
Risk factors and efficacy outcomes of early-onset severe neutropenia due to paclitaxel or nanoparticle albumin-bound paclitaxel combined with ramucirumab in advanced gastric cancer: a multicenter retrospective cohort study
Introduction
The standard first-line chemotherapy for human epidermal growth factor receptor 2 (HER2)-negative advanced gastric cancer (AGC) is a combination of fluoropyrimidine and platinum-based chemotherapy (FP) (1-3). Recently, FP plus nivolumab combination therapy has better progression-free survival (PFS) or overall survival (OS) compared with fluoropyrimidine alone (4,5). Regarding second-line treatment, the RAINBOW trial demonstrated that paclitaxel plus ramucirumab (PTX + RAM) had significantly better OS compared to paclitaxel plus placebo in patients with previously treated AGC (6) and has been recognized as the standard second-line chemotherapy.
In PTX + RAM therapy, the most common adverse event was neutropenia, and grade 4 neutropenia and febrile neutropenia were reported in 19% and 3%, respectively (6). The RAINBOW-Asia trial, which evaluated the efficacy and safety of PTX + RAM for Asian patients with AGC, showed grade 4 neutropenia and febrile neutropenia in 28% and 6% of the treated patients, respectively (7). Nanoparticle albumin-bound paclitaxel plus ramucirumab (nab-PTX + RAM) therapy, which is conditionally recommended in the Japanese guidelines (8), also presented grade 3 or higher neutropenia and febrile neutropenia in 77% and 5% of patients, respectively, in a phase II study (9). Mainly due to grade 3 or higher neutropenia, the median relative dose intensity of nab-paclitaxel was low at 61.8% (range, 28.5–99.8%), although that of ramucirumab was relatively maintained at 87.6% (range, 33.2–100%).
Several studies on the risk factors for severe chemotherapy-induced neutropenia have been reported in patients with solid tumors or hematologic malignancies (10-15). The risk factors for neutropenia, regardless of the time of onset, were age over 65 years, low hemoglobin, high lactate dehydrogenase (LDH), high creatinine, high bilirubin, and so on. Particularly, early-onset severe neutropenia (EOSN) causes a decrease in the intensity of chemotherapy, such as dose reduction and postponement (16-18). However, neutropenia is commonly reported to be associated with efficacy of cytotoxic chemotherapy. Metastatic colorectal cancer patients who developed neutropenia during cycle 1 and 2 of trifluridine-tipiracil treatment had significantly longer PFS and OS than patients who did not (19).
In this study, we identified the risk factors for EOSN and evaluated their relevance to efficacy in patients with AGC treated with PTX/nab-PTX + RAM. We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-499/rc).
Methods
Patients
Clinical data of patients enrolled in the CROSS SELL study (20), which was a multicenter, retrospective study conducted between January 2017 and June 2020 showing similar PFS and OS in PTX + RAM versus nab-PTX + RAM for AGC using propensity score-matched analysis, were used. The major eligibility criteria in this study were as follows: Eastern Cooperative Oncology Group Performance Status (ECOG PS) of 0–2, refractoriness to first-line chemotherapy with a fluoropyrimidine-based regimen, and no history of previous administration of taxane or angiogenesis inhibitors. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the ethics committees of all participating institutions [No. 2020-065 at Shikoku Cancer Center, R03-022 at University of Tsukuba (No. 2020-046) at Himeji Red Cross Hospital, and No. zn210121 at Kobe City Medical Center General Hospital]. Individual consent for this retrospective analysis was waived.
Treatment
The PTX + RAM regimen consisted of administering 80 mg/m2 of paclitaxel intravenously over 60 min on days 1, 8, and 15, along with 8 mg/kg of ramucirumab intravenously on days 1 and 15 every 28 days. The Nab-PTX + RAM regimen consisted of administering 100 mg/m2 of nab-paclitaxel intravenously over 30 min on days 1, 8, and 15 along with 8 mg/kg of ramucirumab intravenously on days 1 and 15 every 28 days. The 28-day treatment was defined as a cycle. Blood tests including absolute neutrophil count and urine test were performed before every treatment. When the neutrophil count was less than 1,200/m3, PTX/nab-PTX was skipped. Treatment was continued until disease progression, unacceptable toxicity, patient refusal, or physician’s decision to discontinue. Disease progression was radiologically or clinically determined by each physician. The patients underwent radiological examination every 8±2 weeks. Patients without disease progression were censored at the last confirmation of nonprogressive disease by radiological examination. Tumor response was assessed based on the Response Evaluation Criteria in Solid Tumors version 1.1 (21).
