
The prognostic role of fasting plasma glucose levels on survival in advanced colorectal cancer patients with type II diabetes mellitus: a retrospective cohort study
Highlight box
Key findings
• The results of the present study showed that FPG levels may not affect the survival of advanced CRC patients with T2DM.
What is known and what is new?
• Fasting blood glucose levels are associated with the risk of CRC. The relationship between fasting glucose control and survival in colorectal cancer patients with type 2 diabetes is inconclusive. And the population of such studies is mainly concentrated in patients with stage I-III. There are few studies on advanced patients.
• The present study sought to investigate the levels of survival among advanced CRC patients with T2DM stratified according to levels of FPG control.
What is the implication, and what should change now?
• The study means FPG may not guide survival in advanced CRC patients with T2DM. It needs multicenter prospective studies to confirm.
Introduction
Colorectal cancer (CRC) is one of the most common malignancies. In 2021, there were over >1.9 million newly diagnosed cases of CRC. CRC is the 3rd most common cancer in males following lung and prostate cancer, and the 2nd most common cancer in females following breast cancer (1). CRC accounts for 10% and 9.4% of all cancer-related morbidities and mortalities worldwide, respectively (1). Due to its large population, China ranks 1st in the world for new cases of CRC and CRC-related deaths (2). Cancer screening reduces morbidity and mortality; however, 25% of CRC patients are diagnosed at an advanced stage, and 25–50% of patients with an early disease state (stage I–III) will develop metastases (3). Unfortunately, the 5-year survival rate for patients with advanced CRC is only ~14% (4).
Diabetes mellitus (DM) is a major disease burden worldwide and is one of the most common chronic diseases. The prevalence of DM continues to rise, and DM represents a notable health threat to humans, primarily due to its related cardiovascular, renal, and neurological complications (5). DM is also associated with a risk of cancer and a high level of mortality. Observational studies have consistently reported that people with type II diabetes mellitus (T2DM) are at an increased risk of developing multiple cancers, including colorectal, liver, pancreatic, endometrial, breast, and bladder cancers (6). Compared to the general population, patients with diabetes exhibit an increased incidence and risk of CRC, decreased survival rates, and an increased risk of recurrence (7-11).
A previous study of CRC patients demonstrated that fasting plasma glucose (FPG) levels were associated with CRC risk, particularly in Asia, the United States of America, and Europe (12). The results of a dose-response meta-analysis demonstrated that the risk of CRC increased with elevated blood glucose concentrations, suggesting that blood glucose is a dose-dependent risk factor for CRC (13). Hyperglycemia can regulate the signaling pathway of CRC and induce the proliferation of cancer cells. It can also induce the migration and invasion of cancer cells and induce the resistance of cancer cells to chemotherapy to affect the survival of patients (14). Some current survival analysis studies have shown the poor prognosis of hyperglycemia in patients with CRC (15-18). The subjects of these studies on blood glucose levels mostly comprised patients with stage I–III CRC. And there were fewer patients with T2DM. To the best of our knowledge, no studies have examined the association between FPG control status and the prognosis of advanced CRC patients with T2DM.
The present study sought to investigate the levels of survival among advanced CRC patients with T2DM stratified according to levels of FPG control. We present the following article in accordance with the REMARK reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1124/rc).
Methods
Patients
A retrospective analysis was conducted of the survival data of advanced CRC patients with T2DM who received first-line treatment at Harbin Medical University Cancer Hospital from May 2010 to May 2019. All the patients were treated with advanced 1st-line therapy according to the current guidelines following diagnosis. To be eligible for inclusion in this study, patients had to meet the following inclusion criteria: (I) have an endoscopic biopsy or postoperative pathological diagnosis of CRC; (II) have been diagnosed with T2DM; (III) have received advanced 1st-line therapy; and (IV) have had their FPG levels measured before undergoing the 1st-line therapy and during the follow-up treatment. Patients were excluded from the study if they met any of the following exclusion criteria: (I) had a history of other malignancies; and/or (II) the time from the end of postoperative adjuvant chemotherapy to recurrence and metastasis was <6 months. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics board of Harbin Medical University Cancer Hospital (No. KY2022-32), and individual consent for this retrospective analysis was waived.
