
Outcomes of patients with esophageal cancer after allogeneic hematopoietic stem cell transplantation
Introduction
The number of patients undergoing allogeneic hematopoietic stem cell transplantation (aHCT) has significantly increased worldwide in the last 3 to 4 decades (1,2). This, coupled with an improving survival of these patients with time, has warranted an increased focus on the long-term complications of allogeneic HCT, especially secondary malignancies. Secondary malignancies form an important part of the long-term complications after aHCT and are a prominent cause of late mortality (3).
The risk of developing secondary solid tumors has been reported across studies to be 2 to 6 times higher than the age and gender-matched general population (4,5). The risk of developing esophageal carcinoma (Ca) after aSCT is thought to be much higher than the risk of developing other types of secondary solid tumors. The EBMT study by Kolb and colleagues showed a standardized incident ratio (SIR) of >30 for esophageal cancer in patients after allogeneic HCT (6) while studies in the Japanese population (known to have a lower risk of chronic Graft versus host disease), showed an SIR ranging between 8.5–23.4 (7,8). This increased risk was also found to persist life-long, warranting a need for continued surveillance for these malignancies (7).
Esophageal carcinoma after aHCT has mostly been described as part of large registry-based studies or in the form of case reports or small case series. There is very little data focusing entirely on esophageal cancers after allogeneic HCT with information on the staging, treatment modalities, causes of death, and other survival parameters in this rare cohort of patients. We conducted this study to describe the baseline characteristics, treatment received and outcomes of patients who developed esophageal cancer after allogeneic HCT. We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-700/rc)
Methods
For this retrospective case series, records of adult (≥18 years) patients who underwent aSCT at Princess Margaret Cancer Centre, University Health Network, Toronto from Jan 1990–Dec 2020 were screened to identify patients who subsequently developed primary esophageal cancer. Patients with cancer of the oral cavity or stomach extending into the esophagus were excluded. Patients with genetic syndromes predisposing them to secondary malignancies (e.g., dyskeratosis congenita) were also excluded. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of University Health Network , Toronto, Canada and individual consent for this retrospective analysis was waived.
Disease and treatment characteristics including previous hematological malignancy and transplantation details, details of esophageal cancer including treatment received and outcomes were collected from electronic patient records. Tumor–node–metastasis classification was done according to the International Union against Cancer (UICC) criteria (9).
Statistical analysis
Estimates of survival were calculated using the Kaplan-Meier method. Overall survival (OS) was defined as the time from diagnosis of esophageal cancer to date of death from any cause or date of the last follow-up. SIR was defined as the ratio of the observed number of cancer cases to the expected number of cases multiplied by 100. All analysis was done using SPSS v22.0.
Results
Baseline and transplant characteristics of all patients are summarized in Table 1. During the 30-year study period, a total of 2,883 allogeneic hematopoietic stem cell transplants were performed at our institute. In this population, 10 patients were diagnosed to have esophageal cancer after aHCT. The incidence rate per year would be 10.41 per 100,000 population. Thus the SIR of esophageal cancer after aHCT would be 1.96. This is in comparison to incidence in province of Ontario Canada (5.3 cases per 100,000 population) (10).