Data collection
The following data were collected; sex, age, ECOG PS, presence of primary tumor, number of metastatic organ sites, liver metastasis, peritoneal metastasis, massive ascites, measurable lesions, prior first-line chemotherapy, initial dose reduction, neutrophil counts at the start of second-line chemotherapy, and aspartate aminotransferase (AST), alkaline phosphatase (ALP), LDH, and albumin levels before initial administration. Adverse events were graded based on the Common Terminology Criteria for Adverse Events version 5.0 (22).
Statistical analyses
EOSN was defined as grade 4 neutropenia during the first 28 days of PTX/nab-PTX + RAM. The risk factors for EOSN were analyzed using a multivariate logistic regression model. In this analysis, all patients’ background data were used as variables, except for tumor differentiation and HER2 status, which were unlikely to affect EOSN. Variables included grade of neutropenia before initiation (grade 1 vs. grade 0), age (≥65 vs. <65 years), sex, ECOG PS (2 vs. 0–1), tumor differentiation (undifferentiated vs. differentiated), presence of primary tumor (yes vs. no), number of metastatic sites (≥2 vs. 0–1), liver metastasis (yes vs. no), peritoneal metastasis (yes vs. no), massive ascites (yes vs. no), measurable lesions (yes vs. no), first-line chemotherapy (FP vs. fluoropyrimidine alone), second-line chemotherapy (nab-PTX + RAM vs. PTX + RAM), initial dose reduction (yes vs. no), high AST level (yes vs. no), high ALP level (yes vs. no), high LDH level (yes vs. no), and low albumin level (yes vs. no). AST, LDH, ALP, and albumin levels were dichotomized as normal and abnormal based on hospital laboratory standards. PFS was defined as the time from the initiation of the study treatment to disease progression or death due to any cause. OS was defined as the time from the initiation of the study treatment to death due to any cause. Survivors were censored at the last contact. The relationship between EOSN and treatment outcomes (PFS and OS) was determined by multivariate analysis using a Cox proportional hazards model. HR of EOSN was adjusted with various patient background factors, adding tumor differentiation (undifferentiated vs. differentiated) to the variables in the logistic regression analysis. The relationships between the risk factors and PFS or OS were evaluated using an interaction test. The follow-up period was estimated using the reverse Kaplan–Meier method. All P values were two-sided, and statistical significance was set at P<0.05. IBM SPSS Statistics (version 25.0; SPSS Inc., Chicago, IL, USA) was used for statistical analyses.
Results
Patients
A flowchart of patient selection is shown in Figure 1. Data of 265 patients treated with PTX/nab-PTX + RAM as second-line chemotherapy were collected from four institutions. After excluding patients who were ineligible and had grade 2 or 3 neutropenia, data from 244 patients were analyzed. Fifty-one (20.9%) patients developed an EOSN. The background characteristics of patients with or without EOSN are shown in Table 1. Age ≥65 years; presence of primary tumor; liver metastasis; massive ascites; high serum levels of AST, ALP, and LDH; and low level of albumin were more common in the EOSN group than in the non-EOSN group. The proportions of other background factors did not differ between the two groups. The median follow-up times was 24.2 months, and 178 patients (73%) had died.
