
Nestin protein affects the maintenance of stem characteristics of colorectal cancer cells based on p53 dependent pathway
Introduction
Colorectal cancer (CRC) is the most common malignancy with a high worldwide incidence and mortality (1,2). Despite curative surgery, chemotherapy, and other comprehensive therapies, CRC mortality remains high. In the past few decades, the 5-year survival rate remained very poor (3,4). Hence, patients die due to the rapid progression of this disease and its frequent recurrent metastasis. However, the pathological mechanism of CRC remains unclear. Identifying the pathogenic target gene that facilitates this disease is of the utmost importance if physicians are to provide effective CRC diagnosis and treatment.
Recently, multiple factors, including oncogenes and tumor suppressors, have been found to function importantly on the progression of malignancies (5,6). Growing evidence shows that protein is a crucial cell regulator (7,8). Neuronal stem cell protein (nestin) is a class VI intermediate filament protein widely expressed in tumors and stem cells. For example, Wang et al. demonstrated that nestin regulated cellular redox homeostasis through the Keap1-Nrf2 feedback loop in lung cancer (9). A study by Lasič et al. found that nestin affected fusion pore dynamics in mouse astrocytes (10). Additionally, nestin was reported to regulate neurogenesis by regulating notch signaling in mice (11). Nevertheless, the role and mechanism of nestin in CRC cells have rarely been explored.
Research has shown that stem cell characteristics play a role in tumor invasion, metastasis, and resistance induced by radiotherapy or chemotherapy (12,13). Nestin, as a member of intermediate filament protein family, has been demonstrated to be association with cancer stem-like property. For example, Nestin is a biomarker of cancer stem cell of triple negative breast cancer (14). While, p53 regulates stemness of colorectal cancer cells via WNT/β-catenin pathway (15). Tschaharganeh et al. suggest that there is interaction between nestin and p65 in liver cancer (16). Cancer stem cells (CSCS) display self-renewal, asymmetric cell division, anti-apoptosis, tumorigenicity, and high metastatic potential (17-19). While, the function of nestin and p65 in CRC was still unknown.
Herein, this study planned to expound the involvement of nestin and p53 in CRC progression. Nestin and p53 expressions in CRC tissues and cell lines were detected, and the relationship between nestin/p53 and the development of CRC was investigated. This study is the first to explore the Nestin-dependent p53 pathway to regulate colorectal cancer cell-like characteristics and regulate the malignant biological behavior of tumor cells. Our findings may provide a promising prognostic and therapeutic marker and offer new insights for improving CRC treatment. We present the following article in accordance with the MDAR reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-788/rc).
Methods
Tissues samples
Together 25 pairs of tumor and para-carcinoma tissues were gained from CRC patients admitted to Meizhou People’s Hospital. All tissues were rapidly frozen in liquid nitrogen, and the tissue samples were stored at −80 °C until needed. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Meizhou People’s Hospital (No. 2022-CY-1). Written informed consent was gained from each patient.
Cell culture
The human CRC cell lines Caco-2, RKO, HT29, HCT116, and LoVo were purchased from the National Collection of Authenticated Cell Cultures (Shanghai, China). The cells were cultured with Dulbecco’s Modified Eagle Medium (DMEM, Gibco, USA) containing 10% fetal bovine serum (FBS, Invitrogen, USA) in a humidified atmosphere with 5% CO2 at 37 °C.
Cell transfection
The siRNAs nestin #1 (siRNA #1), nestin #2 (siRNA #2), nestin #3 (siRNA #3), and negative control (NC), plus pcDNA 3.1 nestin (nestin), pcDNA 3.1 p53 (p53), and control empty vector were purchased from RiboBio (Guangzhou, China). The synthetic oligonucleotides or plasmids were transfected into the CRC cells using Lipofectamine® 2000 (Invitrogen).
Cell counting kit-8 (CCK-8) assay
The Caco-2 and RKO cells (1×104/well) were inoculated in 96-well plates and cultured for 48 h to assess cell viability, which was detected with a CCK-8 kit (Beyotime Biotechnology, China). Subsequently, we used a microplate reader to measure the optical density (OD) at 450 nm.
