
Liver resection for hepatocellular carcinoma beyond the BCLC: are multinodular disease, portal hypertension, and portal system invasion real contraindications?
Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver cancer, and its incidence has increased in recent decades (1). HCC is the leading cause of cancer-related deaths in Asia and Sub-Saharan Africa, and is the fourth worldwide (2). In the USA, the incidence rate of HCC has increased over the past three decades, resulting in 6.7 cases/per 100,000 inhabitants in 2012 and 11,073 deaths in 2016 (3).
Cirrhosis, especially due to chronic viral hepatitis, followed by alcoholic hepatitis and non-alcoholic steatohepatitis (NASH), is the leading risk factor for the development of HCC (3). In 90% of patients, HCC is related to chronic liver disease (4), making the treatment a challenge for hepatologists and liver surgeons, once there are many variables to be considered, such as liver function, tumor size and extension, availability of organs for transplantation, and patients’ performance status (5-8). Many algorithms have been developed to standardize staging and treatment allocation for patients with HCC. The Barcelona Clinic Liver Cancer (BCLC) guidelines, created in 1999 and updated since then (6), are one of the most recognized and endorsed staging systems by both the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver (9,10).
The BCLC staging system allocates patients with HCC into five different groups (0, A, B, C, and D), enabling the selection of the best candidates for the best therapies currently available. It is based on the tumor number and size, presence of vascular invasion, liver function, and clinical conditions, suggesting the ideal treatment among six different options: ablation, liver transplantation, resection, chemoembolization, systemic therapy, and supportive care (1,6). The BCLC classification is accepted worldwide as a good and practical staging system for HCC because of its ability to categorize patients according to their prognosis, resulting in treatment recommendations. However, this algorithm has been criticized by many authors because of its very restrictive character, especially regarding indications for resection that condemn patients with good health conditions and preserved liver function to palliative treatment (7-9,11-15). In addition, the diverse availability of liver grafts in different regions of the world is significant, and this impacts the feasibility of liver transplantation (14).
Many reports from Eastern and European centers, as well as from our group, are less dogmatic and restrictive regarding the indications for HCC resection, unlike the BCLC guidelines, since many patients with large tumors, oligonodular involvement (≤3 nodules), or regional portal vein tumor invasion may benefit from resection (7,8,11-14,16).
This study aimed to evaluate the results of HCC resection in our institution to understand whether there is room for a more liberal indication for resection than proposed by the BCLC guidelines. We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-833/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics committee of Hospital das Clínicas, University of São Paulo School of Medicine, Brazil, with the number 05892819.4.0000.0068. Individual consent for this retrospective analysis was waived.
This retrospective cohort study was based on a prospective database of all patients who underwent HCC resection at our institution between April 2008 and November 2018. The inclusion criteria were patients older than 18 years of age, with a single nodule, and absence of extrahepatic disease. Patients with chronic liver disease and compensated liver function were considered eligible as follows: Child-Pugh A and Model of End Stage Liver Disease (MELD) ≤10 (B7 or MELD >10 only when minor peripheral resection was required), without clinically significant portal hypertension (small-caliber esophageal varices and platelets >100,000/mL), and future liver remnant ≥40% (13). The exclusion criteria were the presence of extrahepatic disease, R2 resection, portal vein trunk tumor invasion, and absence of data.
All the patients underwent medical and laboratory evaluations. Preoperative workup included abdominal computed tomography (CT), magnetic resonance imaging (MRI), and thoracic CT. Preoperative diagnosis was based on image characteristics (10,17), and biopsy was indicated only if diagnostic doubt persisted after radiological evaluation. When CT or MRI showed signs of portal hypertension, an upper digestive endoscopy was performed. The clinical background and imaging scans of all the patients were discussed on a weekly multidisciplinary board, where liver resection was indicated.
The following preoperative characteristics were evaluated: age, sex, body mass index (BMI), preoperative laboratory tests and tumor markers, etiology of liver disease, presence of chronic liver disease/cirrhosis, presence of portal hypertension, Child-Pugh and MELD classifications, number and size of lesions, presence of satellite nodules, presence of tumor pseudocapsule, portal vein invasion, and type of resection (anatomical vs. non-anatomical; minor vs. major; open vs. laparoscopic).
According to the US-National Cancer Institute, we called satellite nodules those located within 2 cm of the primary tumor. Satellite nodules were not considered as another lesion.
