
A descriptive analysis of the characteristics, treatment response and prognosis of hepatic dominant solid tumors undergoing selective internal radiation therapy (SIRT)
Introduction
Primary liver tumors, of which hepatocellular carcinoma (HCC) accounts for 80%, represent 6% of global cancer incidence and 9% of global cancer-associated mortality (1). Primary liver tumor is potentially curable with tumor resection or liver transplantation at a local stage. However, 65–70% of diagnosed primary liver tumors are not suitable for resection due to large or multifocal lesions (2). For these patients, local therapies, such as transcatheter arterial chemoembolization (TACE) or selective internal radiation therapy (SIRT) are appropriate in intermediate stages. In patients with advanced and metastatic tumors, however, systemic therapy, such as sorafenib, is indicated (3).
Secondary liver tumors by solid organ metastasis are even more common than primary liver tumors (4). Of all cancer patients, about 5% show liver metastases at the time of initial diagnosis (5). The most common primary tumors are lung, pancreas and colorectal cancers with 10.7%, 35.6% and 13.8% showing liver metastases at initial diagnosis (5). However, neuroendocrine tumors (NETs) also metastasize frequently and early to the liver, with 82% of all metastatic NETs having a liver metastasis (6).
The treatment of liver metastases essentially depends on the primary tumor, the degree and tumor load of metastasis. For example, colorectal liver metastasis (CRLM) can be treated by resection with curative intent in 20% to 25%, but the remaining majority is usually managed by systemic therapies (7-9). For liver-dominant metastases that do not meet resection criteria or did not show a response to systemic agents, local therapies may be considered for symptomatic control or even downstaging aspects, including local ablation with radiofrequency (RFA) or ethanol, TACE, and SIRT (9,10).
Hence SIRT with yttrium-90 (90Y) is a therapy that can be used to treat primary liver tumors and secondary liver metastases (11). The principle of SIRT is an internal radiation therapy (brachytherapy), in which radioactive particles are delivered to the liver tumor with a catheter via the selective tumor-supplying arteries, whereby the vessel is embolized and the tumor locally irradiated by deposition of the particles in the tumoral microvasculature (11).
SIRT is currently used worldwide and the value of this treatment within the treatment lines of primary liver cancer and liver metastases is the subject of increasing clinical research (12). The European Society for Medical Oncology (ESMO) clinical guidelines for HCC do not recommend SIRT as a first line treatment in intermediate and advanced stages, but argue, that SIRT may be considered in early and intermediate stage patients with liver-confined disease for whom neither TACE nor systemic therapy is possible (13,14). This is based on a phase II trial in early and intermediate stage patients, which found that SIRT can significantly prolong local liver-specific progression-free time compared to TACE and is newly supported by a recent study that showed SIRT resulting in an excellent local tumor growth control in early, unresectable HCC stages (15,16).
For hepatic metastasized colorectal cancer, the position of SIRT in the therapy lines is not clearly defined in clinical guidelines and is only recommended as a possible option in patients with liver-limited, chemotherapy-resistant metastases (9). For CRLM the combined analysis of the three phase III trials FOXFIRE, SIRFLOX, and FOXFIRE-Global set a pivotal statement (17). It showed that the early use of SIRT combined with chemotherapy in hepatic metastasized colorectal cancer is not recommended (17). However, SIRT may be a possible option for salvage therapy, consolidation therapy or allowing patients to undergo chemotherapy breaks (18).
Therefore, the appropriate use of SIRT in the treatment lines of primary and secondary liver cancer is still only vaguely defined and part of an ongoing investigation. Based on the potential advantages of SIRT, there is an increased clinical interest in defining subgroups for this application. This analysis aims to identify patients with different solid liver dominant tumors who might benefit from SIRT in the respective treatment concept. We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-122/rc).
Methods
This was a single-centre retrospective analysis of a consecutive patient cohort with unresectable primary or secondary liver tumors who received selective internal radioembolization at the Comprehensive Cancer Center Zurich (CCCZ) between January 2015 to May 2019.
Patients
All patients who received SIRT at the Department of Nuclear Medicine at the University Hospital Zurich (USZ) from January 2015 to May 2019 were consecutively included in this study. The corresponding 209 patients were referred to the USZ by their oncologist and discussed at a multidisciplinary SIRT board. Indications were unresectable primary liver tumors as well as liver metastases of solid tumors. Contraindications were a life expectancy of less than 1 month, severe chronic lung disease, liver failure, severe chronic renal insufficiency (estimated glomerular filtration rate <30 mL/min), uncontrollable coagulopathy and a severe allergy to contrast media (19). Another contraindication was a pulmonary shunt greater than 20%, which was identified beforehand by a 99m-technetium aggregated albumin (99mTc-MAA) scintigraphy therapy simulation. The assessment of the clinical characteristics of the included patients was performed by the oncologists of the CCCZ.
Data collection
A retrospective search analysis of the electronic patient dossiers of the USZ was performed and the parameters sex, age at intervention, indication for therapy, extrahepatic metastases and previous therapies were recorded. Previous therapies were divided into no prior therapy, systemic therapy/chemotherapy, surgery, surgery and systemic therapy/chemotherapy in sequence, and ablative therapies. The latter includes RFA and TACE. The histologic feature of a NET was additionally recorded. Furthermore, the radioactive activity in Gigabecquerel (GBq) applied during the intervention, the local therapy response and progression and survival data was collected.
Ethics
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and its later amendments, and written informed consent was given by all patients before being included. A formal ethics application was submitted and approved by the Zurich Cantonal Ethics Committee under BASEC number 2021-00743.
SIRT
A SIRT consisted of two procedures: an obligatory preliminary investigation and a subsequent therapeutic intervention. During the preliminary investigation, the pulmonary shunt was determined with 99mTc-MAA in a single photon emission computed tomography (SPECT) scan, and the right gastric artery and gastroduodenal artery were embolized during the same session (19).
