
Rectal tumor fragmentation as a response pattern following chemoradiation
Introduction
Neoadjuvant (chemo)radiation (nCRT) followed by total mesorectal excision (TME) is the standard of care for patients with locally advanced rectal cancer (LARC) (1). In comparison to postoperative treatment, nCRT results in higher rates of compliance, improved local control (LC), tumor down-staging, and lower rates of acute and late toxicity (2,3). However, tumor response, and consequently, prognosis remains heterogeneous (2-4). The extent of residual tumor at time of TME is prognostic, though tumor response is dynamic and affected by many variables, including tumor and treatment related factors (5). The pathologic TN stage, margin status (including circumferential/radial), and degree of tumor response, may identify patients at highest risk for relapse who may benefit most from additional therapy or increased surveillance intensity (6,7).
Although patients with a greater tumor response to nCRT often have a more favorable prognosis, the substantial variability in recurrence and survival amongst partial responders suggests that treatment-induced effects result in prognostic subgroups (8). Several methods to classify tumor response to nCRT have been evaluated for prognostic significance in LARC patients, each with its own strengths and limitations (9). Histopathologic evaluation of tumor response from the TME specimen by ypTN staging and tumor regression grading (TRG) have been shown to be highly prognostic for LARC patients (6,7,10-13). However, the TRG system requires standardization as there are multiple versions describing the proportion of viable residual tumor to fibrosis using different scoring systems (12,14-17). The neoadjuvant rectal (NAR) score, developed from the Valentini nomogram, includes clinical tumor stage (cT) in addition to pathologic tumor (pT) and nodal staging (pN), and results in a pseudo-continuous variable reflecting tumor downstaging (18-20).
However, these methods of tumor response classification can result in discordant findings. As an example, a patient with a clinical T3N+ LARC treated with nCRT followed by TME and histologically ypT3N0, AJCC TRG 1 (single or small group of cells) is expected to have a lower risk of disease recurrence compared to the same patient with ypT3N0, AJCC TRG 3 (minimal tumor response to treatment), indicating that ypTN downstaging methods alone may only partly reflect disease biology and patient outcome. In this way, tumor regression is not directly correlated to ypTN downstaging. A potential explanation for this histologic discrepancy is a tumor response pattern described as fragmentation, as opposed to the more commonly considered response pattern of tumor shrinkage which occurs in the direction of the mucosa (21). Following nCRT, residual tumor can be measured according to distribution of viable tumor cells within submucosa and/or muscularis propria in relation to the proportion of fibrosis. Unexpectedly, viable tumor cells have been shown to be absent within the submucosal layer in 54% of patients with ypT2-4 tumors, indicating that tumor can remain present in the outer layers of the rectal wall, while absent within the inner, superficial layers (9,22,23). This suggest that tumor destruction from nCRT may occur often in a fragmentation pattern, with residual individual or small groups of viable tumor cells dispersed (fragmented) throughout the rectal wall.
The relative prognostic significance of discordant TRG and ypTN stage is not well described (9,13,24-26). Therefore, the present study assessed the prognostic value of ypTN to the AJCC TRG schema in patients with LARC treated with nCRT and TME. We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-477/rc).
Methods
Patients and treatment
This is a single-center, retrospective review of 90 consecutive patients with non-metastatic rectal cancer receiving nCRT followed by TME. Patients eligible for inclusion in the present study had clinical stage II-III (according to the American Joint Committee on Cancer Staging 8th edition) (27) rectal adenocarcinoma without evidence of distant metastasis (DM) who received nCRT followed by TME between 2007 and 2018. Pre-treatment rectal protocol MRI was incorporated into the institutional protocol in 2014, so patients treated from 2007 to 2014 underwent pre-treatment endorectal ultrasound, and all patients treated from 2015 to 2018 underwent pre-treatment rectal protocol MRI. Patient, tumor, and treatment characteristics were obtained from the medical chart. Neoadjuvant chemoradiation (nCRT) consisted of 45–56 Gy delivered in daily fractions of 1.8–2.0 Gy per fraction over 5–6 weeks with concurrent oral capecitabine or continuous venous infusional 5-fluorouracil. The TME was completed 6–12 weeks after the completion of nCRT. Patients were followed with regular clinic visits and labs, including the carcinoembryonic antigen (CEA), every 3–6 months for the first 2 years, then every 6 months for the following 3 years, in addition to computed tomography (CT) of the chest, abdomen, and pelvis every 6 months for the first 2 years, followed by annually for the following 3 years. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the University of South Florida Institutional Review Board (No. 16567) and individual consent for this retrospective review was waived.
