
Circulating lncRNA EGFR-AS1 as a diagnostic biomarker of colorectal cancer and an indicator of tumor burden
Introduction
Colorectal cancer (CRC), a common malignancy affecting the gastrointestinal tract, is characterized by a high recurrence rate and reduced survival and constitutes the second and third most prevalent malignancy in women and men, respectively, accounting for 10% of new cancer diagnoses and cancer-associated deaths worldwide (1,2). About 50–60% of CRC cases metastasize, with 80–90% exhibiting unresectable liver tumors (3,4). CRC treatment mostly includes surgical resection, chemotherapy, as well as radiation and biological therapies. Although these treatments have increased the survival of CRC patients, advanced CRC remains a leading cause of cancer-associated deaths, with only 6% of cases surviving for 5 years or more.
Advanced methods have been developed recently for treating metastatic colorectal cancer (mCRC) (5). Previously, 5-fluorouracil (5-FU) was the unique active compound, conferring an overall survival (OS) approximating 11–12 months. Currently, new treatments could lead to an OS of 3 years (6). Such improvements are mostly attributed to novel active compounds, including oxaliplatin and irinotecan, as well as monoclonal antibodies that suppress angiogenesis and cell division pathways by regulating vascular endothelial growth factor and the epidermal growth factor receptor (7-9). Recently, important insights into tumor immune checkpoints and their suppressors have been provided for the treatment of multiple cancers (10). Programmed cell death-1 (PD-1) and programmed cell death-ligand 1 (PD-L1) suppressors were shown to play essential roles (11).
However, the heterogeneity of mCRCs has hampered efforts by oncologists to design targeted therapeutic approaches and optimize personalized treatment. Therefore, developing predictive tools that are clinically applicable to individual patients prior to treatment initiation is critical (12,13). Additionally, identifying circulating biomarkers is urgently required to improve patient management, since the current approaches, including tissue biopsy and radiological assessment, are very limited in terms of follow-up and prognosis. Thus, in the era of precision medicine, liquid biopsy constitutes a critical decision-making tool for designing therapeutic strategies (14,15).
Detecting blood biomarkers is a promising alternative diagnostic tool in CRC (16). Carcinoembryonic antigen (CEA) has been broadly utilized as a circulating molecular marker for detecting tumor recurrence (17,18). Other circulating biomarkers have also been considered for postoperative CRC surveillance, including cancer antigen 19-9 (CA19-9) and cancer antigen 125 (CA125). Despite their usefulness for patient monitoring, these markers are also associated with non-neoplastic conditions such as inflammatory bowel disease and endometriosis, as well as other types of cancer, including breast, gastric, pancreatic, bladder, and lung cancers (19,20). Presently, no circulating biomarker is available for early CRC detection.
An emerging field of biomarker research is the analysis of ncRNAs. Based on the lengths of their functional transcripts, human ncRNAs are largely grouped into two main classes, including small (<200 nt) and long (>200 nt) ncRNAs. MicroRNAs (miRNAs), as central members of the small ncRNA class, suppress gene expression (21). Diversity is considerably higher in the long ncRNA (lncRNA) class, which includes approximately 19,000 functionally distinct RNA molecules with multiple molecular mechanisms (22). Accumulating evidence suggests that the regulation of lncRNAs is impaired in cancer cells, implying that they might constitute potential biomolecular markers for cancer diagnosis and prognosis (23). Meanwhile, their regulatory effects on cell proliferation, invasion, and apoptosis were detected in CRC cells. Epidermal growth factor receptor (EGFR) antisense RNA1 (EGFR-AS1) is a lncRNA that is 2.8 kb in length. In the present study, circulating EGFR-AS1 levels were examined in 128 CRC patients and 64 healthy individuals by quantitative reverse transcription-polymerase chain reaction (qRT-PCR). We comparatively investigated the circulating EGFR-AS1 levels in CRC patients versus control cases comprising endoscopy-confirmed CRC‑free cases. In addition, pre- and post-surgical plasma levels of EGFR-AS1 were determined in a subset of these patients to examine its value as a biomarker of tumor load. Furthermore, the expression of EGFR-AS1 in the CRC tissues of these surgical patients was significantly higher than that in paracancerous tissues, which may help to further confirm the key role of EGFR-AS1 in the diagnosis and progression of CRC and can be used for early CRC diagnosis. We present the following article in accordance with the STARD reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-968/rc).