Two different methods were used to determine the planned radioactive dose. From 2015 to March 2018 (n=174), the dose was calculated using the body surface method (20). From March 2018 until the end of May 2019 (n=35), contrast magnetic resonance imaging (MRI) or computed tomography (CT) scans determining liver and tumor volume, as well as the 99mTc-MAA/SPECT images, were used to calculate the planned dose with the partition model method (21). Due to the small case number of the partition model method, a subdivision into two subgroups was waived.
For the therapeutic intervention, resin microspheres containing the beta emitter 90Y (90Y-SIR-Spheres; Sirtex Medical, Sidney, Australia) were administered into the predicted hepatic artery through a femoral placed percutaneous catheter. Particle distribution and activity were assessed one hour later using a SPECT scan (19). After the intervention, patients were observed for 5 hours before discharge. Therapy was applied to either the entire liver, individual liver lobes, or combinations of different lobes and liver segments. In some patients, the therapeutic intervention was divided into two sessions. If these two sessions lay within a period of 8 weeks, the applied activity was added together, and both sessions were counted as a single SIRT. The same interventional radiologist performed the preliminary investigation and therapeutic intervention at the USZ.
Response at first follow-up
Therapy response was assessed by either CT, MRI, or positron emission tomography (PET) scans. The scans were performed as a multicenter follow-up in Switzerland, preferably three months and at least 6 weeks after the SIRT. The scans were classified as either regression (partial or complete), stable disease or progression of the predetermined and SIRT targeted liver tumors. A response was defined as either a regression or a stable disease. The categorization was based on the final clinical judgment of the corresponding radiologist. The outcomes were retrospectively read from the electronic patient files and referred only to the intrahepatic localization of the 90Y-microspheres application. Scans obtained at referring institutions were submitted to the USZ for this study after a written request. For some patients, a follow-up scan was not possible due to an early occurring death (<3 months) after SIRT (n=24).
Progression and survival
Of each patient, the overall survival (OS), post-SIRT survival, and progression-free survival were determined.
OS was defined as the period from the initial diagnosis of the primary tumor to the date of death and the post-SIRT survival period from the SIRT to the date of death. The cause of death was not considered. The progression-free survival was defined as the period from the SIRT to the first progression at the location of the 90Y-microspheres application.
For progression assessment, multicenter follow-up CT, MRI, and PET scans were used. The modality and frequency of follow-up imaging depended on the referring oncologist and the clinical condition of the patients. The progression classification was based on the final clinical assessments of the corresponding radiologist.
Subgroup analyses
For the sex subgroup analysis, the sex-specific tumor entities were ignored. For women, these included breast cancer (n=17), cervical cancer (n=2), and endometrial cancer (n=1). In men, prostate cancer (n=2) and testicular cancer (n=1) were ignored.
Statistics
Statistical analysis and graphical representation of the data was performed using Microsoft Excel and R (version 4.2.0) and R Studio (version 2022.02.3) software. Survival analysis was performed with the Kaplan-Meier method, and comparison of time to event was performed with a log-rank test and comparison of frequency of response with the chi-square test. The majority of the analyses were purely descriptive. Metric variables are described by whole numbers as well as medians, with the latter being followed by the range or 95% confidence interval (CI). Nominal and ordinal variables are represented as whole numbers and their relative frequency as percentages.
For SIRT response evaluation we counted either tumor regression or a stable disease by radiologic measurement of the predetermined liver metastasis. In the analysis colon cancers, rectal cancers, and rectosigmoid cancers were combined to form colorectal cancers. Uveal melanomas and cutaneous melanomas were grouped as melanomas.
Results
Patient characteristics
A total of 209 SIRT applications for 182 patients were performed between January 2015 and May 2019. Out of the 182 patients, 30 (17%) were treated more than once with SIRT, of which 15 (50%) had primary liver tumors and 15 (50%) liver metastases. The 182 patients had a median age of 64 years (range, 18–88 years). One hundred and thirteen (62%) were men, and 69 (38%) were women.
Of the 209 SIRT applications, 100 (48%) were performed on primary liver tumors, and 109 (52%) on liver metastases, and of all applications, 98 (47%) had been diagnosed with extrahepatic metastases at the time of intervention. Of the 77 applications to HCC, metastases were present in 7 (9%). The median applied radioactive activity per SIRT application was 1.4 GBq (range, 0.3–2.85 GBq). The seven most common indications for SIRT applications are summarized in Table 1, and the complete list can be found in the supplementary appendix online.
Table 1
| Indications | ICD-10 | N [%] | M | F |
|---|---|---|---|---|
| Hepatocellular carcinoma | C22.0 | 77 [37] | 66 | 11 |
| Colorectal cancer | C18/C19/C20 | 29 [14] | 15 | 14 |
| Neuroendocrine tumors | – | 18 [9] | 11 | 7 |
| Breast cancer | C50 | 17 [8] | 0 | 17 |
| Cholangiocarcinoma | C22.1 | 16 [8] | 7 | 9 |
| Melanoma (skin and uvea) | C43/C69 | 15 [7] | 9 | 6 |
| Gastric cancer | C16 | 5 [2] | 4 | 1 |
| Heterogeneous group of rarely (n<5) treated indications | – | 32 [15] | 21 | 11 |
SIRT, selective internal radiation therapy; ICD-10, International Statistical Classification of Diseases and Related Health Problems-version 10; N, number; M, male; F, female.
Of the 18 NETs, 3 corresponded to a grade 1, 7 to a grade 2, and 7 to a grade 3 tumor, with one additional tumor with an unknown grade. The primary tumors of the NETs are shown in Table 2. Therapies prior to the SIRT were evaluated for the 7 most common indications and are shown in Table 3.
Table 2
| Neuroendocrine primary tumors | N [%] | M | F |
|---|---|---|---|
| Pancreatic cancer | 7 [39] | 5 | 2 |
| Small intestine cancer | 4 [22] | 2 | 2 |
| Malignant neoplasm of other endocrine glands | 4 [22] | 2 | 2 |
| Malignant neoplasm, primary localization unknown | 1 [6] | 0 | 1 |
| Colon cancer | 1 [6] | 1 | 0 |
| Lung cancer | 1 [6] | 1 | 0 |
N, number; M, male; F, female.