Portal hypertension was defined as the presence of collateral circulation or splenomegaly on imaging examinations, low serum platelet count, and/or esophageal varices on endoscopy. Significant portal hypertension was defined as the presence of medium or large esophageal varices or ascites.
The presence of portal vein invasion was evaluated on image scans and categorized into first-order branch (right or left portal vein invasion); second-order branch, which corresponds to the anterior or posterior sector of the right portal vein, and transverse or umbilical portions of the left portal vein; or third-order branch invasion in the segmental portal vein branch (17).
Patients with portal hypertension were eligible for resection when there was no significant portal hypertension (without medium or large esophageal varices or ascites and platelet counts >100,000/mL). We also resected two or three nodules only in cases where the nodules were confined to the same liver section and outside the Milan criteria. Tumor size was a limitation for resection only in patients whose complete resection was not possible or in those with an insufficient liver remnant. Finally, for patients with portal invasion, only trunk portal invasion by the tumor was a contraindication for resection.
For very early tumors (≤2 cm), non-anatomical resections were performed, while anatomical resections were preferred for larger tumors. Major hepatectomy was defined as resection of three or more contiguous liver segments (18). Postoperative morbidity was categorized according to the Clavien-Dindo classification of surgical complications (19).
Follow-up was performed using a standard protocol, with the assessment of serum biomarkers and imaging evaluation every 4–6 months post-resection. Recurrence was determined by the presence of radiological evidence of HCC and/or increasing serum AFP levels. Overall survival (OS) was defined as the length of time between resection and death or the most recent follow-up date. Deaths from other causes were treated as censored. Disease-free survival (DFS) was defined as the time interval between liver resection and recurrence at any site or the most recent follow-up date.
We evaluated our results using both the 2010 and 2018 BCLC guidelines since liver resections in the present study were performed until 2018. The main change between the 2010 and 2018 versions was the withdrawal of the 5-cm tumor size limit for resection in the last edition. Patients were retrospectively classified according to the BCLC 2010 and BCLC 2018 guidelines as 0, A, B, C, and D. Survival of patients who underwent resection was evaluated, in accordance with BCLC guidelines suggested treatment, in three groups: resection, other potentially curative therapies (transplantation or ablation), and palliative treatments (chemoembolization or systemic therapy) (6,10).
To evaluate how the addition of risk factors in our study might have affected patients’ prognoses, we compared overall and disease-free survival after resection in patients with none, one, two, or three of the main risk factors as proposed by the BCLC criteria in 2010 as contraindications to resection, namely, portal hypertension, portal system invasion, and presence of more than one HCC nodule.
Statistical analysis
Continuous data are expressed as median and mean ± standard deviation (SD). Categorical variables are expressed as percentages. Survival was assessed using the Kaplan-Meier method, and measures were calculated using univariate Cox regression. Variables with a P value <0.2 on univariate analysis for OS and DFS were selected for the multiple Cox regression model. Hazard ratios (HR) with 95% confidence intervals (95% CI) were calculated for each studied variable. Statistical significance was set at a P value <0.05.
Results
After applying the exclusion criteria, 150 of the 195 patients who underwent HCC resection were enrolled in our study. The baseline characteristics of the patients are summarized in Table 1. There were 104 male (69.3%) and 46 female patients, with a mean age of 62.8 years (range, 32–86 years). The main causes of chronic liver disease were hepatitis C (55.7%), alcoholic liver disease (20.8%), hepatitis B (10.1%), non-alcoholic steatohepatitis (NASH) (6.7%), and other etiologies (6.7%).