Methods
Study subjects
This trial enrolled individuals with resectable (no distant metastasis) CRC without prior surgery (n=128). The control group comprised age and sex-matched CRC-free individuals (n=64). The absence of CRC in control individuals was verified by endoscopic investigation, and by biopsy in certain cases (if necessary).
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethical Review Board of Second Affiliated Hospital of Navy Medical University (No. 2017SL627) and informed consent was taken from all the patients. Sample collection was conducted at the surgery unit of Second Affiliated Hospital of Navy Medical University from March 2018 to December 2020. Most patients had locally advanced disease. The detailed clinical characteristics of all patients in the CRC group are listed in Table 1. Blood was collected from all CRC patients before partial colorectal surgery and post‑surgically from a subset of patients (n=32), in whom post-surgical blood samples were available (taken within 7–12 days after surgery). Blood specimens (5 mL) were collected in Ethylene Diamine Tetraacetic Acid (EDTA) tubes, kept at 4 ℃ for 2 h, and centrifuged at 1,500 ×g for 10 min at 4 ℃ within 4 h of withdrawal. Following immediate second centrifugation (12,000 ×g for 10 min at 4 ℃), the plasma samples were collected and kept in aliquots at −80 ℃. In total, 32 pairs of CRC tissue and paracancerous tissue specimens were surgically removed in Second Affiliated Hospital of Navy Medical University and stored at −80 ℃.
Table 1
| Patient characteristics | No. of patients, n (%) | Relative plasma EGFR-AS1 level, median (range) | P value |
|---|---|---|---|
| Age, years | 0.941 | ||
| ≥60 | 79 (61.7) | 1.576 (0.968–2.155) | |
| <60 | 49 (38.3) | 1.583 (1.762–1.275) | |
| Gender | 0.461 | ||
| Male | 66 (51.6) | 1.611 (0.860–2.516) | |
| Female | 62 (48.4) | 1.543 (0.897–2.579) | |
| Tumor size, cm | 0.0001** | ||
| ≥5 | 82 (64.1) | 1.739 (0.892–3.579) | |
| <5 | 46 (35.9) | 1.290 (0.860–2.101) | |
| TNM stage | 0.003* | ||
| I/II | 52 (40.5) | 1.412 (0.860–2.275) | |
| III/IV | 76 (59.5) | 1.691 (0.897–3.579) | |
| Lymphatic metastasis | 0.718 | ||
| No | 23 (18.0) | 1.417 (0.860–2.275) | |
| Yes | 105 (82.0) | 1.614 (0.932–3.034) | |
*, P<0.05; **, P<0.001. EGFR-AS1, epidermal growth factor receptor antisense RNA 1; CRC, colorectal cancer; TNM, tumor-node-metastasis.
RNA purification and reverse transcription
Total RNA was extracted from sera or tissues using Trizol reagent (Takara, Dalian, China) according to the manufacturer’s instructions. A One Step Real Time RT-PCR Kit (Takara, Dalian, China) was used for reverse transcription.