Table 3
| Indication | No prior therapy | Syst. therapy/chemotherapy | Surgery | Surgery and syst. therapy/chemotherapy in Sequence | Ablative therapies (RFA/TACE) |
|---|---|---|---|---|---|
| HCC | 31 | 5 | 11 | 1 | 29 |
| Colorectal Ca | 0 | 2 | 1 | 26 | 0 |
| NET | 1 | 6 | 0 | 11 | 0 |
| Breast Ca | 0 | 1 | 0 | 16 | 0 |
| CCA | 3 | 11 | 0 | 2 | 0 |
| Melanomas | 2 | 10 | 0 | 3 | 0 |
| Gastric Ca | 0 | 4 | 0 | 1 | 0 |
SIRT, selective internal radiation therapy; HCC, hepatocellular carcinoma; Ca, cancer; NET, neuroendocrine tumors; CCA, cholangiocarcinoma; syst., systemic; RFA, radiofrequency ablation; TACE, transarterial chemoembolization.
Response at first follow-up
Therapy response was defined as either regression or stable disease and was elicited in the follow-up of 169 out of 209 SIRT applications, of which 88 (52%) showed a response. Response at first follow-up for the 7 most common indications is shown in Table 4.
Table 4
| Indication | Regression | Stable disease | Progression | Response percentage |
|---|---|---|---|---|
| HCC | 20 | 19 | 27 | 59% |
| Colorectal Ca | 4 | 1 | 13 | 28% |
| NET | 7 | 1 | 7 | 53% |
| Breast Ca | 8 | 0 | 4 | 67% |
| CCA | 4 | 5 | 3 | 75% |
| Melanomas | 3 | 2 | 8 | 38% |
| Gastric Ca | 1 | 0 | 2 | 33% |
SIRT, selective internal radiation therapy; HCC, hepatocellular carcinoma; Ca, cancer; NET, neuroendocrine tumors; CCA, cholangiocarcinoma.
Overall survival
OS showed a median of 35.6 months across all tumor entities (95% CI: 32.6–46.8 months). In addition, OS was evaluated for the 7 most common indications and is shown in Table 5.
Table 5
| Indication | Number of SIRT applications | Median survival time in months | 95% CI in months | ||||||
|---|---|---|---|---|---|---|---|---|---|
| <1 y | ≥1, <2 y | ≥2, <3 y | ≥3, <4 y | ≥4, <5 y | ≥5, <10 y | ≥10 y | |||
| HCC | 11 | 31 | 15 | 8 | 3 | 9 | 0 | 32.8 | 23.4–44 |
| Colorectal Ca | 1 | 7 | 12 | 7 | 1 | 1 | 0 | 34.2 | 30.0–NR |
| NET | 1 | 1 | 7 | 1 | 2 | 3 | 3 | 49.4 | 26.0–NR |
| Breast Ca | 0 | 0 | 0 | 1 | 1 | 8 | 7 | 180.6 | 127.3–NR |
| CCA | 5 | 7 | 2 | 0 | 1 | 1 | 0 | 20.8 | 13.4–NR |
| Melanomas | 1 | 3 | 2 | 4 | 1 | 4 | 0 | 61.5 | 42.3–NR |
| Gastric Ca | 0 | 0 | 2 | 3 | 0 | 0 | 0 | 35.6 | 29.3–NR |
SIRT, selective internal radiation therapy; HCC, hepatocellular carcinoma; Ca, cancer; NET, neuroendocrine tumors; CCA, cholangiocarcinoma; CI, confidence interval; NR, not reached; y, years.
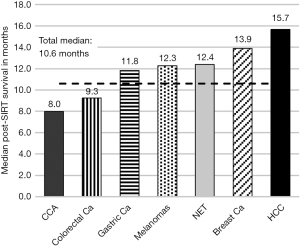
Median post-SIRT survival
Post-SIRT survival across all tumor entities showed a median of 10.6 months (95% CI: 9.55–14.1 months), with a median post-SIRT follow-up of 27 months (range, 1–53 months). The median post-SIRT survival of the 7 most common indications is shown in Figure 1.
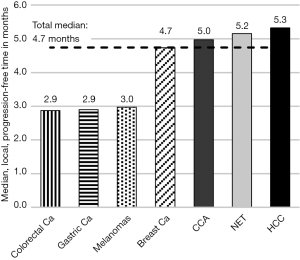
Median local progression-free time
The progression-free time showed a median of 4.7 months across all tumor entities (95% CI: 3.58–5.48 months), with a median follow-up time after SIRT of 27 months (range, 1–53 months). The median local progression-free time of the 7 most common indications is shown in Figure 2.
Subgroup analysis of NETs
The percentage of response to SIRT, median OS, median post-SIRT survival, and median local progression-free time in months are presented separately for the subgroup of pancreatic NETs (p-NETs) and NETs of other tumor entities in Table 6.
Table 6
| Variables | Pancreas NET tumors (n=7) | Remaining NET tumors (n=11) | P value |
|---|---|---|---|
| Percentage of response | 40% | 60% | 0.608 |
| Median overall survival in months (95% CI) | 26.0 (23.6–NR) | 97.5 (32.6–NR) | 0.062 |
| Median post-SIRT survival in months (95% CI) | 7.1 (1.0–NR) | 34.7 (15.2–NR) | 0.001 |
| Median local progression-free time in months (95% CI) | 3.4 (3.2–NR) | 8.5 (5.2–NR) | 0.003 |
NET, neuroendocrine tumors; CI, confidence interval; SIRT, selective internal radiotherapy; NR, not reached.
Subgroup analysis of sex differences
The percentage of response to SIRT, median OS, median post-SIRT survival, and median local progression-free time in months, after excluding sex-specific cancers (breast cancer, cervical cancer, endometrial cancer, prostate cancer, testicular cancer) are presented for each sex separately in Table 7.
