
Effects of sevoflurane and propofol on postoperative nausea and vomiting in patients with colorectal cancer placed under general anesthesia: a systematic review and meta-analysis
Introduction
Postoperative complications are common in colorectal cancer (CRC) (1). With an incidence of 30–70% (2,3), postoperative nausea and vomiting (PONV) is one of the most common complications of general anesthesia. Due to pneumoperitoneum and other factors, the incidence of PONV in laparoscopic surgery is high, and it has been reported that the incidence of PONV may be as high as 50–70% (4). PONV seriously affects the postoperative feelings of patients, and even leads to the dehiscence of incisions, acid-based imbalances, aspiration, and other serious consequences (5). Thus, the prevention of PONV has become a major clinical concern. PONV occurs for variety of reasons, including as a result of general anesthesia, abdominal organ pulling, and surgery (especially laparoscopic surgery); however, carbon trioxide pneumoperitoneum is the main cause of PONV (6). The incidence of PONV is significantly higher in laparoscopic patients than non-laparoscopic patients. Thus, it is important to implement effective measures to reduce the incidence of PONV in laparoscopic abdominal surgery patients to prevent complications after body anesthesia and reduce the risks of surgery.
The causes and nerve conduction of nausea and vomiting are not identical. Nausea is a subjective sensation guided by simple neuro-mediators, which is difficult to simulate in animal models, but its mechanism is not fully understood. Vomiting is a stimulation reaction from the pharynx to the gastrointestinal tract chemoreceptor excitation area or senior cortical center, through a series of neurotransmitters, including norepinephrine, dopamine and 5-HT, to the vomiting center, through the excited motor nerve center, respiratory center, sports center and other nerve reflex formation, vomiting (7). PONV are related to the cost, safety and comfort of postoperative patients. In the eyes of patients, postoperative nausea and vomiting ranked first among the undesirable side effects. In minors, its incidence is highest. Because of obesity, postoperative anxiety, it may also be prolonged surgery makes its incidence increased. It is also associated with specific procedures (such as laparoscopy and abdominal surgery, head and neck surgery, middle ear surgery, strabismus surgery). Potent inhaled anesthetics have not been particularly valuable in altering the occurrence of PONV. Anesthesia that causes sympathetic nerve excitation can increase postoperative nausea and vomiting. Ether combined with opioids, for example, causes nausea and vomiting in more than 70 percent of children. The addition of nitrous oxide to volatile anesthetics can increase the occurrence of PONV, especially desflurane.
PONV is the most common complication of anesthesia. Laparoscopic surgery is widely used in clinical settings but the incidence of nausea and vomiting is high (8). It is higher than that of non-laparoscopic surgery, which is mainly related to patient factors, anesthesia factors, the type and duration of surgery, and carbon dioxide pneumoperitoneum (9). Various inducing factors, such as acetylcholine, norepinephrine, 5-HT, and other transmitters, stimulate peripheral receptors and the vomiting center. Certain 5-HT3 receptor antagonists are widely used in the prevention and treatment of PONV (10), with the development of laparoscopic CRC surgery is increasingly promoted to take effective anti-emetic measures to prevent PONV has attracted more and more attention.
At present, there are some controversies about the effects of sevoflurane and propofol on the incidence of PONV, which may be related to the differences in pharmacological effects and water solubility of sevoflurane and propofol respectively.
Therefore, it is necessary to analyze this issue through systematic review meta-analysis in this study. We present the following article in accordance with the PRISMA reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-783/rc).
Methods
Retrieval strategy
In this study, the PubMed, Cochrane, Web of Science, Embase, clinical research register and CQVIP databases were searched, and the retrieval time was limited from the initial construction time of the databases between October 2000 and October 2021. In addition, other methods, such as website, organization and citation searches, were employed to retrieve the relevant research articles. The key words used included “sevoflurane”, “propofol”, “rectal cancer”, and “nausea and vomiting” (see Figure 1).