Pathology review
Two pathologists retrospectively examined each case to confirm ypTN staging and TRG according to the American Joint Committee on Cancer (AJCC)/College of American Pathologists (CAP) TRG version (14). Tumor regression grade 0 (TRG 0) corresponded to no residual tumor cells, TRG 1 corresponded to a single or small group of cells, TRG 2 corresponded to cancer with fibrotic response, and TRG 3 corresponded to minimal tumor response to treatment. As a combined ypTN downstaging prognostic surrogate, the NAR score was calculated and classified according to prior studies as low (corresponding to an excellent response), intermediate, and high (corresponding to a poor response) score (19,20,24).
Statistical analysis
Descriptive statistics were utilized to evaluate patient, tumor, and treatment characteristics, with quantitative variables presented as a median with range. Time-to-event outcomes, including LC, disease-free survival (DFS), overall survival (OS), and distant metastasis (DM), were estimated with the Kaplan-Meier (KM) method with log-rank testing to assess differences between groups. The Cox proportional hazards ratio model was used to assess the univariate (UVA) and multivariate (MVA) effect of patient, tumor, and treatment factors on DFS. Variables that reached significance upon UVA with a P value less than 0.05 were included for MVA. Statistical analyses were performed using JMP 13 (SAS Institute Inc., Cary, NC, USA).
Results
Patient and treatment characteristics
Ninety patients with LARC underwent nCRT followed by TME (Table 1). Most patients were male (58%) and white (92%) with clinical stage III disease (68%). Most received radiotherapy via three-dimensional conformal radiation therapy (3DCRT, 91%) to a median total dose of 50.4 Gy. Seventy-three patients (81%) underwent low anterior resection (LAR), 17 (19%) underwent abdominoperineal resection (APR), and most patients received adjuvant chemotherapy (ACT) (91%). The median interval from completion of nCRT to surgery was 62 days (IQR: 56–70 days). There were 6 circumferential radial margin positive cases (7%) and 1 distal margin positive case (1%). There were 67 cases (75%) of complete or near complete mesorectal resections, 4 cases (4%) of incomplete mesorectal resection, and 19 cases (21%) in which mesorectum evaluation was unavailable.
Table 1
| Characteristic | No. (%) or median [range] |
|---|---|
| Age | 55 [27–80] |
| Gender | |
| Male | 52 (57.8%) |
| Female | 38 (42.2%) |
| Ethnicity | |
| White | 83 (92.2%) |
| Black | 3 (3.3%) |
| Other | 4 (4.4%) |
| KPS | |
| 90–100 | 80 (88.9%) |
| 70–80 | 10 (11.1%) |
| Clinical T-Stage | |
| cT2 | 3 (3.3%) |
| cT3 | 80 (88.9%) |
| cT4 | 7 (7.8%) |
| Clinical N-Stage | |
| cN0 | 29 (32.2%) |
| cN1 | 48 (53.3%) |
| cN2 | 13 (14.4%) |
| Radiation technique | |
| 3DCRT | 82 (91.1%) |
| IMRT | 8 (8.9%) |
| Total dose (cGy) | |
| 5,600 | 7 (7.8%) |
| 5,040 | 78 (86.7%) |
| 5,000 | 3 (3.3%) |
| 4,860 | 1 (1.1%) |
| 4,500 | 1 (1.1%) |
| Neoadjuvant chemotherapy | |
| 5FU | 42 (46.7%) |
| Capecitabine | 29 (32.2%) |
| 5FU + Sorafenib | 9 (10.0%) |
| Capecitabine + Lenvantinib | 7 (7.8%) |
| FOLFOX | 2 (2.2%) |
| Capecitabine + Oxaliplatin | 1 (1.1%) |
| Interval between completion of neoadjuvant treatment and surgery (days) | 61.5 [36–105] |
| Surgery | |
| LAR | 73 (81.1%) |
| APR | 17 (18.9%) |
| Adjuvant chemo | |
| FOLFOX | 58 (64.4%) |
| Capecitabine + Oxaliplatin | 13 (14.4%) |
| 5FU | 5 (5.6%) |
| Capecitabine | 5 (5.6%) |
| FOLFOX + Avastin | 1 (1.1%) |
| None | 8 (8.9%) |
| Pathologic T-stage | |
| ypT0 | 24 (26.7%) |
| ypT1 | 3 (3.3%) |
| ypT2 | 23 (25.6%) |
| ypT3 | 38 (42.2%) |
| ypT4 | 2 (2.2%) |
| Pathologic N-stage | |
| ypN0 | 53 (58.9%) |
| ypN1 | 26 (28.9%) |
| ypN2 | 11 (12.2%) |
| NAR score | |
| <8 | 29 (32.2%) |
| 8 to 16 | 28 (31.1%) |
| >16 | 33 (36.7%) |
| TRG | |
| 0 | 21 (23.3%) |
| 1 | 47 (52.2%) |
| 2 | 18 (20.0%) |
| 3 | 4 (4.4%) |
| Differentiation | |
| Well/moderate | 88 (95.7%) |
| Poor | 4 (4.3%) |
| LVI | 25 (27.8%) |
| PNI | 19 (21.1%) |
| Distance to anal verge (cm) | 5 [0–15] |
| Pretreatment longitudinal tumor size (cm) | 5 [1.5–11] |
| Circumferential radial margin | |
| Positive | 6 (6.7%) |
| Negative | 84 (93.3%) |
| Distal resection margin | |
| Positive | 1 (1.1%) |
| Negative | 89 (98.9%) |
| Completeness of mesorectum | |
| Complete | 53 (58.9%) |
| Near complete | 14 (15.6%) |
| Incomplete | 4 (4.4%) |
| N/A | 19 (21.1%) |
KPS, Karnofsky Performance Status; 3DCRT, three-dimensional conformal radiation therapy; IMRT, intensity-modulated radiation therapy; cGy, centigray; 5FU, fluorouracil; FOLFOX, fluorouracil, oxaliplatin, leucovorin; LAR, low anterior resection; APR, abdominoperineal resection, NAR, neoadjuvant rectal score; TRG, tumor regression grade; LVI, lymphovascular invasion; PNI, perineural invasion; N/A, not available.