Table 7
| Variables | Women (n=56) | Men (n=130) | P value |
|---|---|---|---|
| Percentage of response | 52% | 51% | 1.000 |
| Median overall survival in months (95% CI) | 37.1 (32.1–90.7) | 32.6 (29.1–35.6) | 0.043 |
| Median post-SIRT survival in months (95% CI) | 10.6 (9.5–19.6) | 10.8 (9.3–15.3) | 0.970 |
| Median local progression-free time in months (95% CI) | 4.2 (3.2–6.0) | 5.0 (3.4–5.7) | 0.451 |
SIRT, selective internal radiation therapy; CI, confidence interval.
Discussion
SIRT is currently used worldwide for a broad array of solid primary and secondary liver tumors. However, its extent of application and value within treatment lines of the respective tumor entities often remains unclear. Thus, the right timing of SIRT within the knowledge of each disease entity and their peri-interventional systemic treatment is of utmost interest. Therefore, we investigated in this retrospective study a consecutive group of patients who received SIR therapy at the USZ from 2015 to mid-2019 about their characteristics, liver-specific response, OS, post-SIRT survival, and local progression-free time. In addition, subgroup analysis of NETs and sex differences were performed.
Similar studies with a comparable consecutive and heterogeneous group of patients showed relatively congruent patient characteristics. Paprottka et al. showed a median age of 64.1 years in their study of 389 patients, compared with 64 years in our study (22). The sex distribution of 60% males is similar to ours (62%) (22). The larger male representation can probably be attributed to the indications most treated with SIRT in this study, such as colorectal cancer and HCC, which are also significantly more common in men than in women (23). Differences, however, are found in the lower percentage of primary liver metastases in the Paprottka et al. study with 26% compared to ours with 48%, and in the percentage of extrahepatic metastases with 70% compared with our 47%. This is most likely explained by the stricter indication and handling of SIRT within our comprehensive cancer center.
The median applied radioactive activity of 1.4 GBq (range, 0.3–2.85 GBq) recorded in our study is in a similar range to Paprottka et al. with 1.199 GBq (range, 0.798–1.681 GBq) and Omed et al. with 1.476 GBq (range, 0.85–2.18 GBq) (22,24). However, the maximum applied activity in our study with 2.85 GBq is considerably higher than the 1.681 GBq in the study of Paprottka et al., which might be attributed to the fact that Paprottka et al. used the body surface method to calculate the planned activity, which however tends to result in underdosing the patient (22,25). The maximum value of 2.85 GBq measured in our study was calculated using the newer partition model method, which from clinical experience leads to higher applied activities. Our study and that of Paprottka et al. showed the same four most frequent indications, such as HCC, colorectal cancer, NETs, and breast cancer. Our study recorded an HCC and colorectal cancer proportion of 37% and 14%, respectively. However, Paprottka et al. showed an inverse distribution with HCC 17% and colorectal cancer 35%, whereby this difference probably origins also in stricter indication of treatment. The NET metastases treated in this study were most frequently from pancreatic and small bowel cancers, which is consistent with the results seen in other studies investigating the use of SIRT for NET (26-28).
The local response rate after SIR therapy across all indications was 52% in our study. Paprottka et al. investigated the response 3 months after SIRT application according to the “Response Evaluation Criteria in Solid Tumors (RECIST)” criteria and resulted in a similar overall response rate across all indications of 63% (22). Kucuk et al. examined the response rate at week 6 after SIRT application, defined it as a 20% decrease in standardized uptake value on 2-fluoro-2-deoxy-D-glucose (FDG) PET/CT imaging, and resulted in an overall response rate of 55% (29).
HCC was the most frequently treated entity in this study and was treated more in the early stages, in accordance with the ESMO and European Association for the Study of the Liver (EASL) guidelines (13,30). Considering therapies prior to SIRT, European studies showed similar results to ours. Sangro et al. showed in a multicenter study with 325 HCC patients in different stages that 36.6% received ablative pre-therapy and 18.2% surgical pre-therapy (31). Bauschke et al. showed that in 52 patients with non-resectable HCC, 34% received ablative, 17% surgical, 44.6% none, and 2% prior systemic therapy (32). Current guidelines, as from ESMO for example, recommend SIRT only in the Barcelona Clinic Liver Cancer (BCLC) stages A and B, which correspond to stages without invasive or metastatic disease and only if a prior TACE was without response or systemic therapy is not feasible (13). This explains the high observed proportion of patients without prior therapies in our as well as the mentioned studies, which probably corresponds to the patient group for which systemic therapy was not feasible. From clinical experience, the ablative pretreated group consists primarily of patients for whom TACE or RFA showed an insufficient response and tumor progression was subsequently treated with SIRT. Surgically pretreated patients were initially mostly in a resectable stage, which was operated with curative intent. Subsequently, however, these patients often developed a relapse, which could no longer be operated, but treated with SIRT. The ESMO does not recommend SIRT in metastatic BCLC stages C and D (13). This is in line with the results of this study, as metastases were present in only 9% of all HCC patients, thus further indicating that SIRT is generally applied for HCC at the CCCZ according to the current European guidelines.
On the other hand, in comparable American studies, a considerably higher proportion of patients received systemic pre-therapy. Two studies of patients with unresectable HCC showed that 34.2% and 78%, respectively, received sorafenib as a systemic therapy before SIRT (33,34). This suggests that in the United States, SIRT is considered after failure of systemic therapy, whereas in accordance to European guidelines, this option is not recommended (13,30). This transatlantic difference in SIRT indications can likely be attributed to a lack of clarifying literature as to what extent the use of SIRT after systemic therapy failure is plausible. Therefore, studies investigating the use of SIRT after a failed systemic therapy are demanded to provide much needed clarity. However, due to tumor heterogeneity as well the setup for SIRT evaluation after systemic treatment failure, a clean study design for SIRT in that setting would be a major challenge.
HCC showed a 59% response rate, compared to 63% and 82% in two studies with unresectable HCC, which defined response according to RECIST criteria after 3 months (12,15). The median local progression-free time in HCC in this study was 5.3 months (95% CI: 4.13–8.23 months). Comparable studies showed similar results in their analyses with 2.5 months (95% CI: 1.9–3.1 months), 7 months (range, 0–41 months), 9.8 months (95% CI: 6.8–14.8 months), and 10.0 months (95% CI: 6.1–16.4 months) (32-35). In HCC, our analysis showed a median post-SIRT survival of 15.7 months (95% CI: 9.87–22.0 months). Studies investigating the use of SIRT in unresectable HCC showed similar values of 11.7 months (95% CI: 6.6–16.8 months), 13.1 months (95% CI: 10.3–18.4 months), and 16.4 months (95% CI: 12.1–∞ months) (33-35).