Inclusion criteria
To be eligible for inclusion in this meta-analysis, the studies had to meet the following inclusion criteria: (I) report on a randomized controlled trial (RCT); (II) include subjects who had rectal cancer; (III) have the full text of the article available, and have the general basic data of the clinical patients available or directly available upon request; (IV) PICOS principle was adopted to clarify the criteria for the inclusion of patients. We used the PICOS principle to assist the logical framework or thinking in the construction of clinical research questions.
Exclusion criteria
Studies were excluded from this meta-analysis if they met any of the following exclusion criteria: (I) the data could not be extracted for the analysis; (II) the article concerned a case report, review, meta-analysis, etc.; (III) the subjects were non-human subjects or the study did not include a control group; (IV) the patients had mental disorders or did not cooperate with management; (V) the patients had a severe heart disease, malignant tumors, or severe infections.
Data extraction and collation
Data from the studies that met the above-mentioned criteria were extracted blindly. The following data were extracted: name of the first author, study area, year of publication of article, study design type, sample content (experimental group and control group), source of control group, thyroid function index data during pregnancy, odds ratio (OR) values for the influential factors, and 95% confidence intervals (CIs).
They were assessed according to the Cochrane Collaborative Network’s risk of bias assessment criteria. The completeness of the results, data, and other sources of bias (e.g., selection bias). The quality assessments were made, and the studies were assessed as having, “low bias”, “high bias”, or “unclear” bias (if there was a lack of relevant information or bias uncertainty). All the evaluation results were input into RevMan 5.2.6 software to generate a risk of bias assessment map (see Figure 2).
Heterogeneity analysis
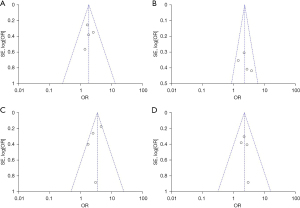
Stata 15 statistical software was used for the funnel plot analysis, and Egger’s test was used to determine whether there was any publication bias in the included articles. If heterogeneity was observed, a subgroup analysis and sensitivity analysis were conducted to explore the source of the heterogeneity. If there was no significant statistical heterogeneity between the studies, the fixed-effects model was used for the pooled analysis. If there was significant statistical heterogeneity among the outcome index data, but there was no clinical heterogeneity, the random-effects model method was used to combine the data (see Figure 3).
Statistical analysis
The Cochrane Collaboration provided Review Manager 5.2 software (Cochrane Information Management System) for the statistical analysis. An χ² test (for which a P value <0.05 was set as the test level), a U test represented by a Z value, and a P value were used for the hypothesis tests, and an χ² test was used to test the heterogeneity of the articles.
Results
Literature retrieval results and included research characteristics
The PubMed, Cochrane, Web of Science, Embase, clinical research register and CQVIP databases were searched in this study. The retrieved articles then underwent a preliminary screening. Specifically, 2 researchers screened and reviewed the included articles by reading the abstract and the full text and eliminated duplicate articles or articles for which the relevant detection data could not be obtained, after which 32 articles remained. The 32 full-text articles were critically reviewed, and articles were excluded if they deviated from the content of this study or if there were multiple different reports on the same clinical study. The eligible articles were searched individually to prevent omissions. Ultimately, a total of 12 RCTs (11-22) were included in this study, and there is no obvious selection risk bias in this study (see Table 1).
