
Apparent diffusion coefficient is a good marker in predicting the prognosis in colorectal cancer liver metastases: a diagnostic study
Introduction
Colorectal cancer (CRC) is a common cancer that is estimated to have more than 1 million new cases a year globally (1). Most CRC cases are sporadic with no family history or significant genetic predisposition, and people >50 years are at the highest risk (2). A study has shown that the cumulative incidence of liver metastasis in patients with CRC in 5 years is up to 15% (3), and another study has shown that the incidence of liver metastasis within 5 years after diagnosis of CRC is up to 26.5% (4). Among them, left colon cancer is the most prone to liver metastasis (4). About 10% of CRC patients can have liver metastases at the first diagnosis, and it is important to determine whether there is liver metastasis before operation for the treatment and the prognosis of patients. Therefore, it is of great significance to accurately identify liver metastatic cancer before surgery. Pathological biopsy is the gold standard for diagnosing liver metastatic cancer, but there are some difficulties in preoperative biopsy. At present, clinical diagnosis of CRC liver metastasis (CRCLM) is mostly based on imaging, and there is a certain false-positive rate and false-negative rate. For example, ultrasound is the preferred imaging method for CRCLM. However, ultrasound is not ideal for liver tumors with deep location, small volume or lack of blood supply. Improving the diagnostic accuracy of imaging for CRCLM is highly significant. Due to its high sensitivity and specificity, magnetic resonance diffusion weighted imaging has been widely used to distinguish the benign and malignant characteristics of liver nodules (5-9). However, there are few studies that focus on the diagnostic value of apparent diffusion coefficient on CRCLM. The aim of the present study was to explore the diagnostic value of apparent diffusion coefficients based on magnetic resonance diffusion weighted imaging for CRCLM. We present the following article in accordance with the STARD reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-861/rc).
Methods
General information
The data of 50 patients with CRCLM admitted to Hebei Yanda Hospital from January 2018 to December 2018 were retrospectively collected (a retrospectively diagnostic study), and the data of 50 patients with benign liver nodules were collected at the same time. Inclusion criteria of the CRCLM group were as follows: (I) primary lesion pathologically diagnosed with CRC, and isolated liver nodule postoperative pathological diagnosis as liver metastatic cancer (derived from CRC); (II) aged 18−65 years; (III) magnetic resonance diffusion weighted imaging examination performed at Hebei Yanda Hospital with apparent diffusion coefficient and so on before surgery; (IV) no lung, bone, or brain metastases; and (V) complete clinicopathological data. Exclusion criteria for the CRCLM group were as follows: (I) recurrent CRC; (II) primary liver cancer; (III) concomitant with other malignant tumors; (IV) abnormal portal vein and mesenteric arteriovenous structure; (V) hepatic insufficiency; (VI) special treatments, such as radiotherapy, chemotherapy, immunotherapy, and targeted therapy had been performed before surgery; and (VII) other serious diseases. The benign liver nodule group included patients aged 18−65 years with isolated benign nodules without the above diseases and no other serious diseases. The types of liver nodules in the benign liver nodule group were as follows: focal nodular hyperplasia, hepatic hemangioma, liver cyst, hepatic hamartoma, and mesenchymal tumors with 10 cases for each type. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Hebei Yanda Hospital (No. 20220040) and individual consent for this retrospective analysis was waived.
Examination procedure
Liver MAGNETIC RESONANCE DIFFUSION WEIGHTED IMAGING examination was performed by Siemens 3.0T magnetic resonance imaging (MRI) (Siemens, 3.0T, Shenzhen), and contrast medium (gadolinium spray glucosamine, 0.2 mmol/kg) was administered through the forearm intravenously at a rate of 2.5 mL/s. Dynamic scan was performed once with contrast agent group injection and saline flushing tube. Images were acquired for 6 consecutive times before and after enhancement, and liver MRI diffusion weighted imaging was obtained with apparent diffusion coefficient of the central part of the liver nodule.