Study design
This retrospective study analyzed the medical records of patients treated from May 2010 to May 2019. The follow-up period continued to May 20, 2022. The median follow-up time was 66.3 months. Patients’ follow-up information was obtained from hospital records or from the patients and their families. A diagnosis of T2DM was obtained from the patients’ early medical records. OS was determined as the end point of the study, as OS is the most suitable event for survival analyses. OS was defined as the time from the discovery of recurrence or metastasis with no chance of cure or transformation until death from any cause. All the patients were followed-up for at least 3 years. Clinical data, including age, sex, blood pressure, body mass index (BMI), primary tumor site, T stage, N stage, histological grade, number of metastatic sites, primary tumor surgery, v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS) status, neuroblastoma RAS viral oncogene homolog (NRAS) status, v-Raf murine sarcoma viral oncogene homolog B1 (BRAF) status, mismatch repair (MMR) status, radiotherapy, the ablation of metastatic lesions, and 1st-line medication, were recorded. First, the FPG value measured before the first-line treatment was selected and recorded as the baseline FPG level. Patients were divided into 2 groups according to the baseline FPG level using an FPG cut-off value of 7. Then, collect FPG measured before each admission treatment during advanced chemotherapy. Patients with all FPG <7 mmol/L during treatment were included in FPG-A group, and patients with all FPG >7 mmol/L were included in FPG-B group. Compare the OS.
Statistical analysis
The Chi-square test and Fisher’s exact test were used to analyze the baseline characteristics. A univariate analysis was performed using the Kaplan-Meier method of log-rank, OS survival curves were drawn and compared, and 3-year OS rates were calculated. The Cox proportional hazards regression model was used to evaluate the association between the control FPG level and survival prognosis of patients in the 2 groups. In these groups, the hazard ratios (HRs) for OS and the corresponding 95% confidence intervals (CIs) were estimated. A two-sided P value <0.05 indicated a statistically significant difference. The statistical analysis was performed in May 2022 using SPSS (version, 25.0) software (IBM Corp, USA) and R software (version 4.2.0, New Zealand).
Results
Data
From May 2010 to May 2019, a total of 210 patients were identified who met the inclusion criteria of the present study. These patients had a median age of 66.5 years (range, 46–89 years), and 148 (70.5%) were male. The baseline FPG range was 4.1–20.3 mmol/L. All the patients were followed-up for at least 3 years. As at the date of the last patient follow-up, 151 patients had died, 32 had survived, and 27 had been lost to follow-up. For patients who did not develop an OS event or were lost to follow-up, the time of their last follow-up was recorded as the time at which the OS event occurred.
The patients were divided into 2 groups according to the FPG value of 7. In total, 94 patients had baseline FPG levels ≤7 mmol/L, and 116 patients had baseline FPG levels >7 mmol/L. There were 52 patients in the FPG-A group and 61 in the FPG-B group. There was no significant difference in the distribution of age, sex, blood pressure, BMI, primary tumor site, T stage, N stage, histological grade, number of metastatic sites, primary tumor surgery, KRAS status, NRAS status, BRAF status, MMR status, radiotherapy, the ablation of metastatic lesions, or 1st-line medication between the 2 groups (P>0.05; Table 1).