The median OS of HCC in this study was 32.8 months (95% CI: 23.4–44 months). In the literature, median survival after HCC diagnosis is reported to be between 6–20 months (36). Thus, the HCC patients treated in this study tend to be at the higher end of the survival spectrum. This may be explained in part by a selection bias. Patients who are in end-stage HCC or have a low liver function are excluded from SIR therapy according to the ESMO and EASL guidelines. As a result, patients in the early HCC stages are more likely to be treated, which leads to better measured survival data. Of the OS of HCC patients with a median of 32.8 months (95% CI: 23.4–44.0 months), almost half comes after the SIRT intervention, with a post-SIRT survival median of 15.7 months (95% CI: 9.9–22.0 months). This high proportion of post-SIRT survival in the OS comes on the one hand from the early use of SIRT in the disease course, but on the other hand probably also from the good response of HCC to SIRT. This is shown by the local response of 59% and the local progression-free time of 5.3 months (95% CI: 4.1–8.2 months), both values being among the highest in this study and overall indicating that HCC is a suitable indication for SIRT.
Three patients with HCC in this study underwent liver transplantation after SIRT application. In these cases, SIRT served as a bridging procedure to prevent the patient from dropping out of the transplant list due to tumor progression. These three patients, successfully transplanted after SIRT, exemplify that SIRT can also be used as a bridging therapy until transplantation. This is also in line with the ESMO guidelines on HCC, which mention SIRT as a possible bridging therapy instead of TACE (13).
Overall, in this study the HCC subgroup showed that SIRT could be used especially in early stages without prior therapy or after ineffective TACE with a good response and a good local progression-free time. This raises the question whether SIRT patients, who were treated with TACE and only later with SIRT, would not have benefited more from receiving SIRT initially instead of TACE. This assumption is supported by the paper of Salem et al., which found that the local progression-free time in BCLC stages A and B was significantly longer with SIRT than with TACE (15). However, a meta-analysis of prospective randomized trials comparing the use of SIRT and TACE in patients with unresectable HCC showed very similar effects regarding OS, disease control rate, transplantation rate, and progression rate (37). This implicates that there is a strong need for further trials focusing on defining more specific subpopulations, which could benefit better from either TACE or SIRT.
In contrast to HCC, CRLM were treated with SIRT relatively late in therapy lines. The most common prior therapy for CRLM was surgery with systemic therapy/chemotherapy in sequence. Thus, SIRT was more likely to be used as a second- or third-line therapy. This is also in line with current guidelines, in which SIRT is not recommended as first-line therapy and is only recommended for chemotherapy-resistant metastases (9). The fact that SIRT is used in late lines of therapy or only after other therapies have failed is also reflected in the survival data. Out of the median OS of 34.2 months [95% CI: 30.0 months–not reached (NR)], only a median of 9.3 months (95% CI: 5.3–17.3 months) occurs after SIRT. This relatively low post-SIRT survival could further come to pass due to a relatively poor response of CRC to SIRT, which could be indicated by a low response rate of 26% and a low local progression-free time of 2.9 months (95% CI: 2.5–8.3 months). A possible cause for this could be resistance to radiotherapies, which is a well-known therapeutic problem in colorectal cancers and the subject of current research (38-40). The cause of such radiotherapy resistance may be at the molecular level, with radiotherapy-resistant stem cells being discussed as responsible for resistance and further tumor progression (41).
In conclusion, SIRT is used for CRC at the CCCZ in accordance with the guidelines, and the results and effects on the course of the disease are visible but clearly below average compared with other indications. Thus, using SIRT for CRC should be well considered, as the chances of success are relatively low compared to other indications. However, it would be possible to compare SIRT in a randomized trial as a third-line therapy to TAS-102 and regorafenib, investigating a possible advantage of SIRT in terms of progression-free time and OS. A fundamental advantage of SIRT in such a comparative study would be that it would not have systemic side effects, unlike therapy with TAS-102 and regorafenib. Further, whether SIRT could be used as a consolidation therapy or to enable patients to take chemotherapy breaks could be discussed.
Advanced breast cancer (ABC) represents a favorable disease with prolonged OS by several anti-hormonal as well systemic targets, including multikinase inhibition as well chemotherapies. This was also reflected in this study, where breast cancer showed the longest OS, with a median of 180.6 months (95% CI: 127.3 months–NR). In sight for SIRT analysis, our study showed a long post-SIRT survival of 13.9 months (95% CI: 9.9 months–NR). Since breast cancers have a wide range of possible established systemic therapies, multiple other therapeutic options will be used before SIRT. This is also reflected in the therapies prior to SIRT as well no study protocols have been published with early use of SIRT and compared to the standard of care in ABC. This fact was also represented at our analysis, as most ABC patients were pretreated systemically and with surgery in sequence. SIRT was generally an effective therapy in breast cancer with the second-best response of 67% and a long local progression-free time of 4.7 months (95% CI: 2.19 months–NR). This suggests that breast cancers can still benefit from SIRT in advanced stages and as a late line of therapy.
Non-small cell lung cancer (NSCLC) generally accounts for a majority of secondary liver tumors. However, in our study, only a minority of 4 SIRT applications (2%) had a NSCLC as a SIRT indication. This is because, as described in the methodology, severe chronic lung disease is a contraindication to SIRT as well SIRT to lung liver metastasis are not depicted in available guidelines (42-44). Moreover, lung tumors are mostly non-small cell lung carcinomas, for which the use of SIRT in liver metastases has been exceptionally poorly studied (45).