5-ethynyl-2'-deoxyuridine (EdU) assay
After cell transfection, the Caco-2 and RKO cells (1×104/well) were inoculated in 96-well plates and cultured for 48 h. After washing with PBS, they were incubated with 10 µM EdU for 2 h at 37 °C. Then, the cells were fixed with 4% paraformaldehyde for 20 min. After adding 0.5% Triton X-100 in PBS for a further 20 min of incubation, staining solution was added to each well, and the cells were incubated in the dark for 30 min. After washing twice with PBS, the samples were placed in a 6-diamidino-2-phenylindole (DAPI) solution for a further 30 min. EdU-positive cells were detected under an inverted fluorescence microscope.
Sphere formation analysis
The transfected cells (1×104 cells per well) were seeded into an ultra-low-attachment 6-well plate for 24 h. The cells were supplemented in DMEM containing N2 (1%) and B27 supplements (2%), human platelet growth factor (20 ng/mL), and epidermal growth factor (100 ng/mL). After 7 days of culture, the spheroids were photographed, and their diameters were calculated.
Quantitative real-time polymerase chain reaction (RT-qPCR) assay
The total RNA of Caco-2 and RKO was extracted using Trizol (Invitrogen, CA, USA). Then, cDNA was reversely transcribed from RNA (1 µg) using a Prime Script RT Master Mix kit (TAKARA, Japan). PCR was performed with the StepOnePlus™ Real-Time PCR System (ABI, USA). GAPDH was used as the internal control. The gene transcription level was calculated using the 2−∆∆Ct method in triplicate.
Western blot assay
Protein was isolated from the CRC cells using a radioimmunoprecipitation (RIP) lysis buffer. SDS-PAGE (10%) was used in electrophoretic separation of total proteins, and the separated protein was transferred to a PVDF membrane. Then, the membranes and primary antibody were incubated below 4 °C overnight at a dilution of 1:1,000. Subsequently, an HRP-conjugated IgG-antibody was used for the second incubation (1 h, room temperature). Finally, an enhanced chemiluminescence (ECL) kit (Millipore, Merck, USA) visualized the protein bands.
Immunofluorescence staining
The Caco-2 and RKO cells (1×104/well) were inoculated into 96-well plates for 48 h. Then, cells were fixed with 4% paraformaldehyde for 10 min, and treated with 0.02% Triton X-100 for 10 min. After blocking with 5% bovine serum albumin in PBS, the cells were incubated with Ki-67 primary antibody at 4 °C. After 12 h, the cells were washed and treated with Alexa Fluor® 488-conjugated IgG-antibody at room temperature for 2 h. Negative control consisted of cells without the primary antibody. DAPI was used to stain the nucleus. The images were obtained using a laser scanning confocal microscopy (Leika, Germany).
Transwell assay
After 48-h cell transfection, the Caco-2 or RKO cells in 100 µL cell suspension were inoculated into the upper part of a 24-well plate and cultured in an 8 µm Transwell chamber (Corning Corporation) for 48 h, and culture medium with 15% FBS was added to the lower chamber. For detecting cell invasion, the upper chamber was covered with Matrigel (BD Biosciences, USA). After 24 h of cell culture, the inferolateral cells were fixed with 4% paraformaldehyde and stained using 0.1% crystal violet. Five visual fields were randomly selected to assess the cells’ migration and invasion ability using a microscope (Nikon company).
Statistical analysis
All data were presented as mean ± standard deviation (SD) and the analysis was performed with GraphPad Prism 8.0 (GraphPad Software, USA). A one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc analysis was used to compare the differences between groups. The online database, Kaplan-Meier Plotter (http://kmplot.com/analysis/index.php), was used to analyze the relationship between Nestin expression level and patient prognosis in CRC patients. A P value <0.05 meant statistically significant.
Results
The expression of nestin and p53 in CRC tissues and cells
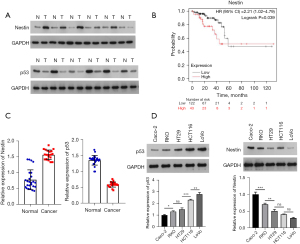
Firstly, we determined the nestin and p53 expression levels in the CRC and normal tissues. The results revealed that nestin protein expression was significantly higher, and p53 expression was significantly lower in CRC tissues than that in para-carcinoma tissues (Figure 1A), suggesting that differentially expressed nestin and p53 are related to CRC occurrence. In addition, the Kaplan Meier database showed that a high level of nestin in tumor tissue was correlated with poor overall survival in CRC patients (P=0.039, Figure 1B). Subsequently, western blot was used to examine the expression of nestin and p53 in CRC cell lines (Caco-2, RKO, HT29, HCT116, and LoVo). As shown in Figure 1C,1D, LoVo and HCT116 exhibited the highest expressions, whereas Caco-2 and RKO showed the lowest expressions and thus were chosen for the following experiments.