Analysis of EGFR-AS1 gene expression
EGFR-AS1 levels were determined from the sera or tissues of study participants. The primers were as follows: glyceraldehyde 3-phosphate dehydrogenase (GAPDH), forward 5'-AGAAGGCTGGGGCTCATTTG-3' and reverse 5'-AGGGGCCATCCACAGTCTTC-3'. EGFR-AS1, forward 5'-CCATCACGTAGGCTTCCTGG-3' and reverse 5'-GCATTCATGCGTCTTCACCTG-3'. Quantitative real-time PCR was performed on a 7900HT (ABI, Arlington, USA) with SYBR Premix Ex TaqTM (Takara, Dalian, China). PCR amplification was performed at 95 ℃ (10 min), followed by 40 cycles of 95 ℃ (30 s), 95 ℃ (10 s), and 60 ℃ (34 s). The relative levels of EGFR-AS1 in circulation were determined by the 2−ΔΔCq method (22). Amplification of the appropriate product was confirmed by melting curve analysis.
Statistical analysis
To determine whether the plasma EGFR-AS1 levels were normally distributed, the Shapiro-Wilk test was performed, which indicated non-normal distribution. Thus, the EGFR-AS1 levels were presented as the median and range with the first and third quartiles. The Mann‑Whitney U test was employed to compare the EGFR-AS1 levels between the study groups and determine associations with clinical features. Receiver operating characteristic (ROC) curve analysis was carried out to examine the diagnostic potential of plasma EGFR-AS1. Pre- and post-operative levels of EGFR-AS1 were compared in a subset of patients with available data (n=32) using the Wilcoxon test. SPSS v.25 (SPSS, USA) was utilized for data analysis, with P<0.05 indicating statistical significance.
Results
Diagnostic value of EGFR-AS1 in CRC
Plasma EGFR-AS1 levels were measured in all study participants. As shown in Figure 1A, circulating EGFR-AS1 levels were higher in CRC cases compared to CRC-free control patients (median levels relative to GAPDH, 1.578 vs. 0.780, P<0.001). The ROC curve in Figure 1B illustrates the diagnostic power of circulating EGFR-AS1, with an area under the curve (AUC) of 93.8% (P<0.001).

Associations between EGFR-AS1 levels and clinicopathological parameters
The associations between plasma EGFR-AS1 levels and clinicopathological parameters in CRC patients were evaluated (Table 1). The results revealed that individuals with larger tumors (≥5 cm) exhibited significantly higher plasma EGFR-AS1 levels compared to those with smaller tumors (<5 cm, relative median levels 1.739 vs. 1.290, P<0.001, Figure 2A). Accordingly, the expression of serum EGFR-AS1 in stage III/IV CRC was higher than that in stage I/II CRC (relative median levels, 1.691 vs. 1.412, P=0.003, Figure 2B). Similarly, patients with no lymphatic metastasis (N0) exhibited lower plasma EGFR-AS1 levels compared to those with lymphatic metastasis (N1–3), although statistical significance was not reached (median relative levels 1.417 vs. 1.614, P=0.718). No associations were identified between plasma EGFR-AS1 and other parameters, including patient age and gender.

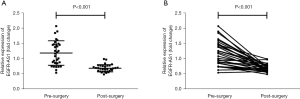
Circulating EGFR-AS1 may be a marker of tumor load
Pre-and post-operative levels of circulating EGFR-AS1 were compared in a subset of CRC patients (n=32). The results showed that EGFR-AS1 levels declined markedly following surgical tumor removal, from 0.544–2.077 to 0.493–0.995 [relative median levels, 1.192 vs. 0.692 (pre-surgery), P<0.001, Figure 3A]. In the vast majority of individuals, plasma EGFR-AS1 levels were decreased following surgery. These data indicated that circulating EGFR-AS1 may be an indicator of tumor burden (Figure 3B).
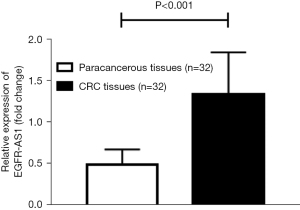
Differential expression of EGFR-AS1 in CRC tissues and paracancerous tissues
The expression of EGFR-AS1 in the CRC tissues of 32 surgical patients was significantly higher than that in paracancerous tissues (n=32, median levels relative to GAPDH, 1.336 vs. 0.487, P<0.001) (Figure 4). This result confirmed that EGFR-AS1 may play a key role in the early diagnosis of CRC.