Table 1
| Characteristics | Value |
|---|---|
| Sex, n (%) | |
| Female | 46 (30.7) |
| Male | 104 (69.3) |
| Age (years) | |
| Mean (SD) | 62.8 (9.6) |
| Median [min – max] | 63 [32–86] |
| Body mass index (kg/m2) | |
| Mean (SD) | 25.4 (4.5) |
| Median [min – max] | 25.1 [15.6–36.7] |
| Chronic liver disease, n (%) | |
| No | 23 (15.3) |
| Yes (without cirrhosis) | 44 (29.3) |
| Yes (with cirrhosis) | 83 (55.3) |
| Child-Pugh, n (%) | |
| A | 143 (95.3) |
| B | 7 (4.7) |
| MELD | |
| Mean (SD) | 8.4 (2.5) |
| Median [min – max] | 8 [6–16] |
| Portal hypertension, n (%) | |
| No | 108 (72.0) |
| Yes | 42 (28.0) |
| Alpha-fetoprotein | |
| Mean (SD) | 1,914.73 (8,041.94) |
| Median [min – max] | 18.60 [1–60,500] |
| Nodule size in imaging studies (cm) | |
| Mean (SD) | 6.3 (5.1) |
| Median [min – max] | 4.5 [1.2–27.0] |
| ≤5 cm, n (%) | 88 (58.7) |
| 5.1–10 cm, n (%) | 36 (24.0) |
| >10 cm, n (%) | 26 (17.3) |
| Number of HCC nodules in imaging studies, n (%) | |
| One | 142 (94.7) |
| More than one | 8 (5.3) |
| Satellite nodules in imaging studies, n (%) | |
| No | 139 (92.7) |
| Yes | 11 (7.3) |
| Pseudocapsule in imaging studies, n (%) | |
| No | 124 (82.7) |
| Yes | 26 (17.3) |
| Portal invasion (CT or MRI), n (%) | |
| No | 123 (82.0) |
| Yes | 27 (18.0) |
| Minor vs. major hepatectomy, n (%) | |
| Minor hepatectomy | 108 (72.0) |
| Major hepatectomy | 42 (28.0) |
| Anatomic vs. nonanatomic resection, n (%) | |
| Nonanatomic | 47 (31.3) |
| Anatomic | 91 (60.7) |
| Anatomic and nonanatomic | 12 (8.0) |
| Clavien-Dindo classification, n (%) | |
| 0 | 84 (56.0) |
| I | 19 (12.6) |
| II | 26 (17.3) |
| III | 5 (3.2) |
| IV | 6 (3.9) |
| V | 10 (6.6) |
HCC, hepatocellular carcinoma; MELD, Model of End Stage Liver Disease; SD, standard deviation; Min, minimum value; Max, maximum value.
The median nodule size in the imaging studies was 4.5 cm (1.2–27.0 cm). Eight patients (5.3%) had more than one HCC nodule (one had three nodules, while the others had two nodules), 26 (17.3%) presented with pseudocapsules, and 11 (7.3%) had satellite nodules. In the entire series, 143 patients (95.3%) had Child-Pugh A, and 86 patients (57.3%) met the Milan criteria. Seven patients (4.7%) were Child-Pugh B; furthermore, the alpha-fetoprotein level was higher than 200 ng/mL in 34 (22.6%) patients and higher than 1,000 ng/mL in 21 (14%) patients. Portal system tumor invasion was detected using CT or MRI in 26 patients (17.3%), half of them in first-order branches, 11 in second-order branches, and 2 patients in third-order branches. According to the BCLC 2010 guidelines, 18 patients (12%) were classified as 0, 64 (42.7%) as A, 41 (27.3%) as B, and 27 (18%) as C; however, according to the BCLC 2018 guidelines, 18 patients (12%) were classified as 0, 101 (67.3%) as A, 4 (2.7%) as B, and 27 (18%) as C.
The types of liver resection and Clavien-Dindo classification of surgical complications are presented in Table 1. Major hepatectomy and anatomical resection were performed in 42 (28 %) and 90 (60.7%) patients, respectively. Sixty-six patients (44%) presented with complications, of which 7.1% were classified as Clavien-Dindo grades 3 and 4. The main postoperative complications were ascites (22 cases, 14.7%), biliary fistula (15, 10%), pneumonia (7, 4.7%), and surgical wound infection (6, 4%). Ninety-day postoperative mortality occurred in 10 cases (6.7%), 5 of which were due to septic shock, 2 due to hypovolemic shock, two due to peritonitis, and one due to myocardial infarction.
The median follow-up was 23.95 months (mean 32.1 months; range, 5–136.28 months). The incidence of recurrence was 43.3% (65 patients), with a median time to recurrence of 12.68 months (range, 3–127.87 months). Median OS was 57.4 months, the median DFS was 30.4 months; 5-year OS was 47.7%, and the 5-year DFS was 39.7%.