Clinical outcomes
There were 18 cases with a complete pathologic response, ypT0ypN0 (20%) (Table 1). There were 29 cases (32%) of low NAR score, 28 cases (31%) of intermediate NAR score, and 33 cases (37%) of high NAR score. There were 21 cases (23%) of AJCC-TRG 0, 47 cases (52%) of AJCC-TRG 1, 18 cases (20%) of AJCC-TRG 2, and 4 cases (4%) of AJCC-TRG 3. Of note, all 4 patients with AJCC-TRG 3 received ACT.
Median follow-up after surgery was 46 months (95% CI: 41–50 months). The 4-year OS, DFS, and LC was 92.4%, 74.4%, and 90.2%, respectively (Figure 1). Upon MVA, female gender was predictive of superior DFS, and APR was associated with inferior DFS (Table 2). While ypT stage and NAR score were not significant prognostic factors, AJCC-TRG score of 3 was predictive for inferior DFS (3-year DFS 79% vs. 25%, P=0.0002, Figure 2). Patients with AJCC-TRG 3 had a significantly higher risk of both local (75% vs. 5%) and distant failure (75% vs. 19%). Though AJCC-TRG score of 3 was a significant prognostic factor for OS upon UVA, there were no prognostic factors that reached significance upon MVA for OS. Receipt of ACT did not predict for OS, DFS, or LC.
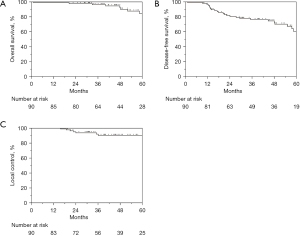
Table 2
| Characteristic | DFS | OS | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age | ⎯ | ⎯ | 1.06 (1.00–1.13) | 0.070 | |
| Gender | |||||
| Male (reference) | 1.00 | ⎯ | ⎯ | ||
| Female | 0.28 (0.11–0.72) | 0.008 | ⎯ | ⎯ | |
| KPS | |||||
| 90–100 (reference) | ⎯ | ⎯ | ⎯ | ⎯ | |
| 70–80 | ⎯ | ⎯ | ⎯ | ⎯ | |
| Clinical T-Stage | |||||
| cT2 (reference) | ⎯ | ⎯ | ⎯ | ⎯ | |
| cT3 | ⎯ | ⎯ | ⎯ | ⎯ | |
| cT4 | ⎯ | ⎯ | ⎯ | ⎯ | |
| Clinical N-Stage | |||||
| cN0 (reference) | ⎯ | ⎯ | ⎯ | ⎯ | |
| cN1 | ⎯ | ⎯ | ⎯ | ⎯ | |
| cN2 | ⎯ | ⎯ | ⎯ | ⎯ | |
| Interval between completion of neoadjuvant treatment and surgery (days) | ⎯ | ⎯ | ⎯ | ⎯ | |
| Surgery | |||||
| LAR (reference) | 1.00 | 1.00 | |||
| APR | 2.59 (1.02–6.58) | 0.045 | 2.07 (0.54–8.03) | 0.291 | |
| Adjuvant chemo | ⎯ | ⎯ | ⎯ | ⎯ | |
| Pathologic T-stage | |||||
| ypT0 (reference) | ⎯ | ⎯ | ⎯ | ⎯ | |
| ypT1 | ⎯ | ⎯ | ⎯ | ⎯ | |
| ypT2 | ⎯ | ⎯ | ⎯ | ⎯ | |
| ypT3 | ⎯ | ⎯ | ⎯ | ⎯ | |
| ypT4 | ⎯ | ⎯ | ⎯ | ⎯ | |
| Pathologic N-stage | |||||
| ypN0 (reference) | ⎯ | ⎯ | ⎯ | ⎯ | |
| ypN1 | ⎯ | ⎯ | ⎯ | ⎯ | |
| ypN2 | ⎯ | ⎯ | ⎯ | ⎯ | |
| NAR score | |||||
| <8 (reference) | ⎯ | ⎯ | ⎯ | ⎯ | |
| 8 to 16 | ⎯ | ⎯ | ⎯ | ⎯ | |
| >16 | ⎯ | ⎯ | ⎯ | ⎯ | |
| TRG | |||||
| 0 (reference) | 1.00 | 1.00 | |||
| 1 | ⎯ | ⎯ | ⎯ | ⎯ | |
| 2 | ⎯ | ⎯ | ⎯ | ⎯ | |
| 3 | 22.8 (4.40–119) | 0.0002 | 4.10 (0.23–73.8) | 0.338 | |
| LVI | ⎯ | ⎯ | ⎯ | ⎯ | |