Table 1
| Study | Age (mean ± standard deviation) | Gender (male), % |
Experimental group (N) |
Control group (N) |
NOS score |
Research type |
|---|---|---|---|---|---|---|
| Kim KH 2017 | 63.71±2.2 | 41.25 | 40/60 | 20/60 | 8 | RCT |
| Takemoto H 2017 | 65.65±3.4 | 69.12 | 75/120 | 45/120 | 7 | RCT |
| Hata T 2021 | 63.12±4.5 | 45.72 | 244/386 | 142/386 | 8 | RCT |
| van der Vorst MJDL 2021 | 62.15±4.5 | 44.12 | 100/189 | 89/189 | 8 | RCT |
| Avallone A 2021 | 62.85±1.4 | 51.89 | 21/30 | 9/30 | 8 | RCT |
| Li M 2021 | 64.36±1.2 | 63.45 | 133/233 | 100/233 | 7 | RCT |
| Liu Q 2020 | 62.62±2.2 | 78.10 | 40/70 | 30/70 | 9 | RCT |
| Lenz HJ 2017 | 62.61±3.0 | 48.75 | 30/56 | 26/56 | 9 | RCT |
| Huang D 2020 | 67.25±4.5 | 59.23 | 45/80 | 35/80 | 7 | RCT |
| Haruethaivijitchock P 2020 | 68.22±5.2 | 56.22 | 15/25 | 10/25 | 8 | RCT |
| Gu B 2021 | 61.35±1.1 | 53.16 | 68/100 | 32/100 | 8 | RCT |
| Boulianne M 2020 | 61.25±1.0 | 66.34 | 38/62 | 24/62 | 8 | RCT |
NOS, Newcastle-Ottawa Scale; RCT, randomized controlled trial.
Changes in the postoperative heart rate
The heterogeneity test of the included 12 RCTs that examined postoperative heart rate change showed that the heterogeneity of the studies was small enough to allow a meta-analysis to be conducted of the selected articles using a fixed-effects model. After the meta-analysis, forest plots were drawn of the postoperative heart rate changes of the 4 included articles, which showed that there was a statistical difference between the postoperative heart rate changes of the experimental and control groups (OR =3.55, 95% CI: 2.40, 5.27, P<0.00001, I2=0%, Z=6.30; see Figure 4).

Mean artery pressure (MAP)
The heterogeneity test of the included 12 RCTs that examined MAP showed that the heterogeneity of the studies was small enough to allow a meta-analysis to be conducted of the selected articles using a fixed-effects model. After the meta-analysis, forest plots were drawn of the MAP of the 4 included articles, which showed there was a statistical difference in the MAP between the experimental and control groups [OR =2.58, 95% CI: 2.04, 3.26, P<0.00001, I2=58%, Z=7.87] (see Figure 5).
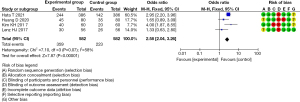
Incidence of PONV
The heterogeneity test of the included 12 RCTs that examined the incidence of PONV showed that the heterogeneity of the studies was small enough to allow a meta-analysis to be conducted of the selected articles using a fixed-effects model. After the meta-analysis, forest plots were drawn of the incidence of PONV of the 4 included articles, with the rhombus plot away from the vertical line, which showed that there was a statistical difference in the incidence of PONV between the experimental and control groups (OR =1.73, 95% CI: 1.38, 2.17, P<0.00001, I2=46%, Z=4.78; see Figure 6).

Incidence of postoperative disturbance of consciousness
The heterogeneity test of the included 12 RCTs that examined the incidence of postoperative disturbance of consciousness showed that the heterogeneity of the studies was small enough to allow a meta-analysis to be conducted of the selected articles using a fixed-effects model. After the meta-analysis, forest plots were drawn made of the incidence of postoperative conscious disturbance of the 4 included articles, with the rhombus plot away from the vertical line, which showed that there was a statistical difference in the incidence of the postoperative conscious disturbance between the experimental and control groups (OR =2.09, 95% CI: 1.62, 3.07, P<0.00001, I2=63%, Z=5.67; see Figure 7).