Table 1
| Patient characteristics | N (%) |
|---|---|
| Median [range] age at time of HCT (years) | 53.5 [39–70] years |
| Gender | |
| Males | 6 (60.0) |
| Females | 4 (40.0) |
| Transplant indication | |
| AML | 5 (50.0) |
| ALL | 2 (20.0) |
| CML | 1 (10.0) |
| NHL | 1 (10.0) |
| CLL | 1 (10.0) |
| Smoking history before HCT | |
| Smoking history present | 5 (50.0) |
| Non smoker | 5 (50.0) |
| HLA match | |
| 10/10 matched unrelated | 4 (40.0) |
| 6/6 or 10/10 matched related | 6 (60.0) |
| Stem cell source | |
| Peripheral blood stem cells | 7 (70.0) |
| Bone marrow stem cells | 3 (30.0) |
| Conditioning regimen | |
| Flu(2)-Bu(2)-TBI(200) | 4 (40.0) |
| Flu(4)-Bu(4)-TBI(400) | 2 (20.0) |
| Bu(4)-Cy(2) | 2 (20.0) |
| Others | 2 (20.0) |
| GVHD prophylaxis regimen | |
| CSA-MMF | 5 (50.0) |
| CSA MTx (+/- prednisone) | 3 (30.0) |
| Others | 2 (20.0) |
| Acute GVHD | |
| No aGVHD | 4 (40.0) |
| Grade 2-4 | 6 (60.0) |
| Chronic GVHD | |
| No cGVHD | 1 (10.0) |
| cGVHD present | 9 (90.0) |
| Severity of cGVHD (n=9) | |
| Mild cGVHD | 1 (11.1) |
| Severe cGVHD | 8 (88.9) |
HCT, hematopoietic stem cell transplant; AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; CML, chronic myeloid leukemia; NHL, non-Hodgkins lymphoma; CLL, chronic lymphocytic leukemia; HLA, human leukocyte antigen; Flu, fludarabine; Bu, busulfan; TBI, total body irradiation; CSA, cyclosporine A; MMF, mycophenolate mofetil; MTx, methotrexate; GVHD, graft versus host disease.
Among the 10 patients with esophageal Ca, males were 60% (n=6) and the median (range) age at the time of Allo HCT was 53.5 [39–70] years. The most common indication of transplant was acute myeloid leukemia (AML) in 50% (n=5) patients followed by acute lymphoblastic leukemia (ALL) in 20% (n=2) patients. Six patients (60%) received a myeloablative conditioning regimen and four patients (40%) received a reduced intensity conditioning regimen. Seven patients (70%) received total body irradiation (TBI) as part of their conditioning regiment. The most common GVHD prophylaxis used was cyclosporine—mycophenolate in 50% (n=5) patients followed by cyclosporine methotrexate in 20% (n=2) patients. Grade 2-3 acute GVHD (aGVHD) was seen in 60% (n=6) patients and most common site of aGVHD was skin (83.3%; n=5) followed by gastrointestinal (50%; n=3). All except one patient (90%; n=9) had chronic GVHD (cGVHD). The common sites affected by cGVHD were mouth (100%) and skin (88.9%). Chronic GVHD was severe in 88.9% (n=8) of patients and mild in one patient (11.1%).
Esophageal cancer was diagnosed in these 10 patients after a median (range) duration of 5.8 (1–18.9) years after allogeneic HCT. The median age at the time of diagnosis of esophageal carcinoma was 62 [50–75] years. The histology of esophageal Ca was squamous cell carcinoma (SCC) in 80% (n=8) of patients, adenocarcinoma in 10% (n=1) of patients, and poorly differentiated in 10% (n=1) of patients. The site of esophageal cancer was proximal (upper) esophagus in 40% (n=4) of patients, mid esophagus in 20% (n=2) of patients, and distal esophagus in 40% (n=4) of patients. Staging CT scans were done for all patients at diagnosis and only 1 patient (10%) had distant metastasis at the time of diagnosis. Five patients (50%) had a history of smoking before transplant (median =12 pack-years), but only 1 patient (10%) had a history of smoking after allogeneic HCT.
All patients underwent treatment for esophageal cancer with surgery, chemotherapy, and/or radiation therapy. Esophagectomy was part of the therapeutic armamentarium in 50% (n=5) patients with 2 patients (20%) having surgery as their only treatment. The remaining 3 patients received neoadjuvant chemo-radiotherapy as part of the CROSS protocol (11) before undergoing Esophagectomy. Two patients (20%) were treated with palliative intent with radiation therapy +/− chemotherapy. Remission was defined as no evidence of disease by imaging 6 weeks after the end of planned treatment. At the end of planned treatment, 70% of patients (n=7) achieved remission while 1 patient had progressive disease with new brain metastasis. This patient was subsequently treated with a palliative intent. Others (n=2) achieved only partial disease control which subsequently progressed. All patients of esophageal cancer who underwent surgery (including patients treated under CROSS protocol) went into remission at end of treatment. There were no cases of relapse of the disease once remission was achieved. Individual patient characteristics, disease particulars, and treatment received by all the patients with esophageal cancer after allogeneic HCT are summarized in Table 2.