Variables with a significant impact on decreasing OS evaluated by univariate analysis were the presence of portal hypertension, increased alpha-fetoprotein levels, presence of satellite nodules, and more than one HCC nodule in imaging studies. Variables with a significant impact on decreasing DFS evaluated by univariate analysis were elevated alpha-fetoprotein levels, more than one HCC nodule, and the presence of satellite nodules on imaging examinations. Table 2 summarizes the univariate analyses of OS and DFS.
Table 2
| Variable | Overall survival | Disease-free survival | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Sex | |||||||
| Female | 1 | 1 | |||||
| Male | 1.03 | 0.59–1.81 | 0.917 | 1.05 | 0.63–1.75 | 0.858 | |
| Age at surgical procedure (years) | 1.02 | 0.99–1.05 | 0.221 | 0.99 | 0.96–1.01 | 0.401 | |
| Body mass index (kg/m2) | 0.98 | 0.92–1.04 | 0.570 | 0.95 | 0.90–1.01 | 0.105 | |
| Chronic liver disease | |||||||
| No | 1 | 1 | |||||
| Yes, with cirrhosis | 0.81 | 0.30–2.14 | 0.669 | 2.38 | 0.90–6.30 | 0.081 | |
| Yes, no cirrhosis | 1,29 | 0.54–3.09 | 0.563 | 2.26 | 0.89–5.76 | 0.088 | |
| Preoperative Child-Pugh | |||||||
| A | 1 | 1 | |||||
| B | 1.31 | 0.40–4.21 | 0.655 | 1.78 | 0.55–5.73 | 0.332 | |
| Preoperative MELD | 1.00 | 0.90–1.10 | 0.936 | 0.93 | 0.83–1.04 | 0.219 | |
| Portal hypertension | |||||||
| No | 1 | 1 | |||||
| Yes | 2.42 | 1.43–4.10 | 0.001 | 1.56 | 0.93–2.64 | 0.092 | |
| Preoperative alpha-fetoprotein | 1.00 | 1.00–1.00 | 0.001 | 1.00 | 1.00–1.00 | 0.000 | |
| ≤500 | 1 | 1 | |||||
| >500 | 1.75 | 0.92–3.33 | 0.089 | 1.11 | 0.57–2.19 | 0.753 | |
| Nodule size in imaging studies (cm) | |||||||
| ≤5 cm | 1 | 1 | |||||
| 5.1–10 cm | 1.15 | 0.60–2.19 | 0.675 | 1.00 | 0.55–1.81 | 0.993 | |
| >10 cm | 1.56 | 0.78–3.11 | 0.205 | 0.96 | 0.48–1.93 | 0.911 | |
| Number of HCC nodules in imaging studies | |||||||
| No | 1 | 1 | |||||
| Yes | 2.97 | 1.26–7.04 | 0.013 | 5.09 | 2.28–11.38 | 0.000 | |
| Presence of satellite nodules in imaging studies | |||||||
| No | 1 | 1 | |||||
| Yes | 2.33 | 1.05–5.17 | 0.037 | 2.71 | 0.13–5.53 | 0.006 | |
| Presence of pseudocapsule in imaging studies | |||||||
| No | 1 | 1 | |||||
| Yes | 1.18 | 0.58–2.41 | 0.650 | 0.89 | 0.44–1.80 | 0.750 | |
| Portal invasion (CT or MRI) | |||||||
| No | 1 | 1 | |||||
| Yes | 1.75 | 0.85–3.61 | 0.130 | 1.56 | 0.77–3.19 | 0.218 | |
HCC, hepatocellular carcinoma; MELD, Model of End Stage Liver Disease; HR, hazard ratio; 95% CI, 95% confidence interval.
The results of the multivariate analysis are presented in Table 3. Independent risk factors for OS were portal hypertension, the presence of more than one HCC, or satellite nodules on imaging examinations. Independent risk factors for DFS were increased alpha-fetoprotein levels and more than one HCC nodule. Nodule size and presence of portal invasion in our series did not have a significant impact on OS or DFS.
Table 3
| Variables | HR | 95% CI | P value |
|---|---|---|---|
| OS | |||
| Portal hypertension (ref: no) | 2.57 | 1.50–4.38 | 0.001 |
| Presence of more than 1 HCC nodule in image exam (ref: no) | 2.58 | 1.09–6.14 | 0.032 |
| Presence of satellite nodules in image exam (ref: no) | 2.63 | 1.17–5.94 | 0.020 |
| DFS | |||
| Preoperative alpha-fetoprotein (continuous) | 1.00 | 1.00–1.00 | 0.002 |
| Presence of more than 1 HCC nodule in image exam (ref: no) | 3.14 | 1.20–8.18 | 0.019 |
OS, overall survival; DFS, disease-free survival; HCC, hepatocellular carcinoma; HR, hazard ratio; 95% CI, 95% confidence interval.