Table 1
| Characteristic | Glycemia ≤7 (n=94) | Glycemia >7 (n=116) | χ2 | P value |
|---|---|---|---|---|
| Age, years | 0.542 | 0.461 | ||
| <65 | 35 | 49 | ||
| ≥65 | 59 | 67 | ||
| Gender | 1.303 | 0.254 | ||
| Male | 70 | 78 | ||
| Female | 24 | 38 | ||
| Bp | 0.3 | 0.584 | ||
| Normal | 57 | 66 | ||
| High | 37 | 50 | ||
| BMI | 1.132 | 0.626 | ||
| Underweight | 1 | 4 | ||
| Normal | 41 | 49 | ||
| Overweight | 52 | 63 | ||
| Tumor site | 7.258 | 0.111 | ||
| Missing | 3 | 1 | ||
| Multi-sides | 4 | 3 | ||
| Right sides | 24 | 24 | ||
| Left sides | 29 | 26 | ||
| Rectum | 34 | 62 | ||
| T | 6.081 | 0.107 | ||
| Missing | 26 | 34 | ||
| 2 | 1 | 7 | ||
| 3 | 48 | 62 | ||
| 4 | 19 | 13 | ||
| N | 5.918 | 0.116 | ||
| Missing | 28 | 35 | ||
| 0 | 13 | 29 | ||
| 1 | 30 | 35 | ||
| 2 | 23 | 17 | ||
| Histologic grade | 0.694 | 0.707 | ||
| Missing | 26 | 32 | ||
| Low grade | 46 | 62 | ||
| High grade | 22 | 22 | ||
| Number of metastatic sites | 0.205 | 0.651 | ||
| Single | 53 | 69 | ||
| Multiple | 41 | 47 | ||
| MMR status | 0.309 | 0.94 | ||
| Missing | 77 | 93 | ||
| dMMR | 1 | 2 | ||
| pMMR | 16 | 21 | ||
| KRAS status | 0.049 | 0.976 | ||
| Missing | 68 | 84 | ||
| Mutant | 8 | 9 | ||
| Wild-type | 18 | 23 | ||
| NRAS status | 0.377 | 0.93 | ||
| Missing | 76 | 92 | ||
| Mutant | 1 | 1 | ||
| Wild-type | 17 | 23 | ||
| BRAF status | 1.495 | 0.553 | ||
| Missing | 76 | 95 | ||
| Mutant | 0 | 2 | ||
| Wild-type | 18 | 19 | ||
| Primary tumor surgery | 0.074 | 0.786 | ||
| No | 30 | 35 | ||
| Yes | 64 | 81 | ||
| Radiotherapy | 0.008 | 0.931 | ||
| No | 79 | 98 | ||
| Yes | 15 | 18 | ||
| Ablation of metastatic lesions | 1.479 | 0.224 | ||
| No | 84 | 109 | ||
| Yes | 10 | 7 | ||
| 1st-line medication | 0.119 | 0.730 | ||
| Chemotherapy | 74 | 89 | ||
| Chemotherapy + targeted therapy | 20 | 27 | ||
Bp, blood pressure; BMI, body mass index; MMR, mismatch repair; dMMR, mismatch repair-deficient; pMMR, mismatch repair-proficient; KRAS, Kirsten rat sarcoma viral oncogene homolog; NRAS, Neuroblastoma RAS viral oncogene homolog; BRAF, V-Raf murine sarcoma viral oncogene homolog B1.
Analysis and presentation
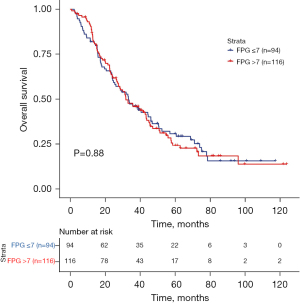
The survival outcomes of the 2 groups of patients were compared separately. The 3-year OS rate of the patients with a baseline FPG ≤7 mmol/L was 46.2%, and that of patients with a baseline FPG >7 mmol/L was 45.8%. The median OS was 33.4 months in the baseline FPG ≤7 mmol/L group, and 31.9 months in the baseline FPG >7 mmol/L group. Compared to the baseline FPG >7 mmol/L group, the OS of patients in the baseline FPG ≤7 mmol/L group was not significantly prolonged (P=0.88; HR, 0.976; 95% CI: 0.708–1.345; Figure 1).