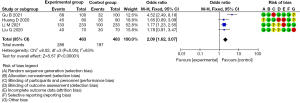
Discussion
Proper fasting before surgery is recommended to prevent vomiting and regurgitation during or after surgery, and to prevent aspiration, lung infection, and suffocation (23). However, surgery patients may experience fluid quantity insufficiency, and after the operation may experience thirst, dizziness, fatigue, nausea, and other symptoms (24). Oral and pharyngeal suction and extubation under shallow anesthesia often cause nausea and vomiting. The tracheal catheter should be removed as soon as possible after spontaneous breathing recovery by patients (25). After extubation, the placement of the oropharyngeal airway should be avoided where possible to avoid excessive stimulation to the pharynx, such as repeated attraction (26). The extubation of patients with a full stomach and intestinal obstructions should be performed with caution to prevent nausea, vomiting, aspiration, and suffocation. As hypoxemia and hypotension can also cause nausea and vomiting, patients’ respiratory and circulatory functions should be maintained throughout and after surgery (27). Oxygen inhalation is an important measure in the treatment of hypoxemia, but the etiological treatment is more important, such as airway obstruction to open the airway and increase tidal volume is the most important. The high incidence of PONV in abdominal surgery may be related to local tissue ischemia and the release of 5-HT from gastrointestinal tissues caused by surgical compression (28). Oxygen inhalation can improve the hypoxia state, thus preventing the release of 5-HT. However, it has also been reported that a high concentration of oxygen inhalation does not effectively reduce the incidence of PONV (29). Hypotension should also be treated with blood transfusion, fluid rehydration, and vasoactive drugs (30).
Despite great developments in anesthesia and anesthesia technology, PONV is still a common complication of surgery with anesthesia. PONV is not only painful for patients, it is also prone to result in poor wound healing, esophageal injury, water and electrolyte disorders, and other complications, thus increasing the length of hospital stay of patients, resulting in increased medical costs. PONV generally occurs within 24 h of surgery, and most frequently occurs within 2 h of surgery, but also occasionally occurs within 48 h of surgery (31). The incidence of PONV is affected by individual factors, the type and duration of the surgery, the anesthetic drugs and methods, preoperative anxiety, and other factors. Different incidence rates have been reported in different articles; however, the incidence rate of PONV is generally about 20–30%. As patients’ demands for comfort after anesthesia and surgery continue to increase, great concern about this issue has been generated.
As a common gastrointestinal tumor, CRC is treated with surgical therapy, chemotherapy, radiotherapy, immunotherapy, and targeted therapy. For CRC patients, various gastrointestinal symptoms after anesthesia can directly affect the efficacy or even end chemotherapy in advance, resulting in a poor prognosis (32). As the most common adverse reaction of the gastrointestinal tract after CRC chemotherapy, chemotherapy induced nausea and vomiting (CINV) may result in a series of serious consequences; for example, patients’ nutritional status may deteriorate, their body microenvironment may change, and their emotional adverse responses may require treatment. The treatments for CINV include anti-emetic drugs, traditional Chinese medicine, external treatment of traditional Chinese medicine, and a combination of Chinese and Western medicine. 5-HT receptor antagonists and neurokinin-1 receptor antagonists can treat the nausea and vomiting of patients after chemotherapy; however, the effects are not good for delayed type nausea and vomiting.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-783/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-783/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen Y, Qi A, Teng D, et al. Probiotics and synbiotics for preventing postoperative infectious complications in colorectal cancer patients: a systematic review and meta-analysis. Tech Coloproctol 2022;26:425-36. [Crossref] [PubMed]