In Table 4, we simulated which therapy the BCLC guidelines (2010 and 2018) would have recommended for our patients who underwent resection. Figure 1 show the survival curves of patients who underwent resection according to the proposed treatment if BCLC 2010 and 2018 were followed, respectively, all divided into three groups: resection, other potentially curative therapies (transplantation or ablation), and palliative treatments (chemoembolization or systemic therapy).
Table 4
| Therapy recommendation | No. of patients | % |
|---|---|---|
| BCLC 2010 | ||
| Resection | 49 | 32.7 |
| Ablation | 18 | 12.0 |
| Transplantation | 13 | 8.7 |
| TACE | 43 | 28.7 |
| Systemic therapy | 27 | 18.0 |
| BCLC 2018 | ||
| Resection | 76 | 50.7 |
| Ablation | 28 | 18.7 |
| Transplantation | 6 | 4.0 |
| TACE | 13 | 8.7 |
| Systemic therapy | 27 | 18.0 |
BCLC, Barcelona Clinic Liver Cancer; TACE, transarterial chemoembolization.
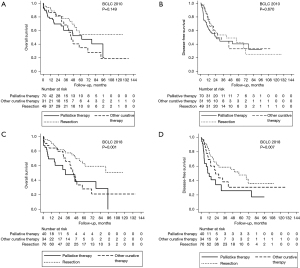
Analysis of the OS and DFS curves demonstrate that there was no significant difference between the survival of patients resected in accordance with BCLC 2010 and from those resected, but that BCLC would have contraindicated surgery (Figure 1A,1B). For the group of patients who underwent resection but would have undergone palliative therapies if the BCLC was applied, the median OS and DFS were 61.5 and 25.3 months, respectively; the 5-year OS was 52.2% and the SLD was 40.3%.
Analysis of the OS and DFS curves demonstrated that patients who underwent resection in accordance with BCLC 2018 guidelines had higher OS and DFS than those in whom the BCLC guidelines would have contraindicated resection (Figure 1C,1D). The median OS and DFS of patients who underwent resection but would have undergone systemic treatment or chemoembolization if the BCLC had been applied, were 32.5 and 12.2 months, respectively; the 5-year OS was 38.0% and the SLD was 25.7%.
The impact of the number of risk factors that are considered as contraindications to resection according to BCLC 2010 recommendations (portal system invasion, portal hypertension, and more than one nodule) on OS and DFS in our series are shown in Figure 2A,2B. Patients with only one risk factor had a median OS of 43.3 months, while those without risk factors had a median OS of 92.1 months. Patients with two risk factors (10 patients) had a shorter survival, with a median OS of 6.5 months, and a median DFS of 5.3 months. Despite an unfavorable prognosis in this group, 2 patients (20%) had 22 and 95 months of DFS, respectively, and 3 patients (30%) had an OS of >33 months. One patient presented with all three risk factors and had a short survival (OS of 17.3 months and DFS of 3.6 months).

Discussion
The treatment of HCC is complex because liver function, presence of portal hypertension, tumor staging, patient status performance, and organ availability should be considered. Liver resection, transplantation, and ablation therapies are considered curative treatments.
The BCLC classification is accepted as a good staging system and treatment algorithm for HCC, and has been adopted by the European Association for the Study of the Liver (EASL) and the American Association for the Study of Liver Diseases (AASLD) based on its ability to categorize patients according to prognosis, resulting in treatment recommendations (9,10). According to the BCLC (2010 and 2018) guidelines, liver resection is limited to patients with early-stage tumors without portal hypertension, which deprives patients with multiple nodules (up to three, if one is >3 cm) or large nodules (>5 cm on the 2010 version), with portal hypertension or vascular invasion from receiving curative treatments (6,10). In contrast, many reports since the beginning of the last decade have shown that selected patients undergoing liver resection for multinodular, large nodules with vascular invasion and portal hypertension seem to benefit from resection (7,8,11-15,20).