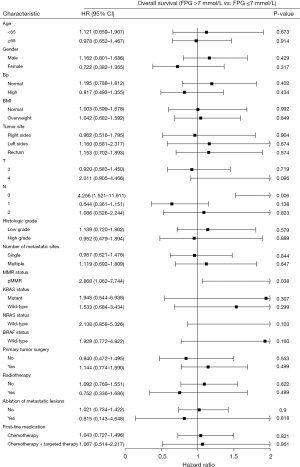
The subgroup analysis showed that in terms of N0 stage (P=0.006; HR, 4.256; 95% CI: 1.521–11.911) and mismatch repair-proficient (pMMR) status (P=0.038; HR, 2.868; 95% CI: 1.062–7.744), the OS of patients with a baseline FPG ≤7 mmol/L was significantly longer than that of patients with a baseline FPG >7 mmol/L (Figure 2).
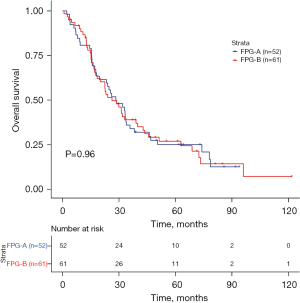
The 3-year OS rate was 34.1% in the FPG-A group, whereas the OS rate was 39% in the FPG-B group. The median OS was 28.6 months in the FPG-A group but was 26.4 months in the FPG-B group. Similarly, there was no significant difference in OS between the FPG-A and FPG-B groups (P=0.96; HR, 0.989; 95% CI: 0.648–1.512; Figure 3).
The subgroup analysis showed that in terms of N0 stage (P=0.031; HR, 2.013; 95% CI: 1.065–3.804), the OS of patients in the FPG-A group was significantly longer than that of patients in the FPG-B group (Figure 4).

Discussion
Diabetes is a global public health problem, which affected 537 million people in 2021. Moreover, this number is expected to reach 643 million by 2030 and 783 million by 2045 (19). The occurrence of T2DM increases the risk of death from cancers of the digestive system, particularly those involving the colorectum, liver, and pancreas (20). However, the association between T2DM and recurrence and death in local or regional CRC remains controversial (21-24). Previous studies have reported that T2DM affects the OS and DFS of CRC patients (21,22). However, other studies have reached contradictory conclusions (i.e., that pre-existing T2DM has no effect on the disease-specific and all-cause survival of CRC patients) (23).
T2DM may not affect the OS and DFS of patients with stage I–III CRC (24). Moreover, the effect of T2DM on the survival of advanced CRC patients is yet to be fully elucidated. Brown et al. demonstrated that diabetes is associated with increased mortality and an increased risk of tumor progression in patients with advanced or metastatic CRC (mCRC) (25). However, a pooled analysis of 2 phase-III clinical trials (NCT00272051 and NCT00305188) by Abdel-Rahman revealed that DM did not affect the OS or progression-free survival of patients with mCRC receiving 1st-line FOLFOX chemotherapy. Following a propensity-score matching analysis between diabetic and non-diabetic patients, the OS and DFS analyses were performed again with similar results (26).
Previously published retrospective studies have shown that patients with stage-III CRC with high blood glucose levels (blood glucose >7 mmol/L) had significantly poorer outcomes in the short term (2 years) than patients with lower blood glucose levels (16). The results of a previous study showed that among patients with colon cancer, patients with DM with well-controlled HbA1c exhibited significantly longer OS than those with uncontrolled DM (15). However, the subjects of these studies on blood glucose levels mostly comprised patients with stage I–III CRC, and further studies need to be conducted with patients with stage IV CRC.
The present study sought to evaluate patient prognosis based on different levels of glycemic control in advanced CRC patients with T2DM. The data were extracted from a central database using strict inclusion/exclusion criteria. The data analysis demonstrated that compared to the FPG bad controlled group, the FPG well controlled group did not exhibit a statistically significant increase in survival time. However, the results of the present study showed that the median OS of the low blood glucose group was prolonged compared to that of the high blood glucose group.