Study variables
The study variables were as follows: (I) general information, including age, sex, body mass index, smoking history, and drinking history; (II) apparent diffusion coefficient and diameter of liver nodules; (III) whether there was a combination of portal vein tumor thrombus; (IV) pathological characteristics of primary lesions, including number of metastatic regional lymph nodes, tumor T stage, primary lesion site, and differentiation degree of primary tumor cells; and (V) long-term prognosis: all patients underwent surgical treatment after diagnosis, and primary lesions and liver metastatic cancer were removed at the same time. Three-year follow up after surgery was conducted in clinic or via phone, and the 3-year survival rate of patients after surgery was observed.
Statistical analysis
The data analysis of this study was completed using SPSS 26.0 (IBM, Armonk, NY, USA), and 2-tailed P<0.05 was considered statistically significant. Measurement data were normally distributed and expressed as mean ± standard deviation. Independent samples t-test was applied in the comparison of the measurement data between 2 groups. Enumeration data, expressed as n (%), were analyzed by Pearson’s χ2-test in the comparison of the measurement data between 2 groups. The diagnostic value of apparent diffusion coefficients for CRCLM and predicting value of apparent diffusion coefficients for death were analyzed using receiver-operating characteristic curves.
Results
Comparison of clinical data of patients in the 2 groups
There was no statistical difference in age, sex, body mass index, smoking history, drinking history, and liver nodule diameter between the 2 groups (P>0.05). Compared with the benign liver nodule group, the apparent diffusion coefficients of patients in the CRCLM group were significantly decreased [(1.14±0.26 vs. 2.06±0.57) ×10−3 mm2/s, P<0.001] (Table 1).
Table 1
| Category | CRCLM group (n=50) | Benign liver nodule group (n=50) | t/χ2 value | P value |
|---|---|---|---|---|
| Age (years) | 52.26±7.78 | 53.00±7.39 | 0.488 | 0.627 |
| Sex | 0.160 | 0.689 | ||
| Male | 25 (50%) | 27 (54%) | ||
| Female | 25 (50%) | 23 (46%) | ||
| Body mass index (kg/m2) | 27.39±4.48 | 27.95±4.98 | 0.598 | 0.551 |
| Smoking history | 10 (20%) | 14 (28%) | 0.877 | 0.349 |
| Drinking history | 17 (34%) | 13 (26%) | 0.762 | 0.383 |
| Apparent diffusion coefficient (×10−3 mm2/s) | 1.14±0.26 | 2.06±0.57 | 10.470 | <0.001 |
| Liver nodule diameter (cm) | 4.24±1.72 | 4.19±1.58 | 0.135 | 0.893 |
Data are expressed as mean ± standard deviation or n (%). CRCLM, colorectal cancer liver metastasis.
Diagnostic value of the apparent diffusion coefficient in distinguishing between benign liver nodules and liver metastatic cancer
The apparent diffusion coefficient was of high value in differentiating between benign liver nodules and liver metastatic cancer. The area under the curve was 0.927 [95% confidence interval (CI): 0.879–0.975, P<0.001], the optimal diagnostic cut-off value was 1.315×10−3 mm2/s, and the sensitivity and specificity were 0.94 and 0.78, respectively (Figure 1).
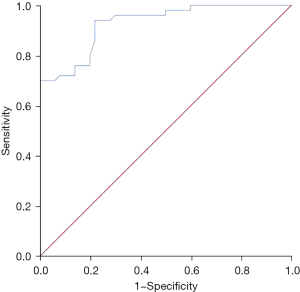
Correlation between the apparent diffusion coefficient of liver nodules and clinical pathological features in patients with CRCLM
The apparent diffusion coefficient of liver nodules in patients with CRCLM was not significantly correlated with portal vein tumor thrombus, T stage, and primary lesion site (P>0.05). Compared with patients with highly differentiated tumors, the apparent diffusion coefficients of patients with moderately and poorly differentiated tumors were significantly reduced [(1.08±0.26 vs. 1.29±0.22)×10−3 mm2/s, P=0.010]. Compared with patients who survived 3 years after operation, the apparent diffusion coefficients of patients who died were significantly lower [(1.05±0.26 vs. 1.23±0.23)×10−3 mm2/s, P=0.011]. There was no significant correlation between the apparent diffusion coefficient of liver nodules in patients with CRCLM and the number of metastatic regional lymph node and the diameter of liver nodules (P>0.05) (Tables 2,3).