- Buschmann D, Brandes F, Lindemann A, et al. Propofol and Sevoflurane Differentially Impact MicroRNAs in Circulating Extracellular Vesicles during Colorectal Cancer Resection: A Pilot Study. Anesthesiology 2020;132:107-20. [Crossref] [PubMed]
- Zhao A, Liu Y. Propofol suppresses colorectal cancer development by the circ-PABPN1/miR-638/SRSF1 axis. Anal Biochem 2021;631:114354. [Crossref] [PubMed]
- Cui X, Feng J, Wu J, et al. Propofol postpones colorectal cancer development through circ_0026344/miR-645/Akt/mTOR signal pathway. Open Med (Wars) 2021;16:570-80. [Crossref] [PubMed]
- Wu L, Zhong Y, Wu D, et al. Immunomodulatory Factor TIM3 of Cytolytic Active Genes Affected the Survival and Prognosis of Lung Adenocarcinoma Patients by Multi-Omics Analysis. Biomedicines 2022;10:2248. [Crossref] [PubMed]
- Ye LL, Cheng ZG, Cheng XE, et al. Erratum: Corregendum to: Propofol regulates miR-1-3p/IGF1 axis to inhibit the proliferation and accelerates apoptosis of colorectal cancer cells. Toxicol Res (Camb) 2021;10:1229. [Crossref] [PubMed]
- Xu K, Tao W, Su Z. Propofol prevents IL-13-induced epithelial-mesenchymal transition in human colorectal cancer cells. Cell Biol Int 2018;42:985-93. [Crossref] [PubMed]
- Lee S, Pyo DH, Sim WS, et al. Early and Long-Term Outcomes after Propofol-and Sevoflurane-Based Anesthesia in Colorectal Cancer Surgery: A Retrospective Study. J Clin Med 2022;11:2648. [Crossref] [PubMed]
- Wu L, Zhong Y, Yu X, et al. Selective poly adenylation predicts the efficacy of immunotherapy in patients with lung adenocarcinoma by multiple omics research. Anticancer Drugs 2022;33:943-59. [Crossref] [PubMed]
- Gao Y, Han T, Han C, et al. Propofol Regulates the TLR4/NF-κB Pathway Through miRNA-155 to Protect Colorectal Cancer Intestinal Barrier. Inflammation 2021;44:2078-90. [Crossref] [PubMed]
- Kim KH, Kim DH, Bae JM, et al. Acupuncture and PC6 stimulation for the prevention of postoperative nausea and vomiting in patients undergoing elective laparoscopic resection of colorectal cancer: a study protocol for a three-arm randomised pilot trial. BMJ Open 2017;7:e013457. [Crossref] [PubMed]
- Takemoto H, Nishimura J, Komori T, et al. Combination antiemetic therapy with aprepitant/fosaprepitant in patients with colorectal cancer receiving oxaliplatin-based chemotherapy in the SENRI trial: analysis of risk factors for vomiting and nausea. Int J Clin Oncol 2017;22:88-95. [Crossref] [PubMed]
- Hata T, Hagihara K, Tsutsui A, et al. Administration Method of Adjuvant Tegafur-Uracil and Leucovorin Calcium in Patients with Resected Colorectal Cancer: A Phase III Study. Oncologist 2021;26:e735-41. [Crossref] [PubMed]
- van der Vorst MJDL, Toffoli EC, Beusink M, et al. Metoclopramide, Dexamethasone, or Palonosetron for Prevention of Delayed Chemotherapy-Induced Nausea and Vomiting After Moderately Emetogenic Chemotherapy (MEDEA): A Randomized, Phase III, Noninferiority Trial. Oncologist 2021;26:e173-81. [Crossref] [PubMed]
- Avallone A, Piccirillo MC, Nasti G, et al. Effect of Bevacizumab in Combination With Standard Oxaliplatin-Based Regimens in Patients With Metastatic Colorectal Cancer: A Randomized Clinical Trial. JAMA Netw Open 2021;4:e2118475. [Crossref] [PubMed]
- Li M, Chen J, Deng Y, et al. Risk prediction models based on hematological/body parameters for chemotherapy-induced adverse effects in Chinese colorectal cancer patients. Support Care Cancer 2021;29:7931-47. [Crossref] [PubMed]