Table 1
| Characteristics | Non-EOSN (n=193), n [%] | EOSN (n=51), n [%] |
P value* |
|---|---|---|---|
| Age | 0.006 | ||
| Median [range], years | 66 [31–90] | 71 [47–84] | |
| <65 years | 82 [42] | 11 [22] | |
| ≥65 years | 111 [58] | 40 [78] | |
| Sex | 0.15 | ||
| Male | 139 [72] | 42 [82] | |
| Female | 54 [28] | 9 [18] | |
| ECOG PS | 0.77 | ||
| 0–1 | 177 [92] | 48 [94] | |
| 2 | 16 [8] | 3 [6] | |
| Tumor differentiation | 0.14 | ||
| Differentiated | 65 [34] | 23 [45] | |
| Undifferentiated | 128 [66] | 28 [55] | |
| Presence of primary tumor | 0.02 | ||
| No | 90 [47] | 14 [27] | |
| Yes | 103 [53] | 37 [73] | |
| Number of metastatic sites | 0.20 | ||
| 0–1 | 82 [43] | 16 [31] | |
| ≥2 | 111 [57] | 35 [69] | |
| Liver metastasis | 0.008 | ||
| No | 147 [76] | 29 [57] | |
| Yes | 46 [24] | 22 [43] | |
| Peritoneal metastasis | 0.43 | ||
| No | 81 [42] | 18 [35] | |
| Yes | 112 [58] | 33 [65] | |
| Massive ascites** | 0.03 | ||
| No | 179 [93] | 42 [82] | |
| Yes | 14 [7] | 9 [18] | |
| Measurable lesions | 1.0 | ||
| No | 97 [50] | 25 [49] | |
| Yes | 96 [50] | 26 [51] | |
| HER2 status | 0.52 | ||
| Negative | 161 [83] | 45 [88] | |
| Positive | 32 [17] | 6 [12] | |
| First-line chemotherapy | 0.49 | ||
| Fluoropyrimidine alone | 28 [15] | 5 [10] | |
| Fluoropyrimidine + platinum | 165 [85] | 46 [90] | |
| Second-line chemotherapy | 0.76 | ||
| Paclitaxel + ramucirumab | 96 [50] | 24 [47] | |
| Nab-paclitaxel + ramucirumab | 97 [50] | 27 [53] | |
| Starting dose of second-line chemotherapy | 0.70 | ||
| No reduction | 156 [81] | 40 [78] | |
| Reduction | 37 [19] | 11 [22] | |
| Neutropenia*** | 0.09 | ||
| Grade 0 | 173 [90] | 41 [80] | |
| Grade 1 | 20 [10] | 10 [20] | |
| AST level | 0.002 | ||
| Normal | 152 [79] | 29 [57] | |
| High | 41 [21] | 22 [43] | |
| ALP level | 0.003 | ||
| Normal | 131 [68] | 23 [45] | |
| High | 62 [32] | 28 [55] | |
| LDH level | 0.02 | ||
| Normal | 123 [64] | 23 [45] | |
| High | 70 [36] | 28 [55] | |
| Albumin level | 0.02 | ||
| Normal | 54 [28] | 6 [12] | |
| Low | 139 [72] | 45 [88] | |
*, Fisher’s exact test; **, ascites existing from the surface of the liver to the pelvic cavity continuously; ***, based on the Common Terminology Criteria for Adverse Events version 5.0. EOSN, early-onset severe neutropenia; ECOG PS, Eastern Cooperative Oncology Group Performance Status; HER2, human epidermal growth factor receptor 2; Nab-paclitaxel, nanoparticle albumin-bound paclitaxel; AST, aspartate aminotransferase; ALP, alkaline phosphatase; LDH, lactate dehydrogenase.
Risk factors for early-onset severe neutropenia (EOSN)
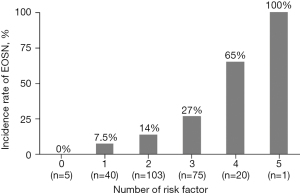
Multivariate analysis revealed the following five significant risk factors for EOSN: age ≥65 years [odds ratio (OR), 2.65; 95% confidence interval (CI), 1.16–6.02; P=0.02], presence of primary tumor (OR, 2.82; 95% CI, 1.12–7.09; P=0.03), peritoneal metastasis (OR, 2.52; 95% CI, 1.01–6.29; P=0.048), grade 1 neutropenia (OR, 3.32; 95% CI, 1.19–9.26; P=0.02), and high ALP level (OR, 2.34; 95% CI, 1.05–5.24; P=0.04) (Table 2). The incidence rate of EOSN increased with an increase in the number of risk factors (Figure 2).