A previous study examined 520 patients with stage I–III CRC, of whom 312 were normoglycemic, 208 were hyperglycemic, 135 had a history of DM, and 385 had no history of DM (17). The patients were grouped according to their blood glucose levels, and the results showed that OS was significantly more decreased in the hyperglycemic group (≥110 mg/dL) than the normoglycemic group (<110 mg/dL). In patients with a history of DM, there was no significant difference in the OS rate between the high blood glucose level group (≥110 mg/dL) and the low blood glucose level group (P=0.5225) (17). The results of this previous study are comparable to those of the present study. Further, a mendelian randomization analysis by Murphy et al. showed that fasting glucose exerted no effect on the risk of developing CRC (27). To date, this is the largest and most comprehensive study examining the effects of multiple glycemic profiles on CRC risk (27).
At present, there are numerous potential mechanisms underlying the association between hyperglycemia and cancer. Glycolysis plays a central role in tumor development, and elevated levels of circulating glucose are highly used by cancer cells (28). Moreover, in a subset of tumor cells, hyperglycemia leads to increased reactive oxygen species produced by mitochondrial respiration, which may lead to cellular deoxyribonucleic acid mutations that play an important role in the initiation and progression of multistage carcinogenesis (29). In addition, enhanced glucose metabolism may also inhibit cytochrome c-mediated apoptosis in cancer cells (30) and lead to resistance to chemotherapy (16), both of which are beneficial for continued tumor growth. Further, when plasma glucose is poorly controlled, oxidative stress may increase. This leads to inflammation, which contributes to all stages of tumorigenesis, including angiogenesis and metastasis (31).
The subgroup analysis of the present study showed that the FPG level of patients with N0 stage was significantly correlated with OS, indicating that this part of the population should pay more attention to blood sugar control. However, this result needs to be proven in a larger sample.
The results of the present study did not establish a significant association between FPG levels and survival in advanced CRC patients with T2DM. This may be because the length of time that the patients had T2DM was unclear, and the effect of hyperinsulinemia on CRC patients could not be determined. T2DM affects cancer outcomes primarily through insulin resistance, hyperglycemia, and inflammation (32). T2DM maintains insulin resistance only in the early stages, which leads to a decline in the biological effects of insulin. The body promote insulin secretion in order to maintain normal blood sugar levels, resulting in hyperinsulinemia. As T2DM progresses, hypoinsulinemia develops due to islet cell dysfunction, at which point the negative effect of hyperinsulinemia on CRC prognosis may be attenuated. Previous studies have revealed that the OS of CRC patients is closely associated to the use of metformin (33,34). The present retrospective analysis was limited, as the information regarding the diabetes medication taken by the patients was incomplete; thus, the effects of metformin and insulin on the prognosis of CRC could not be taken into account.
The present study had a number of limitations. For example, as a retrospective study, there are biases in the data collected. In addition, we didn’t know the severity of diabetes in patients, or the serious complications diabetes may produce. It may affect the prognosis of CRC patients. Moreover, the use of diabetes drugs, such as metformin, insulin, and other drugs, may have an effect on advanced CRC patients with T2DM, which requires further analysis. The present study mainly focused on FPG levels, which reflect daily blood glucose levels; however, this may be biased. Because it cannot reflect the long-term level of blood glucose. HbA1c testing can be performed at any time, regardless of the time of fasting or the content of the previous meal, and HbA1c levels also reflect blood glucose levels during the previous 6–8 weeks (35). HbA1c levels may be more important than FPG for prognosis; however, the HbA1c data for the patients in the present study were incomplete. Thus, further experiments, including experiments with larger sample sizes, and prospective randomized controlled trials should be conducted.
Conclusions
The results of the present study demonstrated that FPG levels did not affect the survival of advanced CRC patients with T2DM. However, special attention should be paid to FPG control in N0 stage patients.
Acknowledgments
We would like to thank Spandidos Publications for undertaking the English-language edit of this article.