| PNI | ⎯ | ⎯ | ⎯ | ⎯ | |
| Grade | |||||
| Low (reference) | ⎯ | ⎯ | ⎯ | ⎯ | |
| High | ⎯ | ⎯ | ⎯ | ⎯ | |
| Distance to anal verge | ⎯ | ⎯ | ⎯ | ⎯ | |
| Pretreatment longitudinal tumor size (cm) | |||||
| Completeness of mesorectum | ⎯ | ⎯ | ⎯ | ⎯ | |
| Complete/near complete (reference) | 1.00 | ⎯ | ⎯ | ||
| Incomplete | 2.37 (0.62–9.05) | 0.206 | ⎯ | ⎯ | |
| Positive circumferential margin | ⎯ | ⎯ | ⎯ | ⎯ | |
DFS, disease-free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval; KPS, Karnofsky Performance Status; LAR, low anterior resection; APR, abdominoperineal resection; NAR, neoadjuvant rectal score; TRG, tumor regression grade; LVI, lymphovascular invasion; PNI, perineural invasion.
A subset analysis of clinical T3 disease who were not downstaged after nCRT and remained ypT3 (n=35, Table 3), AJCC TRG 3 was the only significant predictor of DFS (HR 6.4, P=0.012) The NAR score was not a significant prognostic factor for DFS or OS within this subset.
Table 3
| Characteristic | DFS | |
|---|---|---|
| HR (95% CI) | P value | |
| Age | 1.01 (0.96⎯1.06) | 0.712 |
| Gender | ||
| Male (reference) | 1 | |
| Female | 0.33 (0.10⎯1.11) | 0.073 |
| KPS | ||
| 90–100 (reference) | 1 | |
| 70–80 | 1.05 (0.13⎯8.27) | 0.966 |
| Clinical N-Stage | ||
| cN0 (reference) | 1 | |
| cN1 | 1.20 (0.24⎯5.99) | 0.800 |
| cN2 | 2.62 (0.46⎯14.8) | 0.615 |
| Interval between completion of neoadjuvant treatment and surgery (days) | 0.97 (0.92⎯1.03) | 0.366 |
| Surgery | ||
| LAR (reference) | 1 | |
| APR | 0.45 (0.06⎯3.50) | 0.446 |
| Adjuvant chemo | ⎯ | ⎯ |
| Pathologic N-stage | ||
| ypN0 (reference) | 1 | |
| ypN1 | 5.00 (0.60⎯41.6) | 0.136 |
| ypN2 | 4.64 (0.54⎯40.2) | 0.163 |
| NAR score | ||
| <8 | ⎯ | ⎯ |
| 8 to 16 (reference) | 1 | |
| >16 | 4.84 (0.62⎯37.6) | 0.132 |
| TRG | ||
| 0 | ⎯ | ⎯ |
| 1 (reference) | 1 | |
| 2 | 0.86 (0.21⎯3.43) | 0.828 |
| 3 | 6.44 (1.51⎯27.5) | 0.012 |
| LVI | 2.45 (0.66⎯9.11) | 0.180 |
| PNI | 1.63 (0.52⎯5.13) | 0.402 |
| Grade | ||
| Low (reference) | 1 | |
| High | ⎯ | ⎯ |
| Distance to anal verge | 0.98 (0.83⎯1.15) | 0.853 |
| Pretreatment longitudinal tumor size (cm) | 1.11 (0.79⎯1.49) | 0.520 |
| Completeness of mesorectum | ||
| Complete/near complete (reference) | 1 | |
| Incomplete | ⎯ | ⎯ |
| Positive circumferential margin | 0.72 (0.09⎯5.66) | 0.758 |
DFS, disease-free survival; HR, hazard ratio; CI, confidence interval; KPS, Karnofsky Performance Status; LAR, low anterior resection; APR, abdominoperineal resection; NAR, neoadjuvant rectal score; TRG, tumor regression grade; LVI, lymphovascular invasion; PNI, perineural invasion.