Discussion
Cancer biomarkers represent objectively quantifiable compounds in cells, tissues, and/or body fluids, and reflect the occurrence or prognosis of cancer. Since plasma constitutes the commonest specimen type for clinical diagnosis, multiple reports have focused on identifying efficient circulating biomarkers, with good availability, no requirement for invasive techniques, and low cost (24). Recently, circulating miRNAs were proposed as stably quantifiable and as reliable molecular markers of several cancers (25). In addition, typical circulating amounts of lncRNAs in cancer patients are currently considered a hot topic in molecular target research (22). Timely diagnosis and treatment of cancer, as well as early detection of precancerous lesions, including benign and adenomatous polyps, may significantly prolong the survival of cancer patients (26). The above results showed that EGFR-AS1 could not only discriminate CRC cases from healthy individuals but also help to diagnose CRC at both early and advanced stages, indicating its remarkable diagnostic value in differentiating various disease stages.
The lncRNA EGFR-AS1, found at chromosome 7p11.2, constitutes an antisense transcript of EGFR. EGFR-AS1 and EGFR share a complementary sequence, which provides a basis for EGFR modulation. EGFR plays an essential role in the progression of multiple malignancies, including pulmonary, renal, gastric, and CRCs. In lung cancer, the specific EGFR suppressor gefitinib improves patient survival. Meanwhile, elevated EGFR amounts detected in renal cancer are closely associated with tumor development and metastasis (27). High EGFR levels induce several downstream pathways in cancer, e.g., the MAPK, PLCγ, STAT, and PI3K/AKT pathways (28). Also, EGFR-AS1 may exert a potential function in tumor development and survival via miRNA-133b sponging and EGFR/STAT3 axis modulation in CRC (29). However, the research about those of EGFR-AS1 in CRC remains largely unclear. Here, we have carried some studies, loss-of-function investigations disclosed that EGFR-AS1 silencing impaired CRC cell proliferation, migration and invasion and ascended cell apoptosis, which exhibited the oncogenic property of EGFR-AS1 in CRC.
In the present study, we examined the diagnostic and prognostic value of circulating EGFR-AS1 in CRC. Elevated circulating EGFR-AS1 levels were detected in CRC cases compared with endoscopy-verified CRC-free individuals. In addition, individuals with larger tumors (≥5 cm) exhibited higher plasma EGFR-AS1 levels compared to those with smaller lesions (<5 cm). Finally, plasma EGFR-AS1 levels were markedly reduced after surgical resection of colorectal tumors, as shown in a patient subset.
The current study had some limitations that should be noted. Firstly, only 128 CRC cases were examined, indicating low statistical power. Secondly, the mechanism by which EGFR-AS1 affects CRC was not explored. Thirdly, as a single-center trial, selection bias is unavoidable.
Indeed, many circulating lncRNAs remains have poor performance when taken individually. Several lncRNAs reportedly have either poor sensitivity or poor specificity towards a specific cancer type, affecting their potentials as diagnosis biomarkers. Overall, while the research of circulating lncRNAs is still at an early stage, the emergence of new technologies will improve their detection, specificity, and potential in clinical applications which will allow the early and accurate detection of cancer.