Funding: The present study was supported by grants from Natural Science Foundation of Heilongjiang Province (grant No. LH2021H079 to Guangyu Wang) and the Beijing Medical Award Foundation (grant No. YXJL-2020-0785-1198 to Guangyu Wang).
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1124/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1124/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1124/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that any questions related to the accuracy or integrity of any part of the work have been appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics board of Harbin Medical University Cancer Hospital (No. KY2022-32), and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Yang Y, Han Z, Li X, et al. Epidemiology and risk factors of colorectal cancer in China. Chin J Cancer Res 2020;32:729-41. [Crossref] [PubMed]
- Fan A, Wang B, Wang X, et al. Immunotherapy in colorectal cancer: current achievements and future perspective. Int J Biol Sci 2021;17:3837-49. [Crossref] [PubMed]
- Shen L, Li Q, Wang W, et al. Treatment patterns and direct medical costs of metastatic colorectal cancer patients: a retrospective study of electronic medical records from urban China. J Med Econ 2020;23:456-63. [Crossref] [PubMed]
- Saito E, Charvat H, Goto A, et al. Burden of cancer associated with type 2 diabetes mellitus in Japan, 2010-2030. Cancer Sci 2016;107:521-7. [Crossref] [PubMed]
- Tsilidis KK, Kasimis JC, Lopez DS, et al. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ 2015;350:g7607. [Crossref] [PubMed]
- Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst 2005;97:1679-87. [Crossref] [PubMed]
- Rao Kondapally Seshasai S, Kaptoge S, Thompson A, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 2011;364:829-41. [Crossref] [PubMed]
- Guraya SY. Association of type 2 diabetes mellitus and the risk of colorectal cancer: A meta-analysis and systematic review. World J Gastroenterol 2015;21:6026-31. [Crossref] [PubMed]
- Li J, Liu J, Gao C, et al. Increased mortality for colorectal cancer patients with preexisting diabetes mellitus: an updated meta-analysis. Oncotarget 2017;8:62478-88. [Crossref] [PubMed]
- Petrelli F, Ghidini M, Rausa E, et al. Survival of Colorectal Cancer Patients With Diabetes Mellitus: A Meta-Analysis. Can J Diabetes 2021;45:186-197.e2. [Crossref] [PubMed]
- Xu J, Ye Y, Wu H, et al. Association between markers of glucose metabolism and risk of colorectal cancer. BMJ Open 2016;6:e011430. [Crossref] [PubMed]
- Shi J, Xiong L, Li J, et al. A Linear Dose-Response Relationship between Fasting Plasma Glucose and Colorectal Cancer Risk: Systematic Review and Meta-analysis. Sci Rep 2015;5:17591. [Crossref] [PubMed]
- Supabphol S, Seubwai W, Wongkham S, et al. High glucose: an emerging association between diabetes mellitus and cancer progression. J Mol Med (Berl) 2021;99:1175-93. [Crossref] [PubMed]
- Lee SJ, Kim JH, Park SJ, et al. Optimal glycemic target level for colon cancer patients with diabetes. Diabetes Res Clin Pract 2017;124:66-71. [Crossref] [PubMed]
- Yang IP, Miao ZF, Huang CW, et al. High blood sugar levels but not diabetes mellitus significantly enhance oxaliplatin chemoresistance in patients with stage III colorectal cancer receiving adjuvant FOLFOX6 chemotherapy. Ther Adv Med Oncol 2019;11:1758835919866964. [Crossref] [PubMed]
- Yang IP, Tsai HL, Huang CW, et al. High blood sugar levels significantly impact the prognosis of colorectal cancer patients through down-regulation of microRNA-16 by targeting Myb and VEGFR2. Oncotarget 2016;7:18837-50. [Crossref] [PubMed]
