, we found that its overexpression inhibited the proliferation and migration of CRC cells, while its knockdown showed the opposite results, confirming the idea that TRPV3 expression could inhibit the progression of CRC. In this study, we found that low expression of TRPV3 was significantly associated with poor prognosis of CRC patients. Further experiments revealed that TPRV3 inhibited cell proliferation and migration by repressing MAPK signaling pathway. Therefore, TRPV3 is a potential biomarker for the prediction of prognosis of CRC patients. In addition, it is possible to individualized the use of MAPK inhibitor in CRC.
Although our study provided some intriguing findings, it had some limitations. First, we did not perform any in vivo experiments in this study. In addition, we did not explore why TRPV3 was downregulated in CRC tissues, the mechanisms of which might provide more alternatives for developing therapeutic drugs for CRC. Further study of the mechanisms of TRPV3 deficiency in activating the downstream pathways of MAPK is needed.
In conclusion, we investigated and uncovered the prognostic value and functions of TRPV3 in CRC, revealing it to be a tumor suppressor and a prognostic marker. Our findings demonstrated that the MAPK signaling pathway is a critical mechanism contributing to CRC progression mediated by TRPV3 silencing, which might facilitate future drug development for CRC patients.
Acknowledgments
We thank the GEO database for providing valuable data.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-938/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-938/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-938/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study has been examined and approved by the ethics committee of Fujian Cancer Hospital (No. K2021-045-01). Written informed consent was obtained from the participants in this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ruan H, Leibowitz BJ, Zhang L, et al. Immunogenic cell death in colon cancer prevention and therapy. Mol Carcinog 2020;59:783-93. [Crossref] [PubMed]
- Ganesh K, Stadler ZK, Cercek A, et al. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat Rev Gastroenterol Hepatol 2019;16:361-75. [Crossref] [PubMed]
- Gong Z, Xie J, Chen L, et al. Integrative analysis of TRPV family to prognosis and immune infiltration in renal clear cell carcinoma. Channels (Austin) 2022;16:84-96. [Crossref] [PubMed]
- Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem 2007;76:387-417. [Crossref] [PubMed]
- Dai W, Bai Y, Hebda L, et al. Calcium deficiency-induced and TRP channel-regulated IGF1R-PI3K-Akt signaling regulates abnormal epithelial cell proliferation. Cell Death Differ 2014;21:568-81. [Crossref] [PubMed]
- Wesselink E, Kok DE, Bours MJL, et al. Vitamin D, magnesium, calcium, and their interaction in relation to colorectal cancer recurrence and all-cause mortality. Am J Clin Nutr 2020;111:1007-17. [Crossref] [PubMed]
- Abbasnezhad A, Falahi E, Ghavamzadeh S, et al. Association between deficient levels of circulating vitamin D, dietary intake of vitamin D, calcium and retinol, and risk of colorectal cancer in an Iranian population: A case control study. Asia Pac J Clin Oncol 2022;18:118-26. [Crossref] [PubMed]
- Jiang X, Wang C, Ke Z, et al. The ion channel TRPV1 gain-of-function reprograms the immune microenvironment to facilitate colorectal tumorigenesis. Cancer Lett 2022;527:95-106. [Crossref] [PubMed]
- Liu X, Zhang P, Xie C, et al. Activation of PTEN by inhibition of TRPV4 suppresses colon cancer development. Cell Death Dis 2019;10:460. [Crossref] [PubMed]
- Sozucan Y, Kalender ME, Sari I, et al. TRP genes family expression in colorectal cancer. Exp Oncol 2015;37:208-12. [Crossref] [PubMed]
- Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science 2005;310:1139-43. [Crossref] [PubMed]
- Iskratsch T, Wolfenson H, Sheetz MP. Appreciating force and shape—the rise of mechanotransduction in cell biology. Nat Rev Mol Cell Biol 2014;15:825-33. [Crossref] [PubMed]
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol 2003;4:517-29. [Crossref] [PubMed]
- Fels B, Bulk E, Pethő Z, et al. The Role of TRP Channels in the Metastatic Cascade. Pharmaceuticals (Basel) 2018;11:48. [Crossref] [PubMed]
- Vangeel L, Voets T. Transient Receptor Potential Channels and Calcium Signaling. Cold Spring Harb Perspect Biol 2019;11:a035048. [Crossref] [PubMed]
- Tsagareli MG, Nozadze I. An overview on transient receptor potential channels superfamily. Behav Pharmacol 2020;31:413-34. [Crossref] [PubMed]
- Pedersen SF, Owsianik G, Nilius B. TRP channels: an overview. Cell Calcium 2005;38:233-52. [Crossref] [PubMed]
- Ziglioli F, Frattini A, Maestroni U, et al. Vanilloid-mediated apoptosis in prostate cancer cells through a TRPV-1 dependent and a TRPV-1-independent mechanism. Acta Biomed 2009;80:13-20. [PubMed]
- Wang X, Li G, Zhang Y, et al. Pan-Cancer Analysis Reveals Genomic and Clinical Characteristics of TRPV Channel-Related Genes. Front Oncol 2022;12:813100. [Crossref] [PubMed]
- Siveen KS, Nizamuddin PB, Uddin S, et al. TRPV2: A Cancer Biomarker and Potential Therapeutic Target. Dis Markers 2020;2020:8892312. [Crossref] [PubMed]
- Santoni G, Farfariello V, Amantini C. TRPV channels in tumor growth and progression. Adv Exp Med Biol 2011;704:947-67. [Crossref] [PubMed]
- Huang T, Xu T, Wang Y, et al. Cannabidiol inhibits human glioma by induction of lethal mitophagy through activating TRPV4. Autophagy 2021;17:3592-606. [Crossref] [PubMed]
- Li X, Zhang Q, Fan K, et al. Overexpression of TRPV3 Correlates with Tumor Progression in Non-Small Cell Lung Cancer. Int J Mol Sci 2016;17:437. [Crossref] [PubMed]
- Vyklicka L, Boukalova S, Macikova L, et al. The human transient receptor potential vanilloid 3 channel is sensitized via the ERK pathway. J Biol Chem 2017;292:21083-91. [Crossref] [PubMed]
- Qu Y, Wang G, Sun X, et al. Inhibition of the Warm Temperature-Activated Ca2+-Permeable Transient Receptor Potential Vanilloid TRPV3 Channel Attenuates Atopic Dermatitis. Mol Pharmacol 2019;96:393-400. [Crossref] [PubMed]
(English Language Editor: A. Muijlwijk)