Table 2
| Variable | Objective vs. reference | Multivariate analysis | ||
|---|---|---|---|---|
| Odds ratio | 95% CI | P value | ||
| Age | ≥65 vs. <65 years | 2.75 | 1.18–6.36 | 0.02 |
| Sex | Male vs. female | 1.49 | 0.61–3.66 | 0.38 |
| ECOG PS | 0–1 vs. 2 | 0.48 | 0.11–2.12 | 0.33 |
| Presence of primary tumor | Yes vs. no | 2.82 | 1.12–7.09 | 0.03 |
| Number of metastatic sites | 0–1 vs. ≥2 | 0.90 | 0.35–2.31 | 0.82 |
| Liver metastasis | Yes vs. no | 2.40 | 0.85–6.80 | 0.10 |
| Peritoneal metastasis | Yes vs. no | 2.52 | 1.01–6.29 | 0.048 |
| Massive ascites | Yes vs. no | 1.57 | 0.46–5.43 | 0.47 |
| Measurable lesions | Yes vs. no | 0.82 | 0.32–2.13 | 0.69 |
| First-line chemotherapy | Fluoropyrimidine + platinum vs. fluoropyrimidine alone | 0.84 | 0.24–2.91 | 0.79 |
| Second-line chemotherapy | Nab-paclitaxel + ramucirumab vs. paclitaxel + ramucirumab | 1.18 | 0.56–2.49 | 0.66 |
| Initial dose reduction | Yes vs. no | 0.76 | 0.29–1.98 | 0.58 |
| Neutropenia* | Grade 0 vs. 1 | 3.32 | 1.19–9.26 | 0.02 |
| High AST level | Yes vs. no | 1.55 | 0.63–3.79 | 0.34 |
| High ALP level | Yes vs. no | 2.34 | 1.05–5.24 | 0.04 |
| High LDH level | Yes vs. no | 1.39 | 0.65–2.98 | 0.39 |
| Low albumin level | Yes vs. no | 1.76 | 0.64–4.85 | 0.27 |
*, based on the Common Terminology Criteria for Adverse Events version 5.0. CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group Performance Status; AST, aspartate aminotransferase; ALP, alkaline phosphatase; LDH, lactate dehydrogenase.
Efficacy outcomes and EOSN
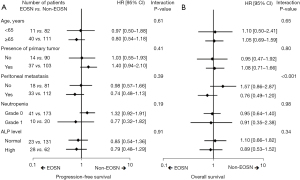
The PFS and OS curves according to EOSN are shown in Figure 3. The median PFS was 5.1 (95% CI, 3.3–6.9) and 4.4 (95% CI, 3.9–4.9) months in the EOSN and non-EOSN groups, respectively (P=0.28, Figure 3A). The median OS was 10.7 (95% CI, 9.0–12.4) and 11.4 (95% CI, 9.0–13.8) months in the EOSN and non-EOSN groups, respectively (P=0.88, Figure 3B). In multivariate analysis, PFS was longer in the EOSN group than in the non-EOSN group (adjusted HR, 0.61; 95% CI, 0.41–0.92; P=0.02) (Table 3). The OS tended to be longer in the EOSN group than in the non-EOSN group (adjusted HR, 0.73; 95% CI, 0.47–1.12; P=0.15) (Table 3). Subgroup analyses of PFS and OS according to risk factors are shown in Figure 4A,4B. Patients with peritoneal metastasis showed favorable OS in the EOSN group (HR, 0.76; 95% CI, 0.49–1.20), whereas those without it favored in the non-EOSN group (HR, 1.57; 95% CI, 0.86–2.87; interaction P<0.001).