Table 2
| Patient No. | Histology of esophageal cancer | Site of esophagus | TNM Stage | Clinical (TNM) stage group | Treatment received | Response to treatment | Follow-up (months) | Outcomes |
|---|---|---|---|---|---|---|---|---|
| 1 | Poorly differentiated | Proximal | T2N1M0 | II | High dose Cisplatin + radiation therapy | Progressive disease with new brain metastasis – subsequently treated with palliative intent | 15 | Died due to disease progression |
| 2 | SCC | Middle | T?N?M1 | IVB | Radiation therapy – (2,500 in 10#) | Palliative intent treatment | 6 | Died due to disease progression |
| 3 | SCC | Distal | T3N1M0 | III | Neo adjuvant carboplatin + paclitaxel + radiation therapy (41.4 Gy in 23#) f/b Ivor-Lewis esophagectomy | Remission following surgery | 44 | Alive at time of data censor |
| 4 | SCC | Distal | T3N0M0 | II | Ivor-Lewis esophagectomy | Remission following surgery | 84 | Died due to unrelated causes – cGVHD lungs + infection |
| 5 | SCC | Distal | Unknown | Unknown | Carboplatin + paclitaxel f/b palliative radiation therapy (1,000 Gy in 5#) | Remission not achieved – subsequently treated with palliative intent | 10 | Died due to disease progression |
| 6 | SCC | Proximal | T3N1M0 | III | Carboplatin + paclitaxel + radiation therapy 45+8 Gy boost | Remission following treatment | 34 | Alive at time of data censor |
| 7 | SCC | Proximal | T2N1M0 | II | Cisplatin+ 5 FU + radiation therapy (50 Gy 25#) | Remission following treatment | 44 | Died due to unrelated causes - CGVHD lungs + infection |
| 8 | SCC | Middle | T1N0M0 | I | Ivor-Lewis esophagectomy | Remission following surgery | 11 | Died due to unrelated causes |
| 9 | SCC | Proximal | T3N1M0 | III | Neo adjuvant chemo + radiation therapy (41.4 Gy in 23#) f/b McKeown esophagectomy | Remission following surgery | 20 | Alive at time of data censor |
| 10 | Adeno carcinoma | Distal | T3N0M0 | III | Neo adjuvant carboplatin + paclitaxel + radiation therapy (41.4 Gy in 23#) f/b Ivor-Lewis esophagectomy | Remission following surgery | 74 | Alive at time of data censor |
SCC, squamous cell carcinoma; cGVHD, chronic graft versus host disease; 5FU, 5-Fluro Uracil.
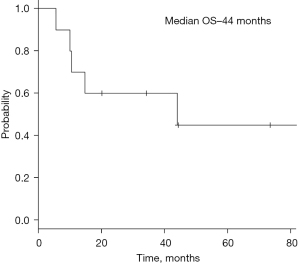
After a median follow-up of 27 months after diagnosis of esophageal Ca (median follow-up after allogeneic HCT =9 years), 60% (n=6) of patients died. The causes of death were progressive esophageal cancer in 50% (n=3) of patients and cGVHD lung with superimposed infection in 40% (n=2) patients. One patient succumbed as a result of a third primary malignancy of the oral cavity. Estimated 2- and 5-year OS after diagnosis of esophageal cancer was 60% and 45% respectively (median survival: 44.1 months). Estimated 10-year OS after Allo HCT was not reached (median survival: 10.5 years). Kaplan-Meier curve for overall survival after diagnosis of esophageal cancer is depicted in Figure 1.