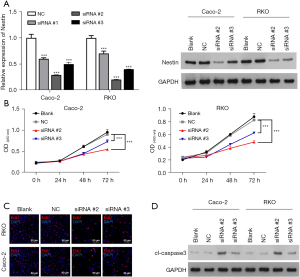
Nestin promoted cell proliferation and suppressed cell apoptosis in CRC
To further examine the role of nestin in CRC progression, we knocked down nestin expression by transfecting nestin siRNAs into Caco-2 and RKO cells and detected their knockout efficiency. We clearly saw a decreased expression of nestin compared with cells transfected with NC, especially in siRNA #2 and siRNA #3 (Figure 2A). Subsequently, to show nestin influencing proliferation of CRC cells, CCK-8 and EdU staining was conducted. The results indicated that knockdown of nestin significantly inhibited cell growth, showing decreased OD450nm values (Figure 2B). Additionally, the results of the EdU staining showed that knockdown of nestin in the Caco-2 and RKO cells significantly reduced the EdU positive cells (shown in red) compared with the NC group (Figure 2C). The protein expression level of apoptotic factor, cleaved-caspase 3, was significantly increased (Figure 2D).
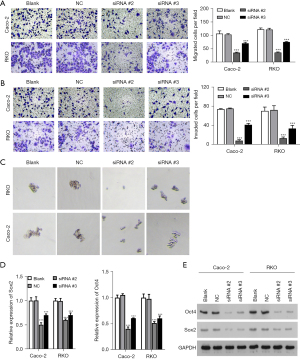
Nestin promoted the migration and invasion of CRC cells
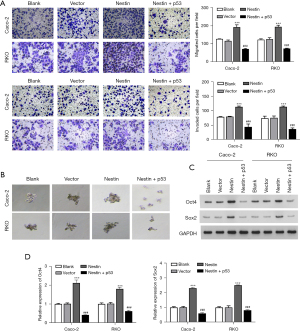
A Transwell assay was applied to assess the role of nestin in the metastatic abilities of Caco-2 and RKO cells. Compared with the NC group, nestin siRNAs significantly reduced the number of migrated cells (Figure 3A). Similarly, the Transwell assay results indicated that the number of invasive Caco-2 and RKO cells was markedly reduced after transfection with nestin siRNAs, compared with those in the NC group (Figure 3B). The number and diameter of spheres were also assessed using a sphere formation analysis. The number of spheres was significantly decreased in the group of nestin-knocked down (Figure 3C). In conjunction with the above results, the protein expressions of the stemness markers, Oct4 and Sox2, were also markedly downregulated when nestin was knocked down in the Caco-2 and RKO cells (Figure 3D,3E).
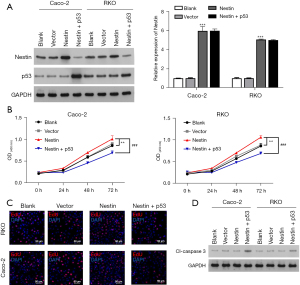
P53 overexpression attenuated nestin functioning in CRC
A recent study has confirmed that p53 mutations occur in malignant tumors and are interrelated to aggressive tumor behavior (20). To verify whether nestin mediated cellular function via p53, we first constructed the nestin and p53 overexpression vector. Caco-2 and RKO cells were co-transfected with nestin and p53. The western blot assay results showed that nestin protein was significantly stimulated by nestin overexpression and attenuated by p53 and showed a significantly increased expression of p53 in the nestin + p53 group. There was no obvious change in p53 levels between the nestin and vector cells (Figure 4A). As the CCK-8 assay shows in Figure 4B, the proliferation activity of Caco-2 and RKO cells was significantly enhanced after single transfection with nestin. However, after co-transfection with p53, its proliferative activity decreased. Similarly, the EdU staining revealed the same results (Figure 4C). As expected, p53 reversed the protein expression changes of cleaved-caspase 3 due to nestin overexpression (Figure 4D).