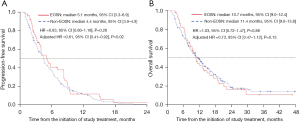
Table 3
| Variable | Objective vs. reference | Progression-free survival | Overall survival | |||||
|---|---|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P value | Hazard ratio | 95% CI | P value | |||
| EOSN | Yes vs. no | 0.61 | 0.41–0.92 | 0.02 | 0.73 | 0.47–1.12 | 0.15 | |
| Age | ≥65 vs. <65 years | 1.00 | 0.72–1.38 | 0.98 | 1.04 | 0.72–1.50 | 0.86 | |
| Sex | Male vs. female | 1.50 | 1.08–2.09 | 0.02 | 1.31 | 0.91–1.88 | 0.15 | |
| ECOG PS | 2 vs. 0–1 | 2.50 | 1.44–4.33 | 0.001 | 2.86 | 1.54–5.30 | 0.001 | |
| Tumor differentiation | Undifferentiated vs. differentiated | 1.61 | 1.13–2.29 | 0.01 | 2.06 | 1.39-3.04 | 0.0001 | |
| Presence of primary tumor | Yes vs. no | 1.40 | 1.01–1.94 | 0.047 | 1.42 | 0.97–2.10 | 0.08 | |
| Number of metastatic sites | ≥2 vs. 0–1 | 1.28 | 0.91–1.81 | 0.15 | 1.65 | 1.11–2.46 | 0.01 | |
| Liver metastasis | Yes vs. no | 1.15 | 0.76–1.74 | 0.51 | 1.46 | 0.92–2.33 | 0.11 | |
| Peritoneal metastasis | Yes vs. no | 1.20 | 0.84–1.72 | 0.31 | 1.59 | 1.06–2.39 | 0.03 | |
| Massive ascites | Yes vs. no | 1.66 | 0.99–2.77 | 0.053 | 1.03 | 0.59–1.80 | 0.92 | |
| Measurable lesions | No vs. yes | 1.45 | 1.01-2.08 | 0.04 | 1.51 | 1.00–2.27 | 0.05 | |
| First-line chemotherapy | Fluoropyrimidine + platinum vs. fluoropyrimidine alone | 1.43 | 0.92–2.26 | 0.12 | 1.26 | 0.76–2.09 | 0.37 | |
| Second-line chemotherapy | Nab-paclitaxel + ramucirumab vs. paclitaxel + ramucirumab | 0.86 | 0.64–1.17 | 0.34 | 0.82 | 0.59–1.14 | 0.23 | |
| Initial dose reduction | Yes vs. no | 0.89 | 0.62–1.28 | 0.52 | 0.96 | 0.64–1.44 | 0.85 | |
| Neutropenia* | Grade 0 vs. 1 | 0.95 | 0.61–1.48 | 0.82 | 0.78 | 0.47–1.28 | 0.32 | |
| High AST level | Yes vs. no | 0.88 | 0.61–1.27 | 0.49 | 0.89 | 0.60–1.34 | 0.58 | |
| High ALP level | Yes vs. no | 0.96 | 0.69–1.34 | 0.81 | 1.01 | 0.70–1.46 | 0.95 | |
| High LDH level | Yes vs. no | 1.61 | 1.17–2.21 | 0.004 | 1.73 | 1.23–2.42 | 0.002 | |
| Low albumin level | Yes vs. no | 1.13 | 0.80–1.59 | 0.48 | 1.35 | 0.90–2.03 | 0.14 | |
*, based on the Common Terminology Criteria for Adverse Events version 5.0. CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group Performance Status; EOSN, early-onset severe neutropenia; AST, aspartate aminotransferase; ALP, alkaline phosphatase; LDH, lactate dehydrogenase.
Discussion
In this study, we found that approximately one-fifth of patients showed EOSN and identified the risk factors for EOSN in patients with AGC treated with PTX/nab-PTX + RAM as second-line chemotherapy. PFS was significantly better in patients with EOSN than in those without EOSN.
Our study identified the following five risk factors for EOSN: age ≥65 years, presence of primary tumor, peritoneal metastasis, grade 1 neutropenia, and high ALP level. The incidence rate of EOSN increased as the number of risk factors increased. Risk factors for severe neutropenia have been investigated in several malignancies, including gastrointestinal cancer, the identified factors partly depend on the cancer types and regimens (14-19).
The commonly reported risk factors for this disease are age, neutropenia, and high ALP level before chemotherapy. In the RAINBOW trial, a high incidence of grade 3 or higher neutropenia was noted in patients aged ≥65 years during all cycles (23). This finding supports the notion that this treatment for elderly patients should be performed with attention to EOSN. Low neutrophil count at the start of second-line chemotherapy has been a risk factor for severe neutropenia during chemotherapy, regardless of the regimen (19,24). Neutropenia at the start of second-line therapy reflects exhausted bone marrow function by previous therapy and requires preparation for EOSN. A high ALP level was also a risk factor for severe neutropenia in a prospective observational study that analyzed data from 3,760 patients with solid tumors or malignant lymphoma (25). High ALP level indicates bile stasis and liver damage. This suggests that the EOSN in our study may be due to the delayed metabolism of PTX and nab-PTX in the liver.
The presence of primary tumor and peritoneal metastasis have rarely been reported as risk factors for EOSN. Both factors are commonly accompanied by malnutrition, malabsorption of nutrients, and occasional bleeding from tumors. This may be related to EOSN. Massive ascites was not identified as a risk factor for EOSN, despite its high incidence in patients with massive ascites. The reason for this is unclear, but a small number of patients with ascites and other confounding variables might be involved.