Discussion
The incidence and risk factors of esophageal cancers are extremely variable and are influenced by gender (males > females), race (increased in whites), country (increased in Japan, Iran, northern China when compared to North America) (12-14). The risk factors for developing esophageal cancer vary both on the geographical location of patients and also on the histology of esophageal cancer (squamous cell vs. adenocarcinoma). Tobacco smoking and previous history of radiation are risk factors for both types of esophageal cancer (15,16). Additional risk factors for developing esophageal cancer after aHCT seem to be related to the presence of oral chronic graft versus host disease and long-term use of immunosuppressive drugs (17,18). Almost all of our patients have squamous cell cancer (SCC) of esophagus (90%) and all of these patients have a history of cGVHD of the oral cavity and oropharynx. Thus, cGVHD maybe the reason for increased proportion of SCC (when compared to adenocarcinoma) and increased proportion of proximal esophageal cancer (40%) in this cohort of patients after aHCT. The use of TBI in the conditioning regimen of transplant has been proposed as a risk factor in some studies (18,19), while no differences were found in other studies (20,21).
The mechanism of development of esophageal Ca after aHCT has not been extensively studied. The pathogenesis for esophageal SCC is thought related to chronic irritation and inflammation of esophageal mucosa (13). cGVHD is thought to predispose to SCC by chronic injury and inflammation of the mucosal epithelium (17). This, coupled with the decreased anti-tumor immunity conferred by long-term immunosuppressive agents possibly leads to SCCs of the esophagus (8). There is scant data on the biological and genetic differences between de novo malignancies and secondary malignancies after aHCT. Akiyama and colleagues studied the genetic profile of a single patient with esophageal Ca after aHCT. They proposed that cGVHD and prolonged used of immunosuppressives led to accumulation of mutations (including TP53), which in turn led to epigenetic modifications, and impairment of checkpoint and DNA repair mechanisms leading to esophageal SCC (22). The additive role played by other risk factors (smoking, alcoholism, achalasia, etc.) has not been studied separately in patients after aHCT and needs to be explored further.
The overall survival in patients with esophageal cancer is thought to be poor with the SEER data [2011–2017] showing only a 5-year relative survival of 19.9% (12). The survival of patients with esophageal cancer is thought to be dependent on TNM stage and metastasis at the time of diagnosis with advanced stages (stage IV/with metastasis) showing a 5-year relative survival of only 5.2%. However, more than 40% of patients are diagnosed in advanced stages of esophageal cancer (12,23). The estimated 5-year survival of patients in our cohort is 45%, possibly because of the relatively early diagnosis of esophageal cancer. Only 10% of our patients had distant metastasis at the time of diagnosis and 50% of our patients were candidates to undergo surgical resection of the tumor (with or without neoadjuvant therapy). Thus, the relatively favorable survival seen in our cohort of patients with esophageal cancer after aHCT may be attributable to the early stage at diagnosis of the malignancy. This underlines the need for constant surveillance for esophageal cancer (and other malignancies) in patients who undergo Allogeneic HCT (8,24).
Large population-based cancer registry studies from Japan did not show a difference in survival between patients with de novo esophageal ca versus esophageal ca after aHCT (7,25). The EBMT study by Tichelli and colleagues showed however, that there was a significant decrease in expected mortality in esophageal cancer patients after aHCT when compared to patients from the general population (SMR 0.49; 95% CI: 0.33–0.68) (26). Despite being poly-treated for primary malignancy with chemotherapy and aHCT, the survival of patients with esophageal ca after aHCT seems comparable (if not better) to that of the general population. One possible explanation for the same may be that most centers across the world, including our own, mandate annual visits to a dentist, and with a transplant physician focused on looking for long-term complications after HCT. These visits facilitate timely reporting of symptoms and may trigger a diagnosis of secondary malignancies including esophageal cancer in their early stages, thus leading to aggressive treatments and favorable outcomes.