Furthermore, the promotional effects of nestin overexpression on migration and invasion were notably reversed by p53 upregulation in Caco-2 and RKO cells (Figure 5A). Next, the number and diameter of spheres were assessed in each group. The data revealed that nestin-overexpressing significantly increased the number and diameter of spheres compared with the vector. However, these effects were reversed when the cells were transfected with overexpressed p53 (Figure 5B). Finally, the western blot assay results demonstrated that the Oct4 and Sox2 stem marker protein and mRNA levels were significantly increased by nestin transfection, whereas this effect was reversed by p53 overexpression (Figure 5C,5D).
Discussion
Protein coding genes have major biological functions and play an important role in tumor progression (20-23). In recent years, a number of reports on CRC and protein-coding genes have emerged. During CRC progression, many protein-coding genes act as oncogenes, such as myeloid differentiation factor 88 (MyD88) (24) ubiquitin‑like modifieractivating enzyme 2 (UBA2) (25). EIF3H (26), MCCC2 (27), SLC38A1 (28) and many protein-coding genes function as tumor suppressors, such as FH535 (29), ubiquitin-specific protease 44 (USP44) (30), phosphatase of regenerating liver-3 (PRL-3) (31), CPEB3 (32), etc. Even so, the investigation of CRC is still challenging, and many relevant questions remain to be answered.
Nestin, a human dysregulated protein in several cancer types, is reported to have prominent effects on cancer cell proliferation and cell stems. Stemness of cancer are closely related to tumor metastasis. Tang et al. discover that in colorectal cancer, tumor cells maintain stemness-like characteristics through the Wnt/β-catenin/c-MYC/SOX2 pathway, and regulate the metastasis and recurrence of colorectal cancer (33). Besides, a related study reported that nestin was significantly associated with overall survival in non-small cell lung cancer (34). Additionally, Nowak et al. found that nestin expression in blood vessels was associated with shorter overall survival of patients with breast cancer (35). Bokhari et al. demonstrated that knock nestin down impeded cancer cell proliferation and metastatic potential by inactivating the TGF-β signaling pathway (36). Similarly, in this study, we demonstrated that the protein level of nestin was significantly increased in CRC tissues and cancer cell lines. Our results are consistent with previous reports, indicating that an abnormal nestin expression might act as an oncogene in CRC.
Although the abnormal regulation and diagnostic value of nestin in malignant diseases has been reported previously, its biological function and molecular mechanism in CRC have remained unclear. A previous study has shown that nestin overexpression correlates to worse prognosis and that nestin inhibition suppresses tumor stemness (37). Nestin expression has also been reported in lung cancers, where it was shown to regulate the proliferation, migration, invasion, and stemness of lung adenocarcinoma (38). Tschaharganeh et al. also demonstrated that p53-regulated nestin expression was important to tumor suppression of HCC (16). P53 acts as a tumor suppressor gene due to its transcription factor function in many malignant tumors. As such, p53 is lost or mutated in most human cancers. A previous study reported that p53 restricted the expression of nestin, a protein indicating cell stemness, and which resulted in inhibition of tumor initiation in vivo (16). In addition, P53 also regulates the expression of other genes related to cell stemness, for example, Nanog and CD44 (39,40). In view of this, the present study assessed the biological functions of nestin in Caco-2 and RKO cells using RT-qPCR, CCK-8, EdU, transwell migration, invasion, and western blot analyses. As expected, the in vitro experiments demonstrated that nestin silencing dramatically inhibited cell proliferation, migration, invasion, and stemness in CRC cells. Subsequently, we provided evidence that nestin interacted with p53, and both proteins participated in CRC progression.
In summary, our findings showed that the expression of nestin was significantly elevated in CRC tissues and related cancer cell lines, and silencing of nestin by regulating p53 suppressed the cell viability, proliferation, stemness, migration, and invasive ability of CRC (Figure 6). Collectively, these results indicated that nestin functions importantly in the development of CRC. Nestin and p53 could potentially be considered therapeutic targets for diagnosing and treating CRC. The interaction between ncRNAs (lncRNA or miRNA, etc.) and Nestin/P53 will be focused in the future investigation.