NETs showed overall excellent treatment results. They showed a median OS of 49.4 months (95% CI: 26.0 months–NR). This result is in good accordance with an analysis of the American Cancer Institute’s “Surveillance, Epidemiology, and End Results” database on NETs of various origins, which showed a median survival of 41 months (46). On the one hand, the prolonged OS can be explained by the biological pattern of the tumor entity, which has a known good survival in low proliferation grades. On the other hand, however, the SIRT specific data also suggests general good results of SIRT in NET. For example, NET showed a 53% response and showed the second longest median local progression-free time of 5.2 months (95% CI: 4.0–17.2 months). NET also showed a long post-SIRT survival at 12.4 months (95% CI: 9.7 months–NR). However, the extent to which this is due to the SIRT or the naturally prolonged survival of this tumor entity remains open.
A sub analysis revealed that p-NET show a median OS of 26 months (95% CI: 23.6 months–NR), compared to the median OS of the remaining NET of 97.5 months (95% CI: 32.6 months–NR) (P=0.062). p-NET survival is in good accordance with the results of an analysis of distant pancreatic islet cell cancer, which showed an OS of 23 months (95% CI: 20–26 months) (47). p-NETs compared to the remaining NET also showed with a P value of 0.001 a lower post-SIRT survival and with a P value of 0.003 a lower local progression-free time. Our study showed no significant difference in response rate between p-NETs compared to the remaining NETs (P=0.608), which is contrary to Devcic et al., who found a significantly worse response for p-NET (48). The difference in median post-SIRT survival found in our study between p-NETs with 7.1 months (95% CI: 1.0 months–NR) and the remaining NET with 34.7 months (95% CI: 15.2 months–NR) further contradicts two published studies suggesting that the origin of NET does not significantly affect post-SIRT survival (49,50).
The p-NETs generally showed considerably worse values than the remaining NET, which is also in line with clinical experience. With a response rate of 40% and a local progression-free time of 3.4 months (95% CI: 3.2 months–NR) months, p-NET also performed poorly compared to other indications. However, this may also be primarily due to the aggressive nature of this entity. However, the post-SIRT survival of p-NETs with a median of 7.1 months (95% CI: 1.0 months–NR) months can be considered as a substantial benefit for the patient in view of the aggressive nature of this tumor entity, with a resulting low median OS of 26 months (95% CI: 23.6 months–NR). Thus, despite the poor outcomes seen in absolute terms, SIRT in p-NETs may potentially provide a crucial life-prolonging benefit to the patient.
In contrast, non-p-NET showed excellent results in absolute terms with a response rate of 60%, a local progression-free time of 8.5 months (95% CI: 5.2 months–NR), and a post-SIRT survival of 34.7 months (95% CI: 15.2 months–NR). Since post-SIRT survival accounts for about one third of the OS of 97.5 months (95% CI: 32.6 months–NR), it can be concluded that SIRT is not used as first-line therapy in this entity and is instead used later, but still with a high survival gain. In conclusion, the NET tumor entities showed very good results. The non-p-NET can definitely, and the p-NET probably benefit from this therapy, despite poorer results.
Considering sex differences to SIRT a separate analysis for women and men showed very similar results. Our study found no evidence (P=0.97) for a difference in the median post-SIRT survival between men with 10.8 months (95% CI: 9.3–15.3 months) and women with 10.6 months (95% CI: 9.5–19.6 months). This is consistent with previously published literature investigating the influence of sex on post-SIRT survival in SIRT patients (31,51,52). Likewise, no differences were found in the response (P=1.0), corresponding to 52% in women and 51% in men, as well as median local progression-free time (P=0.45) with 4.2 months (95% CI: 3.2–6.0 months) in women and 5.0 months (95% CI: 3.4–5.7 months) in men.
In conclusion, no difference was found between the sexes regarding the response, post-SIRT survival data, and local progression-free time. In contrast, recent results suggest different effectiveness and efficacy of chemotherapeutic agents between the sexes, probably due to different molecular causes (53). This would give SIRT an advantage over chemotherapeutic therapies, as SIRT shows sex-neutral results and would therefore be equally suitable for both sexes.
Limitations of this study include a selection bias, caused by patients showing an excellent response after SIRT being treated again with SIRT at a later relapse, thus leading to an overrepresentation of patients with a favorable biological tumor type, whereas we did not identify such clinical or pathological biomarkers yet. Such clinical characteristics as well tumor biomarkers in future analysis would be of utmost interest for a stratification to prospective controlled study protocols. Further limitations arise from the use of two different methods for calculating radioactive activity, the lack of data for calculating the applied radioactive dose and the lack of measured tumor volume with the consequent omission of a standardized radiological assessment of tumor response. In addition, it must be noted that the multicenter design of this study resulted in different durations between intervention and follow-up and between follow-ups, reducing the accuracy of measured response rate and progression-free time. Out of the response at first follow-up and the local progression-free time, 42 data points were missing each because the corresponding imaging was non-existent, representing 20.1% of all SIRT applications analyzed in this study. Of these 42, 24 had died within 3 months after SIRT, 15 were lost to follow-up, 2 had only imaging performed less than 6 weeks after SIRT, and one patient underwent liver transplantation shortly after SIRT intervention.
Conclusions
SIRT is a commonly used method for the treatment of primary as well for secondary liver tumors. The patient group and indications for SIRT are very heterogeneous. The position of SIRT within the therapy lines differs greatly between the different indications most depended by tumor origin, extrahepatic tumor load and biologic features of the primary tumor. The response to SIRT is highly dependent on the indication. HCCs, NETs, and breast cancers have responded particularly well. Gastric cancers, melanomas, and colorectal cancers have responded particularly poorly. The HCCs in the early stages are successfully treated with SIRT at the CCCZ in accordance with the guidelines. However, further studies are needed to define subpopulations of HCC patients, which would either benefit more from SIRT or TACE. CRLM are treated with SIRT at the CCCZ according to the guidelines. However, compared to other indications, this entity shows considerably worse results. Thus, the indication for SIRT should be reviewed more closely for colorectal cancers. p-NET showed significantly lower progression and survival outcomes compared to the rest of NET. Nevertheless, SIRT seems to be able to achieve a therapeutic benefit for all NET. A sex-specific subgroup analysis showed no relevant differences in SIRT results, possibly giving an advantage to SIRT over chemotherapeutic agents, as it would be equally well suited in a sex-neutral manner.