In conclusion, the present study confirmed previous data suggesting increased circulating EGFR-AS1 levels in CRC patients. However, this study also provided novel insights. Firstly, the establishment of a defined control group is considered to be important in determining the exact role of EGFR-AS1 in CRC. Indeed, establishing appropriate control groups is a universal challenge in case-control studies (29). Therefore, confirming the absence of malignancy in control individuals in the current study indicated that the obtained expression levels were accurate. Secondly, EGFR-AS1 expression was analyzed with respect to tumor load; following surgery, plasma EGFR-AS1 levels were decreased substantially in most patients, indicating that a significant proportion of plasma EGFR-AS1 originates from the tumor tissue. Furthermore, we examined the expression of EGFR-AS1 in 32 cases of paracancerous and CRC tissues and found that its expression in cancer was significantly higher than that in paracancerous tissues, further confirming that EGFR-AS1 in cancer tissues can be released into the plasma. The changing trend of EGFR-AS1 in cancer tissue and plasma is consistent, confirming that EGFR-AS1 can be used as a practical non-invasive biomarker. Despite certain limitations, including the small sample size and imbalanced case numbers in the subgroups, the present study revealed EGFR-AS1 as a marker with potential diagnostic usefulness in CRC. It may also be relevant in the assessment of therapeutic efficiency, which requires further investigation.
Acknowledgments
Funding: This study was supported by the National Natural Science Foundation of China (Grant No. 81800545) and the Technology and Innovation Foundation of Shang Hai Jiao Tong University (Grant No. YG2021QN114).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-968/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-968/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-968/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethical Review Board of Second Affiliated Hospital of Navy Medical University (No. 2017SL627) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Brenner H, Chen C. The colorectal cancer epidemic: challenges and opportunities for primary, secondary and tertiary prevention. Br J Cancer 2018;119:785-92. [Crossref] [PubMed]
- Xi Y, Yuefen P, Wei W, et al. Analysis of prognosis, genome, microbiome, and microbial metabolome in different sites of colorectal cancer. J Transl Med 2019;17:353. [Crossref] [PubMed]
- Biller LH, Schrag D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA 2021;325:669-85. [Crossref] [PubMed]
- Tsilimigras DI, Brodt P, Clavien PA, et al. Liver metastases. Nat Rev Dis Primers 2021;7:27. [Crossref] [PubMed]
- Seeber A, Gastl G. Targeted Therapy of Colorectal Cancer. Oncol Res Treat 2016;39:796-802. [Crossref] [PubMed]
- Bensmaïne. Factors predicting for efficacy of oxaliplatin in combination with 5-fluorouracil (5-FU)+/-folinic acid (FA) in a compassionate-use cohort of 370 5-FU-resistant advanced colorectal cancer (CRC) patients. Eur J Cancer 2000;36:2335-43. [Crossref] [PubMed]
- Midgley R, Kerr D. Conventional cytotoxic and novel therapeutic concepts in colorectal cancer. Expert Opin Investig Drugs 2001;10:1011-9. [Crossref] [PubMed]
- Thomaidis T, Maderer A, Formentini A, et al. Proteins of the VEGFR and EGFR pathway as predictive markers for adjuvant treatment in patients with stage II/III colorectal cancer: results of the FOGT-4 trial. J Exp Clin Cancer Res 2014;33:83. [Crossref] [PubMed]
- Napolitano S, Matrone N, Muddassir AL, et al. Triple blockade of EGFR, MEK and PD-L1 has antitumor activity in colorectal cancer models with constitutive activation of MAPK signaling and PD-L1 overexpression. J Exp Clin Cancer Res 2019;38:492. [Crossref] [PubMed]