Discussion
nCRT prior to mesorectal excision for LARC allows for an in vivo assessment of tumor response once resected (1). Several methods to assess response to nCRT, including ypStage, NAR, and TRG, have been evaluated as prognostic factors and surrogate oncologic endpoints (9). However, the relative prognostic value of these three methods remains incompletely defined (9,13,24-26). In this study, our results not only demonstrate that TRG is an independent predictor of DFS in patients treated with nCRT, but also, they highlight that AJCC TRG 3 represents an especially poor prognosis irrespective of ypTN (Table 3, Figure 2).
Several studies have confirmed that ypStage is a significant prognostic factor in rectal cancer patients (7,11-13,26). In a pooled analysis of 3,105 LARC patients, Maas et al. demonstrated the significance of ypStage, with 5-year DFS 83.3% for patients achieving a pCR and 65.6% for those who did not (11). While the present study did not find ypStage to be a significant prognostic factor, this was likely due to limited numbers of particular ypStage categories (3 cases of ypT1, for example). However, there are important limitations to the prognostic value of ypStage. For example, in cases in which the tumor responds to nCRT via fragmentation versus shrinkage, ypStage alone is likely an inaccurate assessment of prognosis (9). In addition, the accuracy of ypN stage may be dependent upon the number of lymph nodes retrieved at TME (28). Finally, ypStage does not necessarily account for the degree of response to nCRT, a known significant predictor of DFS (24,25,29). The limitations of ypStage may be partly addressed by considering at least two patterns in which rectal cancer responds to neoadjuvant therapy: fragmentation and tumor shrinkage (9).
Nagtegaal and Glynne-Jones have described fragmentation as a pattern of residual tumor cells distributed (fragmented) throughout the rectal wall in isolated or groups of small viable tumor cells with resolution of the dominant tumor mass (9). This contrasts with the more commonly recognized concept of tumor response occurring in a shrinkage pattern, that is, concentrically inward towards the direction of the mucosa (epicenter) of the tumor (9,30). These patterns of residual disease partly explain the anatomic and biologic relationship between ypT stage and TRG. These observations have clinical implications, as tumor scatter among radiation-induced fibrosis remains a point of special oncologic caution before pursuing a rectal organ preserving transanal local excision approach. These distinct patterns of residual disease also explain variability in prognosis among patients within similar downstaging subgroups (30-34). In comparison to TN downstaging, the TRG method offers a more accurate evaluation of the fragmentation response through the evaluation of the overall grade of tumor regression rather than largest tumor extent alone. Therefore, patients with clinical T3 disease treated with nCRT subsequently undergoing TME and pathologically T1-4 reflects a wide spectrum of recurrence risk according to AJCC TRG, with TRG 3 (minimal or no histologic tumor regression) strongly associated with both local and distant failure in our study. Mace et al. used the Cleveland Clinic rectal cancer database to show that AJCC TRG influences oncologic outcome independent of other significant prognostic factors, with each AJCC TRG score increase associated with a statistically significant worse survival outcome (14).
Our results demonstrate that AJCC-TRG is a significant independent predictor of DFS (Table 2, Figure 2), consistent with prior studies (6,7,10,13,24,25,35). In a study of 522 LARC patients, Sun et al. also reported AJCC TRG 3 was a significant predictor of DFS upon MVA (HR 2.81, P=0.047) (24). Our results also show that AJCC-TRG is a significant prognostic variable even in cases of clinical T3 disease that fail to show response by ypStage (Table 3), suggesting that the combination of TRG and ypStage may improve prognostication. Similarly, Song et al. in a study of 331 LARC patients, found that TRG and ypStage were significant prognostic factors for DFS and OS (35). They demonstrated that TRG was predictive of DFS even amongst ypStage III cases, and the addition of TRG to ypStage improved patient prognostication. These findings support pathologic assignment of AJCC TRG, quantifying proportions of residual tumor to fibrosis, provides important prognostic information. Tumor resistance to nCRT portends a higher risk of disease recurrence, reflected as AJCC TRG 3, and highlights our poor understanding of the biologic mechanisms mediating local non-responsiveness and distant failure.