Acknowledgments
We thank Anne-Laurène Wenger and Susanne Baalbaki for their help with data collection.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-122/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-122/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-122/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Stewart BW, Wild C. International Agency for Research on Cancer. World Health Organization. World Cancer Report 2014. Lyon, France, Geneva, Switzerland: International Agency for Research on Cancer, WHO Press, 2014:630.
- Sotiropoulos GC, Lang H, Frilling A, et al. Resectability of hepatocellular carcinoma: evaluation of 333 consecutive cases at a single hepatobiliary specialty center and systematic review of the literature. Hepatogastroenterology 2006;53:322-9. [PubMed]
- Grandhi MS, Kim AK, Ronnekleiv-Kelly SM, et al. Hepatocellular carcinoma: From diagnosis to treatment. Surg Oncol 2016;25:74-85. [Crossref] [PubMed]
- Centeno BA. Pathology of liver metastases. Cancer Control 2006;13:13-26. [Crossref] [PubMed]
- Horn SR, Stoltzfus KC, Lehrer EJ, et al. Epidemiology of liver metastases. Cancer Epidemiol 2020;67:101760. [Crossref] [PubMed]
- Riihimäki M, Hemminki A, Sundquist K, et al. The epidemiology of metastases in neuroendocrine tumors. Int J Cancer 2016;139:2679-86. [Crossref] [PubMed]
- Engstrand J, Nilsson H, Strömberg C, et al. Colorectal cancer liver metastases - a population-based study on incidence, management and survival. BMC Cancer 2018;18:78. [Crossref] [PubMed]
- Hubbard JM, Alberts SR. Treatment of liver-limited metastatic colorectal cancer. Cancer J 2010;16:235-40. [Crossref] [PubMed]
- Van Cutsem E, Cervantes A, Nordlinger B, et al. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25:iii1-9. [Crossref] [PubMed]
- Sangha BS, Nimeiri H, Hickey R, et al. Radioembolization as a Treatment Strategy for Metastatic Colorectal Cancer to the Liver: What Can We Learn from the SIRFLOX Trial? Curr Treat Options Oncol 2016;17:26. [Crossref] [PubMed]
- Bilbao JI, Reiser MF. Liver Radioembolization with 90Y Microspheres. 2nd edition. Heidelberg: Springer Berlin, 2014.
- Iñarrairaegui M, Sangro B. Selective Internal Radiation Therapy Approval for Early HCC: What Comes Next? Hepatology 2021;74:2333-5. [Crossref] [PubMed]
- Vogel A, Cervantes A, Chau I, et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29:iv238-55. [Crossref] [PubMed]
- Vogel A, Martinelli EESMO Guidelines Committee. Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO Clinical Practice Guidelines. Ann Oncol 2021;32:801-5. [Crossref] [PubMed]
- Salem R, Gordon AC, Mouli S, et al. Y90 Radioembolization Significantly Prolongs Time to Progression Compared With Chemoembolization in Patients With Hepatocellular Carcinoma. Gastroenterology 2016;151:1155-1163.e2. [Crossref] [PubMed]
- Salem R, Johnson GE, Kim E, et al. Yttrium-90 Radioembolization for the Treatment of Solitary, Unresectable HCC: The LEGACY Study. Hepatology 2021;74:2342-52. [Crossref] [PubMed]
- Wasan HS, Gibbs P, Sharma NK, et al. First-line selective internal radiotherapy plus chemotherapy versus chemotherapy alone in patients with liver metastases from colorectal cancer (FOXFIRE, SIRFLOX, and FOXFIRE-Global): a combined analysis of three multicentre, randomised, phase 3 trials. Lancet Oncol 2017;18:1159-71. [Crossref] [PubMed]
- McFadden NR, Perry LM, Ghalambor TJ, et al. Locoregional Liver-Directed Therapies to Treat Unresectable Colorectal Liver Metastases: A Review Oncology (Williston Park) 2022;36:108-14.
- Aberle S, Kenkel D, Becker AS, et al. Outpatient Yttrium-90 microsphere radioembolization: assessment of radiation safety and quantification of post-treatment adverse events causing hospitalization. Radiol Med 2020;125:971-80. [Crossref] [PubMed]
- Sirtex Medical Limited. SIR-Spheres Y-90 resin microspheres (Yttrium-90 microspheres). Appendix III: Calculation of Individual Dose. US Sales Office: Sirtex Medical Inc., 2017:3.
- Ho S, Lau WY, Leung TW, et al. Partition model for estimating radiation doses from yttrium-90 microspheres in treating hepatic tumours. Eur J Nucl Med 1996;23:947-52. [Crossref] [PubMed]
- Paprottka KJ, Schoeppe F, Ingrisch M, et al. Pre-therapeutic factors for predicting survival after radioembolization: a single-center experience in 389 patients. Eur J Nucl Med Mol Imaging 2017;44:1185-93. [Crossref] [PubMed]
- Statistik für Staatssekretariat für Bildung und Forschung (SBF). Swiss cancer report 2015: current situation and developments. Arndt V. editor. Neuchâtel: Federal Statistical Office, 2016.