Table 2
| Category | Apparent diffusion coefficient (×10−3 mm2/s) | t-value | P value |
|---|---|---|---|
| Portal vein tumor thrombus | 1.498 | 0.141 | |
| Yes (n=9) | 1.02±0.12 | ||
| No (n=41) | 1.16±0.28 | ||
| T-stage | 0.030 | 0.976 | |
| III (n=23) | 1.14±0.28 | ||
| IV (n=27) | 1.14±0.25 | ||
| Primary lesion site | 1.903 | 0.063 | |
| Colon (n=24) | 1.07±0.20 | ||
| Rectum (n=26) | 1.20±0.30 | ||
| Differentiation degree | 2.695 | 0.010 | |
| Moderately and poorly differentiated (n=36) | 1.08±0.26 | ||
| Well differentiated (n=14) | 1.29±0.22 | ||
| 3-year survival | 2.652 | 0.011 | |
| Death (n=25) | 1.05±0.26 | ||
| Survival (n=25) | 1.23±0.23 |
Data are expressed as mean ± standard deviation. CRCLM, colorectal cancer liver metastasis.
Table 3
| Category | r value | P value |
|---|---|---|
| Liver nodule diameter (cm) | 0.067 | 0.507 |
| Metastatic regional lymph node number | 0.025 | 0.862 |
CRCLM, colorectal cancer liver metastasis.
Predictive value of the apparent diffusion coefficient of liver nodules in patients with CRCLM for postoperative 3-year survival
The apparent diffusion coefficients of liver nodules in patients with CRCLM had some diagnostic value for postoperative 3-year survival, and the area under the curve was 0.728 (95% CI: 0.580–0.876, P=0.006). The optimal diagnostic cut-off value was 1.115×10−3 mm2/s, and the sensitivity and specificity were 0.76 and 0.76, respectively (Figure 2).
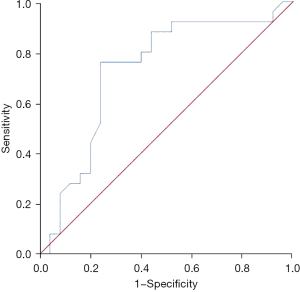
Discussion
CRC is a common malignant tumor of the digestive tract. Preoperative treatment, surgery, and postoperative adjuvant therapy are important treatment strategies for a variety of malignant tumors (10-12). Individualized treatment of CRC needs to be based on detailed preoperative assessment. Currently MRI is a useful means to distinguish between CRCLM and benign liver nodules, and the apparent diffusion coefficient is a quantitative parameter in magnetic resonance diffusion weighted imaging. The findings of the present study indicated that the apparent diffusion coefficient had high value for distinguishing between CRCLM and benign liver nodules, and it had certain value for predicting the 3-year survival of patients after surgery. It was confirmed that the apparent diffusion coefficient was also related to the differentiation degree of tumor cells.
Magnetic resonance diffusion weighted imaging is an imaging method using the special sequence of magnetic resonance to observe the microscopic diffusion motion of water molecules in living tissue, which is the only imaging technology that can detect the free diffusion activity of water molecules in the absence of invasive operation in humans. It has been used for the diagnosis and treatment of a variety of solid tumors (13-15). The apparent diffusion coefficient is used to assess the free diffusion activity of water molecules, especially the movement of water molecules in tissue cells. The cell density of malignant nodules is high, and the tissue gap is small, which leads to the restriction of the free diffusion movement of water molecules manifested by a decreased apparent diffusion coefficient. Conversely, benign tumors have relatively low cell density and a large tissue gap, so the free diffusion movement of water molecules is relatively free, manifested by a high apparent diffusion coefficient. The apparent diffusion coefficient has been confirmed to have high value in identifying various malignant tumors (16-19). Zheng et al. found that the apparent diffusion coefficient had a high predictive value for the response to chemotherapy in gastrointestinal cancer patients with liver metastases (20). Another study showed that the apparent diffusion coefficient was related to the response to chemotherapy in CRC patients (21). These results support those of the present study, but the focus of this study was different from those studies. In the present study, we mainly analyzed the value of apparent diffusion coefficients in distinguishing between liver metastatic cancer and benign liver nodules, and analyzed the correlation between apparent diffusion coefficients and clinical pathological features. The results showed that apparent diffusion coefficients were related to the differentiation degree of tumor cells and prognosis of patients. We found that patients with moderately and poorly differentiated tumors had low apparent diffusion coefficients. This was because that tumor cells of moderately and poorly differentiated tumors multiplied fast, and cell density was high. In patients with bladder cancer and ovarian cancer, it was also found that the apparent diffusion coefficients were correlated with the differentiation degree of tumor cells (22,23), which supports the findings of the present study. In addition, studies on patients with gastric cancer and cervical cancer confirmed that apparent diffusion coefficient plays an important role in predicting the prognosis of patients, and patients with low apparent diffusion coefficients tended to have high tumor cell density and susceptibility to metastasis, resulting in poor prognosis (24,25). However, to the best of our knowledge, there are no studies exploring the correlation between the apparent diffusion coefficient of CRCLM patients and the differentiation degree of tumor cells and prognosis, so our findings have clinical significance.