Acknowledgments
Funding: This work was supported by the Medical Scientific Research Foundation of Guangdong Province of China (No. B2021442).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-788/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-788/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-788/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All patients signed written informed consent. The study was approved by the Ethics Committee of Meizhou People’s Hospital (No. 2022-CY-1).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Qiu H, Cao S, Xu R. Cancer incidence, mortality, and burden in China: a time-trend analysis and comparison with the United States and United Kingdom based on the global epidemiological data released in 2020. Cancer Commun (Lond) 2021;41:1037-48. [Crossref] [PubMed]
- Ladabaum U, Dominitz JA, Kahi C, et al. Strategies for Colorectal Cancer Screening. Gastroenterology 2020;158:418-32. [Crossref] [PubMed]
- Burnett-Hartman AN, Lee JK, Demb J, et al. An Update on the Epidemiology, Molecular Characterization, Diagnosis, and Screening Strategies for Early-Onset Colorectal Cancer. Gastroenterology 2021;160:1041-9. [Crossref] [PubMed]
- Neureiter D, Jäger T, Ocker M, et al. Epigenetics and pancreatic cancer: pathophysiology and novel treatment aspects. World J Gastroenterol 2014;20:7830-48. [Crossref] [PubMed]
- Michalak EM, Burr ML, Bannister AJ, et al. The roles of DNA, RNA and histone methylation in ageing and cancer. Nat Rev Mol Cell Biol 2019;20:573-89. [Crossref] [PubMed]
- Toivola DM, Strnad P, Habtezion A, et al. Intermediate filaments take the heat as stress proteins. Trends Cell Biol 2010;20:79-91. [Crossref] [PubMed]
- Bartrons R, Rodríguez-García A, Simon-Molas H, et al. The potential utility of PFKFB3 as a therapeutic target. Expert Opin Ther Targets 2018;22:659-74. [Crossref] [PubMed]
- Wang J, Lu Q, Cai J, et al. Nestin regulates cellular redox homeostasis in lung cancer through the Keap1-Nrf2 feedback loop. Nat Commun 2019;10:5043. [Crossref] [PubMed]
- Lasič E, Trkov Bobnar S, Wilhelmsson U, et al. Nestin affects fusion pore dynamics in mouse astrocytes. Acta Physiol (Oxf) 2020;228:e13399. [Crossref] [PubMed]
- Wilhelmsson U, Lebkuechner I, Leke R, et al. Nestin Regulates Neurogenesis in Mice Through Notch Signaling From Astrocytes to Neural Stem Cells. Cereb Cortex 2019;29:4050-66. [Crossref] [PubMed]
- Wang L, Liu Y, Zhou Y, et al. Zoledronic acid inhibits the growth of cancer stem cell derived from cervical cancer cell by attenuating their stemness phenotype and inducing apoptosis and cell cycle arrest through the Erk1/2 and Akt pathways. J Exp Clin Cancer Res 2019;38:93. [Crossref] [PubMed]
- Cao S, Wang Z, Gao X, et al. FOXC1 induces cancer stem cell-like properties through upregulation of beta-catenin in NSCLC. J Exp Clin Cancer Res 2018;37:220. [Crossref] [PubMed]
- Feng W, Liu S, Zhu R, et al. SOX10 induced Nestin expression regulates cancer stem cell properties of TNBC cells. Biochem Biophys Res Commun 2017;485:522-8. [Crossref] [PubMed]
- Cho YH, Ro EJ, Yoon JS, et al. 5-FU promotes stemness of colorectal cancer via p53-mediated WNT/β-catenin pathway activation. Nat Commun 2020;11:5321. [Crossref] [PubMed]
- Tschaharganeh DF, Xue W, Calvisi DF, et al. p53-dependent Nestin regulation links tumor suppression to cellular plasticity in liver cancer. Cell 2014;158:579-92. [Crossref] [PubMed]
- Xu X, Zhang X, Wei C, et al. Targeting SLC7A11 specifically suppresses the progression of colorectal cancer stem cells via inducing ferroptosis. Eur J Pharm Sci 2020;152:105450. [Crossref] [PubMed]
- Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature 2004;432:396-401. [Crossref] [PubMed]
- Lytle NK, Barber AG, Reya T. Stem cell fate in cancer growth, progression and therapy resistance. Nat Rev Cancer 2018;18:669-80. [Crossref] [PubMed]