- Omed A, Lawrance JA, Murphy G, et al. A retrospective analysis of selective internal radiation therapy (SIRT) with yttrium-90 microspheres in patients with unresectable hepatic malignancies. Clin Radiol 2010;65:720-8. [Crossref] [PubMed]
- Grosser OS, Ulrich G, Furth C, et al. Intrahepatic Activity Distribution in Radioembolization with Yttrium-90-Labeled Resin Microspheres Using the Body Surface Area Method--A Less than Perfect Model. J Vasc Interv Radiol 2015;26:1615-21. [Crossref] [PubMed]
- Paprottka PM, Hoffmann RT, Haug A, et al. Radioembolization of symptomatic, unresectable neuroendocrine hepatic metastases using yttrium-90 microspheres. Cardiovasc Intervent Radiol 2012;35:334-42. [Crossref] [PubMed]
- Zuckerman DA, Kennard RF, Roy A, et al. Outcomes and toxicity following Yttrium-90 radioembolization for hepatic metastases from neuroendocrine tumors-a single-institution experience. J Gastrointest Oncol 2019;10:118-27. [Crossref] [PubMed]
- Frilling A, Clift AK, Braat AJAT, et al. Radioembolisation with 90Y microspheres for neuroendocrine liver metastases: an institutional case series, systematic review and meta-analysis. HPB (Oxford) 2019;21:773-83. [Crossref] [PubMed]
- Kucuk ON, Soydal C, Lacin S, et al. Selective intraarterial radionuclide therapy with Yttrium-90 (Y-90) microspheres for unresectable primary and metastatic liver tumors. World J Surg Oncol 2011;9:86. [Crossref] [PubMed]
- European Association For The Study Of The Liver. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908-43. [Crossref] [PubMed]
- Sangro B, Carpanese L, Cianni R, et al. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology 2011;54:868-78. [Crossref] [PubMed]
- Bauschke A, Altendorf-Hofmann A, Freesmeyer M, et al. Selektive interne Radioembolisation beim nichtresektablen hepatozellulären Karzinom. Chirurg 2016;87:956-63. [Crossref] [PubMed]
- Mantry PS, Mehta A, Madani B, et al. Selective internal radiation therapy using yttrium-90 resin microspheres in patients with unresectable hepatocellular carcinoma: a retrospective study. J Gastrointest Oncol 2017;8:799-807. [Crossref] [PubMed]
- Gabrielson A, Miller A, Banovac F, et al. Outcomes and Predictors of Toxicity after Selective Internal Radiation Therapy Using Yttrium-90 Resin Microspheres for Unresectable Hepatocellular Carcinoma. Front Oncol 2015;5:292. [Crossref] [PubMed]
- Hilgard P, Hamami M, Fouly AE, et al. Radioembolization with yttrium-90 glass microspheres in hepatocellular carcinoma: European experience on safety and long-term survival. Hepatology 2010;52:1741-9. [Crossref] [PubMed]
- Golabi P, Fazel S, Otgonsuren M, et al. Mortality assessment of patients with hepatocellular carcinoma according to underlying disease and treatment modalities. Medicine (Baltimore) 2017;96:e5904. [Crossref] [PubMed]
- Casadei Gardini A, Tamburini E, Iñarrairaegui M, et al. Radioembolization versus chemoembolization for unresectable hepatocellular carcinoma: a meta-analysis of randomized trials. Onco Targets Ther 2018;11:7315-21. [Crossref] [PubMed]
- Geng L, Wang J. Molecular effectors of radiation resistance in colorectal cancer. Precision Radiation Oncology 2017;1:27-33. [Crossref]
- Ji D, Zhan T, Li M, et al. Enhancement of Sensitivity to Chemo/Radiation Therapy by Using miR-15b against DCLK1 in Colorectal Cancer. Stem Cell Reports 2018;11:1506-22. [Crossref] [PubMed]
- Habiba K, Aziz K, Sanders K, et al. Enhancing Colorectal Cancer Radiation Therapy Efficacy using Silver Nanoprisms Decorated with Graphene as Radiosensitizers. Sci Rep 2019;9:17120. [Crossref] [PubMed]
- Morrison R, Schleicher SM, Sun Y, et al. Targeting the mechanisms of resistance to chemotherapy and radiotherapy with the cancer stem cell hypothesis. J Oncol 2011;2011:941876. [Crossref] [PubMed]
- Besse B, Adjei A, Baas P, et al. 2nd ESMO Consensus Conference on Lung Cancer: non-small-cell lung cancer first-line/second and further lines of treatment in advanced disease. Ann Oncol 2014;25:1475-84. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aisner DL, et al. Non-Small Cell Lung Cancer, Version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017;15:504-35. [Crossref] [PubMed]
- Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29:iv192-237. [Crossref] [PubMed]
- Kuei A, Saab S, Cho SK, et al. Effects of Yttrium-90 selective internal radiation therapy on non-conventional liver tumors. World J Gastroenterol 2015;21:8271-83. [Crossref] [PubMed]
- Man D, Wu J, Shen Z, et al. Prognosis of patients with neuroendocrine tumor: a SEER database analysis. Cancer Manag Res 2018;10:5629-38. [Crossref] [PubMed]
- Yao JC, Eisner MP, Leary C, et al. Population-based study of islet cell carcinoma. Ann Surg Oncol 2007;14:3492-500. [Crossref] [PubMed]
- Devcic Z, Rosenberg J, Braat AJ, et al. The efficacy of hepatic 90Y resin radioembolization for metastatic neuroendocrine tumors: a meta-analysis. J Nucl Med 2014;55:1404-10. [Crossref] [PubMed]
- Ludwig JM, Ambinder EM, Ghodadra A, et al. Lung Shunt Fraction prior to Yttrium-90 Radioembolization Predicts Survival in Patients with Neuroendocrine Liver Metastases: Single-Center Prospective Analysis. Cardiovasc Intervent Radiol 2016;39:1007-14. [Crossref] [PubMed]
- Peker A, Çiçek O, Soydal Ç, et al. Radioembolization with yttrium-90 resin microspheres for neuroendocrine tumor liver metastases. Diagn Interv Radiol 2015;21:54-9. [Crossref] [PubMed]
- Jakobs TF, Paprottka KJ, Raeßler F, et al. Robust evidence for long-term survival with 90Y radioembolization in chemorefractory liver-predominant metastatic colorectal cancer. Eur Radiol 2017;27:113-9. [Crossref] [PubMed]
- Xing M, Prajapati HJ, Dhanasekaran R, et al. Selective Internal Yttrium-90 Radioembolization Therapy (90Y-SIRT) Versus Best Supportive Care in Patients With Unresectable Metastatic Melanoma to the Liver Refractory to Systemic Therapy: Safety and Efficacy Cohort Study. Am J Clin Oncol 2017;40:27-34. [Crossref] [PubMed]
- Li CH, Haider S, Shiah YJ, et al. Sex Differences in Cancer Driver Genes and Biomarkers. Cancer Res 2018;78:5527-37. [Crossref] [PubMed]