In this study, PFS was longer in patients with EOSN than in those without EOSN, and OS tended to be better in patients with EOSN than in those without EOSN. These results are consistent with a previous report that neutropenia is associated with improved prognosis in patients with AGC treated with PTX (26). In the subgroup analysis, patients with any risk factors did not show worse efficacy than those without risk factors. Thus, PTX/nab-PTX + RAM should be carefully recommended to patients with risk factors for EOSN.
The first limitation of our study was its retrospective nature and small sample size. Second, it was unclear whether all risk factors were extracted. This study did not include hemoglobin, total bilirubin, or creatinine clearance, which were reported as risk factors in other studies (15-17,19). Further studies with larger sample sizes are required.
Conclusions
This study identified the risk factors for EOSN and showed that EOSN may be associated with favorable PFS in patients with AGC treated with PTX/nab-PTX + RAM. We should carefully try to treat them keeping the risk factors in mind.
Acknowledgments
We would like to thank Editage (https://www.editage.jp) for English language editing.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-499/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-499/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-499/coif). TM received honoraria for lectures from Chugai, Takeda, Yakult, Taiho, Ono, Daiichi-Sankyo, Bristol Myers Squibb, Lilly, and Merck Serano. YY received honoraria for lectures from Sanofi, Nihon Kayaku, Eisai, Bayer, Lilly, Taiho, Daiichi Sankyo, Yakult, Nihon Servier, Asahi Kasei, and Ono. TK received honoraria for lectures from Taiho, Chugai, Ono, and Lilly. TN received honoraria for lectures from Chugai, Takeda, Yakult-Honsha, Taiho, Ono, Daiichi-Sankyo, Bristol Myers Squibb, Lilly, and Merck Serano, Pharmaceutical and a Data Safety Monitoring Board member from Janssen. TM received honoraria for speakers bureaus from Lilly, Taiho, and Bristol-Myers Squibb, and a grant from Taiho outside the submitted work. IH received honoraria for lectures from Chugai, Takeda, and Yakult, and payment as an Advisory Board member from Asahi-Kasei and a Data Safety Monitoring Board member from Chugai, Taiho, Ono, Daiichi-Sankyo, and Merck Serano. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the ethics committees of all participating institutions (No. 2020-065 at Shikoku Cancer Center, No. R03-022 at University of Tsukuba, No. 2020-046 at Himeji Red Cross Hospital, and No. zn210121 at Kobe City Medical Center General Hospital). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Koizumi W, Narahara H, Hara T, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 2008;9:215-21. [Crossref] [PubMed]
- Yamada Y, Higuchi K, Nishikawa K, et al. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naïve patients with advanced gastric cancer. Ann Oncol 2015;26:141-8. [Crossref] [PubMed]
- Lee KW, Chung IJ, Ryu MH, et al. Multicenter phase III trial of S-1 and cisplatin versus S-1 and oxaliplatin combination chemotherapy for first-line treatment of advanced gastric cancer (SOPP trial). Gastric Cancer 2021;24:156-67. [Crossref] [PubMed]
- Boku N, Ryu MH, Kato K, et al. Safety and efficacy of nivolumab in combination with S-1/capecitabine plus oxaliplatin in patients with previously untreated, unresectable, advanced, or recurrent gastric/gastroesophageal junction cancer: interim results of a randomized, phase II trial (ATTRACTION-4). Ann Oncol 2019;30:250-8. [Crossref] [PubMed]
- Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 2021;398:27-40. [Crossref] [PubMed]
- Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 2014;15:1224-35. [Crossref] [PubMed]
- Xu RH, Zhang Y, Pan H, et al. Efficacy and safety of weekly paclitaxel with or without ramucirumab as second-line therapy for the treatment of advanced gastric or gastroesophageal junction adenocarcinoma (RAINBOW-Asia): a randomised, multicentre, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol 2021;6:1015-24. [Crossref] [PubMed]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer 2021;24:1-21.