- Kou YB, Zhang SY, Zhao BL, et al. Knockdown of MMP11 inhibits proliferation and invasion of gastric cancer cells. Int J Immunopathol Pharmacol 2013;26:361-70. [Crossref] [PubMed]
- Li M, Zheng H, Duan Z, et al. Promotion of cell proliferation and inhibition of ADCC by cancerous immunoglobulin expressed in cancer cell lines. Cell Mol Immunol 2012;9:54-61. [Crossref] [PubMed]
- Shi J, Guan X, Zhan F, et al. CSN6 expression is associated with pancreatic cancer progression and predicts poor prognosis. Cancer Biol Ther 2019;20:1290-9. [Crossref] [PubMed]
- Xu XC, Abuduhadeer X, Zhang WB, et al. Knockdown of RAGE inhibits growth and invasion of gastric cancer cells. Eur J Histochem 2013;57:e36. [Crossref] [PubMed]
- Zhu G, Cheng Z, Huang Y, et al. MyD88 mediates colorectal cancer cell proliferation, migration and invasion via NF-κB/AP-1 signaling pathway. Int J Mol Med 2020;45:131-40. [PubMed]
- He P, Sun X, Cheng HJ, et al. UBA2 promotes proliferation of colorectal cancer. Mol Med Rep 2018;18:5552-62. [PubMed]
- Yu G, Liao J, Wu J, et al. The proliferation of colorectal cancer cells is suppressed by silencing of EIF3H. Biosci Biotechnol Biochem 2018;82:1694-701. [Crossref] [PubMed]
- Dai W, Feng H, Lee D. MCCC2 overexpression predicts poorer prognosis and promotes cell proliferation in colorectal cancer. Exp Mol Pathol 2020;115:104428. [Crossref] [PubMed]
- Zhou FF, Xie W, Chen SQ, et al. SLC38A1 promotes proliferation and migration of human colorectal cancer cells. J Huazhong Univ Sci Technolog Med Sci 2017;37:30-6. [Crossref] [PubMed]
- Tu X, Hong D, Jiang Y, et al. FH535 inhibits proliferation and migration of colorectal cancer cells by regulating CyclinA2 and Claudin1 gene expression. Gene 2019;690:48-56. [Crossref] [PubMed]
- Huang T, Zhang Q, Ren W, et al. USP44 suppresses proliferation and enhances apoptosis in colorectal cancer cells by inactivating the Wnt/β-catenin pathway via Axin1 deubiquitination. Cell Biol Int 2020;44:1651-9. [Crossref] [PubMed]
- Xu H, Zeng Y, Liu L, et al. PRL-3 improves colorectal cancer cell proliferation and invasion through IL-8 mediated glycolysis metabolism. Int J Oncol 2017;51:1271-9. [Crossref] [PubMed]
- Fang Y, Zhong Q, Wang Y, et al. CPEB3 functions as a tumor suppressor in colorectal cancer via JAK/STAT signaling. Aging (Albany NY) 2020;12:21404-22. [Crossref] [PubMed]
- Tang Q, Chen J, Di Z, et al. TM4SF1 promotes EMT and cancer stemness via the Wnt/β-catenin/SOX2 pathway in colorectal cancer. J Exp Clin Cancer Res 2020;39:232. [Crossref] [PubMed]
- Zhang D, Wang J. Nestin is significantly associated with the overall survival of nonsmall cell lung cancer: A meta-analysis. J Cancer Res Ther 2020;16:800-3. [Crossref] [PubMed]
- Nowak A, Grzegrzolka J, Paprocka M, et al. Nestin-positive microvessel density is an independent prognostic factor in breast cancer. Int J Oncol 2017;51:668-76. [Crossref] [PubMed]
- Bokhari AA, Baker TM, Dorjbal B, et al. Nestin suppression attenuates invasive potential of endometrial cancer cells by downregulating TGF-β signaling pathway. Oncotarget 2016;7:69733-48. [Crossref] [PubMed]
- Ishiwata T, Matsuda Y, Naito Z. Nestin in gastrointestinal and other cancers: effects on cells and tumor angiogenesis. World J Gastroenterol 2011;17:409-18. [Crossref] [PubMed]
- Narita K, Matsuda Y, Seike M, et al. Nestin regulates proliferation, migration, invasion and stemness of lung adenocarcinoma. Int J Oncol 2014;44:1118-30. [Crossref] [PubMed]
- Godar S, Ince TA, Bell GW, et al. Growth-inhibitory and tumor- suppressive functions of p53 depend on its repression of CD44 expression. Cell 2008;134:62-73. [Crossref] [PubMed]
- Lin T, Chao C, Saito S, et al. p53 induces differentiation of mouse embryonic stem cells by suppressing Nanog expression. Nat Cell Biol 2005;7:165-71. [Crossref] [PubMed]