Our study is limited by a small heterogenous cohort of patients with esophageal cancer evaluated over a long period of time, increasing the possibility of patients who were lost to follow-up, and subsequently developed esophageal cancer. A significant proportion of patients undergoing aHCT may die due to transplant related causes and may not live long enough to develop secondary cancers (including esophageal). Thus the incidence rates may be underestimated figures, despite being higher than the general population.
In conclusion, there is an increased risk of developing esophageal cancer after aHCT, especially in patients with chronic oropharyngeal GVHD. The prognosis of patients with esophageal GVHD may be comparable to that of the general population. Our data shows that aggressive treatment regimens are tolerated by patients after aHCT especially with diagnosis in the early stages of the disease. With existing data suggesting a continuing increase in the risk of developing these malignancies after aHCT, there is a need for life-long follow-up and surveillance of these patients. Although there is no evidence for routine/surveillance endoscopy after aHCT, patients presenting with symptoms of non-cardiac central chest pain, dysphagia, odynophagia or unexplained weight loss, should be evaluated thoroughly with barium swallow and upper gastrointestinal endoscopy. This will also facilitate early diagnosis of other common esophageal pathologies after aHCT including ulceration, webs and scleroderma-like dysmotility syndromes. Early diagnosis and treatment in the early stages of disease, improves the outcomes of therapy in esophageal cancer after aHCT.
Acknowledgments
We would like to acknowledge the contribution of the Allogeneic BMT data coordinators, especially Ms. Carol Chen for the accurate documentation of BMT data.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-700/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-700/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-700/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-700/coif). LLS has consulting/advisory arrangements with Merck, Pfizer, AstraZeneca, Roche, Symphogen, Seattle Genetics, GlaxoSmithKline, Voronoi, Arvinas, Tessa, Navire, Relay, Rubius, Janpix, Daiichi Sanyko, Coherus, Marengo, InteRNA; stock ownership of Agios (spouse); leadership position in Treadwell Therapeutics (spouse); and institution receives clinical trials support from Novartis, Bristol-Myers Squibb, Pfizer, Boerhinger-Ingelheim, GlaxoSmithKline, Roche/Genentech, Karyopharm, AstraZeneca, Merck, Celgene, Astellas, Bayer, Abbvie, Amgen, Symphogen, Intensity Therapeutics, Mirati Therapeutics, Shattucks, outside of the submitted study. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional ethics board of University Health Network, Toronto, Canada) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- D'Souza A, Lee S, Zhu X, et al. Current Use and Trends in Hematopoietic Cell Transplantation in the United States. Biol Blood Marrow Transplant 2017;23:1417-21. [Crossref] [PubMed]
- Passweg JR, Baldomero H, Chabannon C, et al. Hematopoietic cell transplantation and cellular therapy survey of the EBMT: monitoring of activities and trends over 30 years. Bone Marrow Transplant 2021;56:1651-64. [Crossref] [PubMed]
- Wingard JR, Majhail NS, Brazauskas R, et al. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol 2011;29:2230-9. [Crossref] [PubMed]
- Bhatia S, Louie AD, Bhatia R, et al. Solid cancers after bone marrow transplantation. J Clin Oncol 2001;19:464-71. [Crossref] [PubMed]
- Yokota A, Ozawa S, Masanori T, et al. Secondary solid tumors after allogeneic hematopoietic SCT in Japan. Bone Marrow Transplant 2012;47:95-100. [Crossref] [PubMed]
- Kolb HJ, Socié G, Duell T, et al. Malignant neoplasms in long-term survivors of bone marrow transplantation. Late Effects Working Party of the European Cooperative Group for Blood and Marrow Transplantation and the European Late Effect Project Group. Ann Intern Med 1999;131:738-44. [Crossref] [PubMed]
- Atsuta Y, Suzuki R, Yamashita T, et al. Continuing increased risk of oral/esophageal cancer after allogeneic hematopoietic stem cell transplantation in adults in association with chronic graft-versus-host disease. Ann Oncol 2014;25:435-41. [Crossref] [PubMed]
- Tanaka Y, Kurosawa S, Tajima K, et al. Increased incidence of oral and gastrointestinal secondary cancer after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 2017;52:789-91. [Crossref] [PubMed]
- TNM Classification of Malignant Tumours, 8th Edition. Wiley-Blackwell; 2016.