However, TRG is not without its own limitations as a prognostic variable. Unlike ypStage and the NAR score, TRG does not account for nodal metastasis. In addition, there are currently at least 5 TRG systems commonly used for LARC with 3–5 tiers, each quantifying incomplete response to nCRT slightly differently, and studies comparing the systems have found a low concordance rate (36,37). The AJCC 4-tier system used in the present study has been found to be superior in predicting recurrence and should be adopted as the standard (36).
George et al found that the extent tumor downstaging may be more important than final ypStage for patients with LARC who undergo nCRT (19). The NAR score, taking into account both clinical T-stage and final pathologic T- and N-stage, represents a reproducible prognostic variable with the potential to serve as a surrogate endpoint in clinical trials (8,18). However, the utilization of the NAR score in LARC remains controversial. In the CAO/ARO/AIO-04 phase III trial, Fokas et al found that the NAR score was a significant predictor of DFS upon MVA, and the NAR score was validated as a surrogate endpoint for 3-year DFS (20) However, Deng et al. reported that the NAR score was a valid surrogate for DFS in the FOWARC trial, but was not superior to final pathologic staging (38). The present study did not find the NAR score to be a significant prognostic factor for LARC patients, which is consistent with several prior studies (13,26). Ultimately, the utility of the NAR score as a prognostic factor and surrogate endpoint needs further validation within randomized controlled trials, and several ongoing trials are utilizing it as a primary or secondary endpoint (NCT04406857, NCT02921256, NCT04177602, NCT03300544).
The present study has several important limitations. This was a small, retrospective study at a single center resulting in unmeasured biases. An important limitation of our study was due to the effects of low event rates within the recurrence outcomes, which potentially limited the ability of individual variables to reach statistical significance and limited the power of the study to detect main effects and covariates influencing recurrence and survival outcomes. The most predictive variable, AJCC 3 represented only a minority of patients (4%) within our cohort. Inaccuracies in local clinical staging, variability in time interval between receipt of nCRT and TME as well as receipt of ACT, and pathologist interpretation, may influence the findings within this retrospective study (39). Additionally, mesorectal evaluation was unavailable in 19 pattients (21%). Despite these limitations, our results recognizes prognostic variability among ypT stage with variable AJCC TRG, especially among a subset of patients at particularly high risk for recurrence. The proportion of patients with minimal or no tumor regression following nCRT usually represents a small minority of patients, but can range widely from 5% to 56%, largely depending on the TRG schema applied. Larger series suggest approximately 10% to 20% of patients will be classified as AJCC TRG 3, higher than our study (4%) (14,36,40-42). Our study’s smaller proportion of poor tumor responders (AJCC TRG 3) is likely attributed to the small sample size. Despite this limitation, the strong prognostic association with disease recurrence suggests this subpopulation of patients is not an insignificant number and warrants particular attention to improve outcomes.
The AJCC TRG is prognostic and supports routine reporting in pathologic assessment of rectal cancers treated with nCRT. The AJCC TRG should be considered when discussing prognosis and may factor into treatment and/or surveillance decisions. Ultimately, the biologic mechanisms explaining the poorer outcomes in patients with locally treatment-resistant tumors, represented as AJCC TRG 3, requires further study. Given the changing treatment paradigm which favors completion of all therapy in the neoadjuvant setting, evaluation of AJCC TRG in patients treated with total neoadjuvant therapy (TNT) requires further evaluation.
Conclusions
Minimal tumor response to nCRT reflected as histologic AJCC TRG 3, irrespective of ypTN downstaging, is a pattern of residual disease that is at high risk for recurrence. Although the AJCC TRG provides prognostic information, the optimal tumor response classification method requires further study to best stratify patient risk and management. The biologic differences in rectal tumor response patterns, including shrinkage or fragmentation, appear to strongly influence oncologic outcomes and warrant additional research.