- Liu Q, Lin JY, Zhang YF, et al. Effects of epidural combined with general anesthesia versus general anesthesia on quality of recovery of elderly patients undergoing laparoscopic radical resection of colorectal cancer: A prospective randomized trial. J Clin Anesth 2020;62:109742. [Crossref] [PubMed]
- Lenz HJ, Philip P, Saunders M, et al. Randomized study of etirinotecan pegol versus irinotecan as second-line treatment for metastatic colorectal cancer. Cancer Chemother Pharmacol 2017;80:1161-9. [Crossref] [PubMed]
- Huang D, Song L, Li Y, et al. Posteromedial quadratus lumborum block versus transversus abdominal plane block for postoperative analgesia following laparoscopic colorectal surgery: A randomized controlled trial. J Clin Anesth 2020;62:109716. [Crossref] [PubMed]
- Haruethaivijitchock P, Ng JL, Taksavanitcha G, et al. Postoperative analgesic efficacy of modified continuous transversus abdominis plane block in laparoscopic colorectal surgery: a triple-blind randomized controlled trial. Tech Coloproctol 2020;24:1179-87. [Crossref] [PubMed]
- Gu B, Fang J, Lian Y, et al. Effect of Deep Versus Moderate Neuromuscular Block on Pain After Laparoscopic Colorectal Surgery: A Randomized Clinical Trial. Dis Colon Rectum 2021;64:475-83. [Crossref] [PubMed]
- Boulianne M, Paquet P, Veilleux R, et al. Effects of quadratus lumborum block regional anesthesia on postoperative pain after colorectal resection: a randomized controlled trial. Surg Endosc 2020;34:4157-65. [Crossref] [PubMed]
- Chen X, Wu Q, Sun P, et al. Propofol Disrupts Aerobic Glycolysis in Colorectal Cancer Cells via Inactivation of the NMDAR-CAMKII-ERK Pathway. Cell Physiol Biochem 2018;46:492-504. [Crossref] [PubMed]
- Chen Y, Liang M, Zhu Y, et al. The effect of propofol and sevoflurane on the perioperative immunity in patients under laparoscopic radical resection of colorectal cancer. Zhonghua Yi Xue Za Zhi 2015;95:3440-4. [PubMed]
- Chelazzi C, Consales G, Boninsegni P, et al. Propofol sedation in a colorectal cancer screening outpatient cohort. Minerva Anestesiol 2009;75:677-83. [PubMed]
- Wu L, Zheng Y, Liu J, et al. Comprehensive evaluation of the efficacy and safety of LPV/r drugs in the treatment of SARS and MERS to provide potential treatment options for COVID-19. Aging (Albany NY) 2021;13:10833-52. [Crossref] [PubMed]
- Ki S, Cho Y, Choi Y, et al. Effect of chemotherapy on effect-site concentration of propofol for loss of consciousness in patients with colorectal cancer. Korean J Anesthesiol 2022;75:160-7. [Crossref] [PubMed]
- Oh CS, Park HJ, Piao L, et al. Expression Profiles of Immune Cells after Propofol or Sevoflurane Anesthesia for Colorectal Cancer Surgery: A Prospective Double-blind Randomized Trial. Anesthesiology 2022;136:448-58. [Crossref] [PubMed]
- Hassan C, Rex DK, Cooper GS, et al. Endoscopist-directed propofol administration versus anesthesiologist assistance for colorectal cancer screening: a cost-effectiveness analysis. Endoscopy 2012;44:456-64. [Crossref] [PubMed]
- Riphaus A, Ferreira AO. Reply to the letters to the editor concerning the article: Alexandre Oliveira Ferreira, Andrea Riphaus. Propofol to increase colorectal cancer screening in Portugal. Acta Med Port 2014;27:541-42. Acta Med Port 2014;27:796-8. [Crossref] [PubMed]
- Martins do Vale F. Letters to the editor concerning the article: Alexandre Oliveira Ferreira, Andrea Riphaus. Propofol to increase colorectal cancer screening in Portugal. Acta Med Port 2014;27:541-42. Acta Med Port 2014;27:794-5. [Crossref] [PubMed]
- Wu L, Zheng Y, Ruan X, et al. Long-chain noncoding ribonucleic acids affect the survival and prognosis of patients with esophageal adenocarcinoma through the autophagy pathway: construction of a prognostic model. Anticancer Drugs 2022;33:e590-e603. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)