- Cancer Incidence statistics in Ontario - Cancer Care Ontario. 2018.
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Cancer Statisitcs - Esophagus [Internet]. National Cancer Institute. [cited February 2022]. Available online: https://seer.cancer.gov/statfacts/html/esoph.html
- Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003;349:2241-52. [Crossref] [PubMed]
- Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med 2014;371:2499-509. [Crossref] [PubMed]
- Ahsan H, Neugut AI. Radiation therapy for breast cancer and increased risk for esophageal carcinoma. Ann Intern Med 1998;128:114-7. [Crossref] [PubMed]
- De Stefani E, Barrios E, Fierro L. Black (air-cured) and blond (flue-cured) tobacco and cancer risk. III: Oesophageal cancer. Eur J Cancer 1993;29A:763-6. [Crossref] [PubMed]
- Curtis RE, Metayer C, Rizzo JD, et al. Impact of chronic GVHD therapy on the development of squamous-cell cancers after hematopoietic stem-cell transplantation: an international case-control study. Blood 2005;105:3802-11. [Crossref] [PubMed]
- Nomura K, Iizuka T, Kaji D, et al. Secondary esophageal squamous cell carcinoma after hematopoietic stem cell transplantation. J Cancer Res Clin Oncol 2021;147:2137-44. [Crossref] [PubMed]
- Curtis RE, Rowlings PA, Deeg HJ, et al. Solid cancers after bone marrow transplantation. N Engl J Med 1997;336:897-904. [Crossref] [PubMed]
- Kulkarni S, Powles R, Treleaven J, et al. Melphalan/TBI is not more carcinogeneic than cyclophosphamide/TBI for transplant conditioning: follow-up of 725 patients from a single centre over a period of 26 years. Bone Marrow Transplant 2000;25:365-70. [Crossref] [PubMed]
- Michelis FV, Kotchetkov R, Grunwald RM, et al. Long-Term Incidence of Secondary Malignancies after Allogeneic Hematopoietic Cell Transplantation: A Single-Center Experience. Biol Blood Marrow Transplant 2017;23:945-51. [Crossref] [PubMed]
- Akiyama M, Yamaoka M, Ohyama W, et al. Genetic Profile and Microsatellite Instability in a Case of Secondary Esophageal Squamous Cell Carcinoma 12 Years After Allogeneic Hematopoietic Stem Cell Transplantation for Aplastic Anemia. J Pediatr Hematol Oncol 2020;42:302-6. [Crossref] [PubMed]
- Habbous S, Yermakhanova O, Forster K, et al. Variation in Diagnosis, Treatment, and Outcome of Esophageal Cancer in a Regionalized Care System in Ontario, Canada. JAMA Netw Open 2021;4:e2126090. [Crossref] [PubMed]
- Majhail NS, Rizzo JD. Surviving the cure: long term followup of hematopoietic cell transplant recipients. Bone Marrow Transplant 2013;48:1145-51. [Crossref] [PubMed]
- Inamoto Y, Matsuda T, Tabuchi K, et al. Outcomes of patients who developed subsequent solid cancer after hematopoietic cell transplantation. Blood Adv 2018;2:1901-13. [Crossref] [PubMed]
- Tichelli A, Beohou E, Labopin M, et al. Evaluation of Second Solid Cancers After Hematopoietic Stem Cell Transplantation in European Patients. JAMA Oncol 2019;5:229-35. [Crossref] [PubMed]