Limitations
This study was a retrospective clinical study, and there were some limitations. Only about 10% of CRCLM occurred at the first diagnosis of CRC, and some patients chose preoperative treatment, so the number of cases was relatively insufficient. Moreover, due to the limited number of patients suffered with death in our study, we failed to study the risk factors of death using multiple Cox regression analysis. Further large sample size multicenter studies are needed.
Conclusions
The apparent diffusion coefficient decreased in patients with CRCLM, which was of high value for the diagnosis of CRCLM and was related to the clinicopathological features and prognosis of patients.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-861/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-861/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-861/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Hebei Yanda Hospital (No. 20220040) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Teng D, Xia S, Hu S, et al. miR-887-3p Inhibits the Progression of Colorectal Cancer via Downregulating DNMT1 Expression and Regulating P53 Expression. Comput Intell Neurosci 2022;2022:7179733. [Crossref] [PubMed]
- Landreau P, Drouillard A, Launoy G, et al. Incidence and survival in late liver metastases of colorectal cancer. J Gastroenterol Hepatol 2015;30:82-5. [Crossref] [PubMed]
- Engstrand J, Nilsson H, Strömberg C, et al. Colorectal cancer liver metastases - a population-based study on incidence, management and survival. BMC Cancer 2018;18:78. [Crossref] [PubMed]
- Cai Q, Mao Y, Dai S, et al. The growth pattern of liver metastases on MRI predicts early recurrence in patients with colorectal cancer: a multicenter study. Eur Radiol 2022; Epub ahead of print. [Crossref] [PubMed]
- Sakai N, Hayano K, Mishima T, et al. Fat signal fraction assessed with MRI predicts hepatic recurrence following hepatic resection for colorectal liver metastases. Langenbecks Arch Surg 2022;407:1981-9. [Crossref] [PubMed]
- Reynolds IS, Cromwell PM, Ryan ÉJ, et al. An Analysis of Clinicopathological Outcomes and the Utility of Preoperative MRI for Patients Undergoing Resection of Mucinous and Non-Mucinous Colorectal Cancer Liver Metastases. Front Oncol 2022;12:821159. [Crossref] [PubMed]
- Wang X, Wang Y, Zhang Z, et al. Rim enhancement on hepatobiliary phase of pre-treatment 3.0 T MRI: A potential marker for early chemotherapy response in colorectal liver metastases treated with XELOX. Eur J Radiol. 2021;143:109887. Correction in Eur J Radiol 2022;148:110154. [Crossref] [PubMed]
- Li WH, Wang S, Liu Y, et al. Differentiation of histopathological growth patterns of colorectal liver metastases by MRI features. Quant Imaging Med Surg 2022;12:608-17. [Crossref] [PubMed]
- Chen Y, Qi A, Teng D, et al. Probiotics and synbiotics for preventing postoperative infectious complications in colorectal cancer patients: a systematic review and meta-analysis. Tech Coloproctol 2022;26:425-36. [Crossref] [PubMed]
- Qi A, Li Y, Yan S, et al. Effect of postoperative chemotherapy on blood glucose and lipid metabolism in patients with invasive breast cancer. Gland Surg 2021;10:1470-7. [Crossref] [PubMed]
- Qi A, Li Y, Yan S, et al. Effect of anthracycline-based postoperative chemotherapy on blood glucose and lipid profiles in patients with invasive breast cancer. Ann Palliat Med 2021;10:5502-8. [Crossref] [PubMed]