- Bando H, Shimodaira H, Fujitani K, et al. A phase II study of nab-paclitaxel in combination with ramucirumab in patients with previously treated advanced gastric cancer. Eur J Cancer 2018;91:86-91. [Crossref] [PubMed]
- Choi YW, Jeong SH, Ahn MS, et al. Patterns of neutropenia and risk factors for febrile neutropenia of diffuse large B-cell lymphoma patients treated with rituximab-CHOP. J Korean Med Sci 2014;29:1493-500. [Crossref] [PubMed]
- Mitani Y, Usami E, Kimura M, et al. Risk factors for neutropenia with lenalidomide plus dexamethasone therapy for multiple myeloma. Pharmazie 2016;71:349-51. [PubMed]
- Kawachi S, Shinoda Y, Kimura M, et al. Risk factors for severe neutropenia induced by combination therapy of S-1 and cisplatin in patients with advanced/recurrent gastric cancer. Pharmazie 2018;73:174-7. [PubMed]
- Yasue F, Kimura M, Usami E, et al. Risk factors contributing to the development of neutropenia in patients receiving oral trifluridine-tipiracil (TAS-102) chemotherapy for advanced/recurrent colorectal cancer. Pharmazie 2018;73:178-81. [PubMed]
- Saito Y, Takekuma Y, Kobayashi M, et al. Detection of risk factors related to administration suspension and severe neutropenia in gemcitabine and nab-paclitaxel treatment. Support Care Cancer 2021;29:3277-85. [Crossref] [PubMed]
- Ito G, Kawakami K, Aoyama T, et al. Risk factors for severe neutropenia in pancreatic cancer patients treated with gemcitabine/nab-paclitaxel combination therapy. PLoS One 2021;16:e0254726. [Crossref] [PubMed]
- Taplitz RA, Kennedy EB, Bow EJ, et al. Antimicrobial Prophylaxis for Adult Patients With Cancer-Related Immunosuppression: ASCO and IDSA Clinical Practice Guideline Update. J Clin Oncol 2018;36:3043-54. [Crossref] [PubMed]
- Lyman GH, Abella E, Pettengell R. Risk factors for febrile neutropenia among patients with cancer receiving chemotherapy: A systematic review. Crit Rev Oncol Hematol 2014;90:190-9. [Crossref] [PubMed]
- Pettengell R, Schwenkglenks M, Leonard R, et al. Neutropenia occurrence and predictors of reduced chemotherapy delivery: results from the INC-EU prospective observational European neutropenia study. Support Care Cancer 2008;16:1299-309. [Crossref] [PubMed]
- Yoshino T, Cleary JM, Van Cutsem E, et al. Neutropenia and survival outcomes in metastatic colorectal cancer patients treated with trifluridine/tipiracil in the RECOURSE and J003 trials. Ann Oncol 2020;31:88-95. [Crossref] [PubMed]
- Nakasya A, Hagiwara Y, Ikoma T, et al. Nanoparticle albumin-bound paclitaxel and ramucirumab versus paclitaxel and ramucirumab as second-line chemotherapy for unresectable advanced or recurrent gastric cancer: a multicenter, propensity score-matched analysis (CROSS SELL study). Int J Clin Oncol 2022;27:684-94. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Common Terminology Criteria for Adverse Events (CTCAE v5.0). Available online: http://evsncinihgov/ftp1/CTCAE/CTCAE_403_2010-06-14_QuickReference_5x7pdf; accessed November 27, 2017.
- Muro K, Cho JY, Bodoky G, et al. Age does not influence efficacy of ramucirumab in advanced gastric cancer: Subgroup analyses of REGARD and RAINBOW. J Gastroenterol Hepatol 2018;33:814-24. [Crossref] [PubMed]
- Nomura H, Tsuji D, Demachi K, et al. ABCB1 and ABCC2 genetic polymorphism as risk factors for neutropenia in esophageal cancer patients treated with docetaxel, cisplatin, and 5-fluorouracil chemotherapy. Cancer Chemother Pharmacol 2020;86:315-24. [Crossref] [PubMed]
- Lyman GH, Kuderer NM, Crawford J, et al. Predicting individual risk of neutropenic complications in patients receiving cancer chemotherapy. Cancer 2011;117:1917-27. [Crossref] [PubMed]
- Shitara K, Matsuo K, Takahari D, et al. Neutropenia as a prognostic factor in advanced gastric cancer patients undergoing second-line chemotherapy with weekly paclitaxel. Ann Oncol 2010;21:2403-9. [Crossref] [PubMed]