Acknowledgments
This project was presented in part at the 2021 American Society of Colon and Rectal Surgeons Annual Scientific Meeting.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-477/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-477/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-477/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-477/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the University of South Florida Institutional Review Board and individual consent for this retrospective review was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- NCCN Clinical Practice Guidelines in Oncology: Rectal Cancer. In: Version 2.2021. 2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf
- Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 2012;30:1926-33. [Crossref] [PubMed]
- Roh MS, Colangelo LH, O'Connell MJ, et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol 2009;27:5124-30. [Crossref] [PubMed]
- Kasi A, Abbasi S, Handa S, et al. Total Neoadjuvant Therapy vs Standard Therapy in Locally Advanced Rectal Cancer: A Systematic Review and Meta-analysis. JAMA Netw Open 2020;3:e2030097. [Crossref] [PubMed]
- Fokas E, Glynne-Jones R, Appelt A, et al. Outcome measures in multimodal rectal cancer trials. Lancet Oncol 2020;21:e252-64. [Crossref] [PubMed]
- On J, Shim J, Mackay C, et al. Pathological response post neoadjuvant therapy for locally advanced rectal cancer is an independent predictor of survival. Colorectal Dis 2021;23:1326-33. [Crossref] [PubMed]
- Wei J, Huang R, Guo S, et al. ypTNM category combined with AJCC tumor regression grade for screening patients with the worst prognosis after neoadjuvant chemoradiation therapy for locally advanced rectal cancer. Cancer Manag Res 2018;10:5219-25. [Crossref] [PubMed]
- Glynne-Jones R, Glynne-Jones S. The concept and use of the neoadjuvant rectal score as a composite endpoint in rectal cancer. Lancet Oncol 2021;22:e314-26. [Crossref] [PubMed]
- Nagtegaal ID, Glynne-Jones R. How to measure tumour response in rectal cancer? An explanation of discrepancies and suggestions for improvement. Cancer Treat Rev 2020;84:101964. [Crossref] [PubMed]
- Erlandsson J, Lörinc E, Ahlberg M, et al. Tumour regression after radiotherapy for rectal cancer - Results from the randomised Stockholm III trial. Radiother Oncol 2019;135:178-86. [Crossref] [PubMed]
- Maas M, Nelemans PJ, Valentini V, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol 2010;11:835-44. [Crossref] [PubMed]
- Quah HM, Chou JF, Gonen M, et al. Pathologic stage is most prognostic of disease-free survival in locally advanced rectal cancer patients after preoperative chemoradiation. Cancer 2008;113:57-64. [Crossref] [PubMed]
- Baek JH, Baek DW, Kang BW, et al. Prognostic Impact of the Neoadjuvant Rectal Score as Compared With the Tumor Regression Grade and Yield Pathologic TNM Stage in Patients With Locally Advanced Rectal Cancer After Neoadjuvant Chemoradiotherapy. In Vivo 2020;34:1993-9. [Crossref] [PubMed]
- Mace AG, Pai RK, Stocchi L, et al. American Joint Committee on Cancer and College of American Pathologists regression grade: a new prognostic factor in rectal cancer. Dis Colon Rectum 2015;58:32-44. [Crossref] [PubMed]
- Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis 1997;12:19-23. [Crossref] [PubMed]
- Mandard AM, Dalibard F, Mandard JC, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 1994;73:2680-6. [Crossref] [PubMed]
- Rödel C, Martus P, Papadoupolos T, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol 2005;23:8688-96. [Crossref] [PubMed]
- Valentini V, van Stiphout RG, Lammering G, et al. Nomograms for predicting local recurrence, distant metastases, and overall survival for patients with locally advanced rectal cancer on the basis of European randomized clinical trials. J Clin Oncol 2011;29:3163-72. [Crossref] [PubMed]
- George TJ Jr, Allegra CJ, Yothers G. Neoadjuvant Rectal (NAR) Score: a New Surrogate Endpoint in Rectal Cancer Clinical Trials. Curr Colorectal Cancer Rep 2015;11:275-80. [Crossref] [PubMed]
- Fokas E, Fietkau R, Hartmann A, et al. Neoadjuvant rectal score as individual-level surrogate for disease-free survival in rectal cancer in the CAO/ARO/AIO-04 randomized phase III trial. Ann Oncol 2018;29:1521-7. [Crossref] [PubMed]
- Graham Martinez C, Kus Öztürk S, Al-Kaabi A, et al. Shrinkage versus fragmentation response in neoadjuvantly treated oesophageal adenocarcinoma: significant prognostic relevance. Histopathology 2022;80:982-94. [Crossref] [PubMed]
- Duldulao MP, Lee W, Streja L, et al. Distribution of residual cancer cells in the bowel wall after neoadjuvant chemoradiation in patients with rectal cancer. Dis Colon Rectum 2013;56:142-9. [Crossref] [PubMed]