- Chen Y, Wang J, Zhang X, et al. Correlation between apparent diffusion coefficient and pathological characteristics of patients with invasive breast cancer. Ann Transl Med 2021;9:143. [Crossref] [PubMed]
- Kimura K, Yoshida S, Tsuchiya J, et al. Usefulness of texture features of apparent diffusion coefficient maps in predicting chemoradiotherapy response in muscle-invasive bladder cancer. Eur Radiol 2022;32:671-9. [Crossref] [PubMed]
- Connor S, Anjari M, Burd C, et al. The impact of Human Papilloma Virus status on the prediction of head and neck cancer chemoradiotherapy outcomes using the pre-treatment apparent diffusion coefficient. Br J Radiol 2022;95:20210333. [Crossref] [PubMed]
- Li C, Yin J. Radiomics Based on T2-Weighted Imaging and Apparent Diffusion Coefficient Images for Preoperative Evaluation of Lymph Node Metastasis in Rectal Cancer Patients. Front Oncol 2021;11:671354. [Crossref] [PubMed]
- Alessi S, Maggioni R, Luzzago S, et al. Apparent Diffusion Coefficient and Other Preoperative Magnetic Resonance Imaging Features for the Prediction of Positive Surgical Margins in Prostate Cancer Patients Undergoing Radical Prostatectomy. Clin Genitourin Cancer 2021;19:e335-45. [Crossref] [PubMed]
- Ito K, Chiba E, Oyama-Manabe N, et al. Combining the Tumor Contact Length and Apparent Diffusion Coefficient Better Predicts Extraprostatic Extension of Prostate Cancer with Capsular Abutment: A 3 Tesla MR Imaging Study. Magn Reson Med Sci 2022;21:477-84. [Crossref] [PubMed]
- Anjari M, Guha A, Burd C, et al. Apparent diffusion coefficient agreement and reliability using different region of interest methods for the evaluation of head and neck cancer post chemo-radiotherapy. Dentomaxillofac Radiol 2021;50:20200579. [Crossref] [PubMed]
- Zheng DX, Meng SC, Liu QJ, et al. Predicting liver metastasis of gastrointestinal tract cancer by diffusion-weighted imaging of apparent diffusion coefficient values. World J Gastroenterol 2016;22:3031-7. [Crossref] [PubMed]
- Drewes R, Pech M, Powerski M, et al. Apparent Diffusion Coefficient Can Predict Response to Chemotherapy of Liver Metastases in Colorectal Cancer. Acad Radiol 2021;28:S73-80. [Crossref] [PubMed]
- Liu X, Wang T, Wang Y, et al. Histogram analysis of apparent diffusion coefficient on diffusion weighted magnetic resonance imaging in differentiation between low and high grade serous ovarian cancer. Curr Med Imaging 2022. [Epub ahead of print]. doi:
10.2174/1573405618666220517101012 .10.2174/1573405618666220517101012 - Min JH, Kang TW, Cha DI, et al. Apparent diffusion coefficient as a potential marker for tumour differentiation, staging and long-term clinical outcomes in gallbladder cancer. Eur Radiol 2019;29:411-21. [Crossref] [PubMed]
- Giganti F, Ambrosi A, Chiari D, et al. Apparent diffusion coefficient by diffusion-weighted magnetic resonance imaging as a sole biomarker for staging and prognosis of gastric cancer. Chin J Cancer Res 2017;29:118-26. [Crossref] [PubMed]
- Nakamura K, Kajitani S, Joja I, et al. The posttreatment mean apparent diffusion coefficient of primary tumor is superior to pretreatment ADCmean of primary tumor as a predictor of prognosis with cervical cancer. Cancer Med 2013;2:519-25. [Crossref] [PubMed]
(English Language Editor: R. Scott)