- Tolba MF. Revolutionizing the landscape of colorectal cancer treatment: The potential role of immune checkpoint inhibitors. Int J Cancer 2020;147:2996-3006. [Crossref] [PubMed]
- Almquist DR, Ahn DH, Bekaii-Saab TS. The Role of Immune Checkpoint Inhibitors in Colorectal Adenocarcinoma. BioDrugs 2020;34:349-62. [Crossref] [PubMed]
- Van Schaeybroeck S, Allen WL, Turkington RC, et al. Implementing prognostic and predictive biomarkers in CRC clinical trials. Nat Rev Clin Oncol 2011;8:222-32. [Crossref] [PubMed]
- Zhu M, Dang Y, Yang Z, et al. Comprehensive RNA Sequencing in Adenoma-Cancer Transition Identified Predictive Biomarkers and Therapeutic Targets of Human CRC. Mol Ther Nucleic Acids 2020;20:25-33. [Crossref] [PubMed]
- Zhou E, Li Y, Wu F, et al. Circulating extracellular vesicles are effective biomarkers for predicting response to cancer therapy. EBioMedicine 2021;67:103365. [Crossref] [PubMed]
- Sawabata N. Circulating Tumor Cells: From the Laboratory to the Cancer Clinic. Cancers (Basel) 2020;12:3065. [Crossref] [PubMed]
- Krzystek-Korpacka M, Diakowska D, Kapturkiewicz B, et al. Profiles of circulating inflammatory cytokines in colorectal cancer (CRC), high cancer risk conditions, and health are distinct. Possible implications for CRC screening and surveillance. Cancer Lett 2013;337:107-14. [Crossref] [PubMed]
- Relationship among circulating tumor cells, CEA and overall survival in patients with metastatic colorectal cancer. Ann Oncol 2013;24:2708-10. [Crossref] [PubMed]
- Hampton R, Walker M, Marshall J, et al. Differential expression of carcinoembryonic antigen (CEA) splice variants in whole blood of colon cancer patients and healthy volunteers: implication for the detection of circulating colon cancer cells. Oncogene 2002;21:7817-23. [Crossref] [PubMed]
- Dizman N, Meza L, Pal SK. Biomarker approach harnessed in trials of personalized medicine for bladder cancer. Nat Med 2021;27:761-3. [Crossref] [PubMed]
- Darrigues L, Pierga JY, Bernard-Tessier A, et al. Circulating tumor DNA as a dynamic biomarker of response to palbociclib and fulvestrant in metastatic breast cancer patients. Breast Cancer Res 2021;23:31. [Crossref] [PubMed]
- Spizzo R, Nicoloso MS, Croce CM, et al. SnapShot: MicroRNAs in Cancer. Cell 2009;137:586-586.e1. [Crossref] [PubMed]
- Schmitt AM, Chang HY. Gene regulation: Long RNAs wire up cancer growth. Nature 2013;500:536-7. [Crossref] [PubMed]
- Qian Y, Shi L, Luo Z. Long Non-coding RNAs in Cancer: Implications for Diagnosis, Prognosis, and Therapy. Front Med (Lausanne) 2020;7:612393. [Crossref] [PubMed]
- Sohn E. Diagnosis: Frontiers in blood testing. Nature 2017;549:S16-8. [Crossref] [PubMed]
- Cheng CJ, Bahal R, Babar IA, et al. MicroRNA silencing for cancer therapy targeted to the tumour microenvironment. Nature 2015;518:107-10. [Crossref] [PubMed]
- Vu JV, Sheetz KH, De Roo AC, et al. Variation in colectomy rates for benign polyp and colorectal cancer. Surg Endosc 2021;35:802-8. [Crossref] [PubMed]
- Wang A, Bao Y, Wu Z, et al. Long noncoding RNA EGFR-AS1 promotes cell growth and metastasis via affecting HuR mediated mRNA stability of EGFR in renal cancer. Cell Death Dis 2019;10:154. [Crossref] [PubMed]
- Vitiello PP, Cardone C, Martini G, et al. Receptor tyrosine kinase-dependent PI3K activation is an escape mechanism to vertical suppression of the EGFR/RAS/MAPK pathway in KRAS-mutated human colorectal cancer cell lines. J Exp Clin Cancer Res 2019;38:41. [Crossref] [PubMed]
- Atef MM, Amer AI, Hafez YM, et al. Long non-coding RNA EGFR-AS1 in colorectal cancer: potential role in tumorigenesis and survival via miRNA-133b sponge and EGFR/STAT3 axis regulation. Br J Biomed Sci 2021;78:122-9. [Crossref] [PubMed]
(English Language Editor: A. Kassem)