- Xiao L, Yu X, Deng W, et al. Pathological Assessment of Rectal Cancer after Neoadjuvant Chemoradiotherapy: Distribution of Residual Cancer Cells and Accuracy of Biopsy. Sci Rep 2016;6:34923. [Crossref] [PubMed]
- Sun Y, Zhang Y, Wu X, et al. Prognostic significance of neoadjuvant rectal score in locally advanced rectal cancer after neoadjuvant chemoradiotherapy and construction of a prediction model. J Surg Oncol 2018;117:737-44. [Crossref] [PubMed]
- Maeda K, Shibutani M, Tachimori A, et al. Prognostic Significance of Neoadjuvant Rectal Score and Indication for Postoperative Adjuvant Therapy in Rectal Cancer Patients After Neoadjuvant Chemoradiotherapy. In Vivo 2020;34:283-9. [Crossref] [PubMed]
- van der Valk MJM, Vuijk FA, Putter H, et al. Disqualification of Neoadjuvant Rectal Score Based on Data of 6596 Patients From the Netherlands Cancer Registry. Clin Colorectal Cancer 2019;18:e231-6. [Crossref] [PubMed]
- Tong GJ, Zhang GY, Liu J, et al. Comparison of the eighth version of the American Joint Committee on Cancer manual to the seventh version for colorectal cancer: A retrospective review of our data. World J Clin Oncol 2018;9:148-61. [Crossref] [PubMed]
- Luna-Pérez P, Rodríguez-Ramírez S, Alvarado I, et al. Prognostic significance of retrieved lymph nodes per specimen in resected rectal adenocarcinoma after preoperative chemoradiation therapy. Arch Med Res 2003;34:281-6. [Crossref] [PubMed]
- Mukai T, Uehara K, Aiba T, et al. Importance of the neoadjuvant rectal (NAR) score to the outcome of neoadjuvant chemotherapy alone for locally advanced rectal cancer. Surg Today 2020;50:912-9. [Crossref] [PubMed]
- Gosens MJ, Klaassen RA, Tan-Go I, et al. Circumferential margin involvement is the crucial prognostic factor after multimodality treatment in patients with locally advanced rectal carcinoma. Clin Cancer Res 2007;13:6617-23. [Crossref] [PubMed]
- Hayden DM, Jakate S, Pinzon MC, et al. Tumor scatter after neoadjuvant therapy for rectal cancer: are we dealing with an invisible margin? Dis Colon Rectum 2012;55:1206-12. [Crossref] [PubMed]
- Perez RO, Habr-Gama A, Smith FM, et al. Fragmented pattern of tumor regression and lateral intramural spread may influence margin appropriateness after TEM for rectal cancer following neoadjuvant CRT. J Surg Oncol 2014;109:853-8. [Crossref] [PubMed]
- Hav M, Libbrecht L, Geboes K, et al. Prognostic value of tumor shrinkage versus fragmentation following radiochemotherapy and surgery for rectal cancer. Virchows Arch 2015;466:517-23. [Crossref] [PubMed]
- Smith FM, Wiland H, Mace A, et al. Depth and lateral spread of microscopic residual rectal cancer after neoadjuvant chemoradiation: implications for treatment decisions. Colorectal Dis 2014;16:610-5. [Crossref] [PubMed]
- Song C, Chung JH, Kang SB, et al. Impact of Tumor Regression Grade as a Major Prognostic Factor in Locally Advanced Rectal Cancer after Neoadjuvant Chemoradiotherapy: A Proposal for a Modified Staging System. Cancers (Basel) 2018;10:319. [Crossref] [PubMed]
- Trakarnsanga A, Gönen M, Shia J, et al. Comparison of tumor regression grade systems for locally advanced rectal cancer after multimodality treatment. J Natl Cancer Inst 2014;106:dju248. [Crossref] [PubMed]
- Chetty R, Gill P, Govender D, et al. International study group on rectal cancer regression grading: interobserver variability with commonly used regression grading systems. Hum Pathol 2012;43:1917-23. [Crossref] [PubMed]
- Deng Y, Zhang J, Hu H, et al. Validation of neoadjuvant rectal cancer (NAR) score as a surrogate endpoint for disease free survival in Chinese FOWARC study. Proc Am Soc Clin Oncol 2019;37:e15162. [Crossref]
- Probst CP, Becerra AZ, Aquina CT, et al. Extended Intervals after Neoadjuvant Therapy in Locally Advanced Rectal Cancer: The Key to Improved Tumor Response and Potential Organ Preservation. J Am Coll Surg 2015;221:430-40. [Crossref] [PubMed]
- Kim SH, Chang HJ, Kim DY, et al. What Is the Ideal Tumor Regression Grading System in Rectal Cancer Patients after Preoperative Chemoradiotherapy? Cancer Res Treat 2016;48:998-1009. [Crossref] [PubMed]
- Fokas E, Ströbel P, Fietkau R, et al. Tumor Regression Grading After Preoperative Chemoradiotherapy as a Prognostic Factor and Individual-Level Surrogate for Disease-Free Survival in Rectal Cancer. J Natl Cancer Inst 2017; [Crossref] [PubMed]
- Kalady MF, de Campos-Lobato LF, Stocchi L, et al. Predictive factors of pathologic complete response after neoadjuvant chemoradiation for rectal cancer. Ann Surg 2009;250:582-9. [Crossref] [PubMed]