- Calderillo-Ruiz G, Lopez H, Herrera M, et al. Obesity and hyperglycemia as a bad prognosis factor for recurrence and survival in colon cancer. Ann Oncol 2019;30:iv40-iv41. [Crossref]
- IDF Diabetes Atlas. 10th ed. 2021. Accessed April 8, 2022. Available online: https://www.diabetesatlas.org/en/
- Lo SF, Chang SN, Muo CH, et al. Modest increase in risk of specific types of cancer types in type 2 diabetes mellitus patients. Int J Cancer 2013;132:182-8. [Crossref] [PubMed]
- Jeon JY, Jeong DH, Park MG, et al. Impact of diabetes on oncologic outcome of colorectal cancer patients: colon vs. rectal cancer. PLoS One 2013;8:e55196. [Crossref] [PubMed]
- Zhu B, Wu X, Wu B, et al. The relationship between diabetes and colorectal cancer prognosis: A meta-analysis based on the cohort studies. PLoS One 2017;12:e0176068. [Crossref] [PubMed]
- Amshoff Y, Maskarinec G, Shvetsov YB, et al. Type 2 diabetes and colorectal cancer survival: The multiethnic cohort. Int J Cancer 2018;143:263-8. [Crossref] [PubMed]
- Cheng Y, Cheng YX, Liu XY, et al. The Effect of Type 2 Diabetes Mellitus on the Short-Term Outcomes and Prognosis of Stage I-III Colorectal Cancer: A Propensity Score Matching Analysis. Cancer Manag Res 2022;14:205-14. [Crossref] [PubMed]
- Brown JC, Zhang S, Ou FS, et al. Diabetes and Clinical Outcome in Patients With Metastatic Colorectal Cancer: CALGB 80405 (Alliance). JNCI Cancer Spectr 2020;4:pkz078. [Crossref] [PubMed]
- Abdel-Rahman O. Impact of diabetes comorbidity on the efficacy and safety of FOLFOX first-line chemotherapy among patients with metastatic colorectal cancer: a pooled analysis of two phase-III studies. Clin Transl Oncol 2019;21:512-8. [Crossref] [PubMed]
- Murphy N, Song M, Papadimitriou N, et al. Associations Between Glycemic Traits and Colorectal Cancer: A Mendelian Randomization Analysis. J Natl Cancer Inst 2022;114:740-52. [Crossref] [PubMed]
- Joshi S, Liu M, Turner N. Diabetes and its link with cancer: providing the fuel and spark to launch an aggressive growth regime. Biomed Res Int 2015;2015:390863. [Crossref] [PubMed]
- Duan W, Shen X, Lei J, et al. Hyperglycemia, a neglected factor during cancer progression. Biomed Res Int 2014;2014:461917. [Crossref] [PubMed]
- Vaughn AE, Deshmukh M. Glucose metabolism inhibits apoptosis in neurons and cancer cells by redox inactivation of cytochrome c. Nat Cell Biol 2008;10:1477-83. [Crossref] [PubMed]
- Luo C, Zhang H. The Role of Proinflammatory Pathways in the Pathogenesis of Colitis-Associated Colorectal Cancer. Mediators Inflamm 2017;2017:5126048. [Crossref] [PubMed]
- Cheng HC, Chang TK, Su WC, et al. Narrative review of the influence of diabetes mellitus and hyperglycemia on colorectal cancer risk and oncological outcomes. Transl Oncol 2021;14:101089. [Crossref] [PubMed]
- Christou N, Bergen ES, Canton C, et al. Impact of diabetes and metformin use on recurrence and outcome in stage II-III colon cancer patients-A pooled analysis of three adjuvant trials. Eur J Cancer 2022;166:100-11. [Crossref] [PubMed]
- Ng CW, Jiang AA, Toh EMS, et al. Metformin and colorectal cancer: a systematic review, meta-analysis and meta-regression. Int J Colorectal Dis 2020;35:1501-12. [Crossref] [PubMed]
- Okosun IS, Seale JP, Lyn R, et al. Improving Detection of Prediabetes in Children and Adults: Using Combinations of Blood Glucose Tests. Front Public Health 2015;3:260. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