Thus, many expert centers have a more aggressive approach to HCC treatment in contrast to the very restrictive BCLC guidelines. In this study, we evaluated our results for HCC resection based on a more liberal indication from our group due to a long waiting list time (>9 months) for liver transplantation and the reported benefits of resection, especially from the Eastern groups.
In our single-center study, the criteria for resection since 2008 were less restrictive and included patients with one nodule independent of size, with or without vascular invasion, provided that the portal vein trunk was free, with preserved liver function, and MELD ≤10, without clinically significant portal hypertension, and a future liver remnant volume ≥40%.
Liver resection of multiple HCC nodules remains controversial (7,12,13,18). According to the BCLC guidelines, patients with more than one nodule should undergo a transplant (within the Milan criteria), ablation, or TACE (6). Indeed, in our series, the presence of two or three nodules negatively impacted DFS and OS. However, despite a worse prognosis following resection, Ruzzenente et al. (21) showed that patients with up to three nodules who underwent resection had a higher survival rate than those treated with ablation or TACE. Glantzounis et al. (12), in a systematic review, evaluated patients with multinodular HCC (up to 3 nodules vs. more than 3) and showed a median 5-year OS of 49% (range, 30–64%) when up to three nodules were present, and 23% (range, 0–54%) in patients with more than three nodules. These results suggest that for selected patients, resection of up to three HCC nodules could have long-term survival benefits. In our series, we performed resection of two or three nodules only in selected cases where the nodules were confined to the same liver section and outside the Milan criteria (non-transplantable cases). Of eight patients with more than one nodule, 3 (37.5%) had macroscopic portal vein invasion. The other cases could correspond to a multicentric occurrence of HCC. Survival was similar in both groups (median survival =18 months, with and without portal vein invasion).
In the first versions of the BCLC guidelines [1999–2010], resection was indicated only for patients with one nodule smaller than 5 cm; however, in subsequent versions, this restriction was withdrawn (6,22). Since the early 2000 s, our group did not consider tumor size for single nodules as a contraindication for resection; moreover, in our study, tumor size did not affect either OS or DFS, reinforcing this concept. Tumor size remains a concern for liver resection because it is related to an increased risk of macrovascular invasion and distant metastases (23); however, resection for selected cases can lead to long-term survival (11).
Portal vein invasion, a BCLC contraindication for curative therapies, is one of the strongest predictors of survival in patients with HCC due to an increased risk of intrahepatic or extrahepatic spread (6,23). However, in our series, macrovascular portal invasion did not affect OS and DFS, probably because we excluded patients with portal trunk invasion who underwent resection. In a systematic review of 29 studies that evaluated resection in patients with portal vein invasion (12), the 5-year OS rates were 45%, 19%, and 14.5% when second-order or beyond branches, first-order branches, and the main trunk were affected, respectively. Resection has a higher survival rate compared to non-surgical strategies or best supportive care (12,16). In a recent meta-analysis and systematic review, Ibrahim et al. showed that OS compared to TACE, resection in patients with HCC and portal vein tumor thrombosis, excluding those with portal vein trunk thrombosis, resulted in increased OS when compared to TACE (24).
There is no way to know the origin of other nodules in multinodular presentation of HCC, whether multicentric disease or due to portal invasion. However, both negatively impact prognosis. From 8 patients with more than 1 nodule, 3 (37.5%) had macroscopic portal vein invasion. Survival was similar in both groups (median survival =18 months, with and without portal vein invasion).
Beyond tumor-related factors, the presence of clinically significant portal hypertension has been considered a contraindication for resection by the BCLC guidelines because of the high risk of postoperative complications and lower long-term survival (6,10,22). In many centers, especially in Eastern countries, portal hypertension is not a contraindication for liver resection because major liver resections are avoided (13,20). Authors have reported mortality and morbidity rates from 0.5% to 4.5% and 38% to 43%, respectively, in patients with portal hypertension and from 0.0% to 6.1% and 34.8% to 38.5%, respectively, in those without portal hypertension. Considering the long-term outcomes, the 5-year OS rates following liver resection range from 48% to 51.5% in patients with portal hypertension and from 61.5% to 65% in patients without portal hypertension (7). In fact, in our series, only minor resections were performed in the presence of portal hypertension, and the presence of portal hypertension was associated with a shorter OS but did not affect DFS. Our results showed that the impact of portal hypertension is probably related to perioperative morbidity and mortality rates rather than oncological outcomes.
In our series, if the BCLC 2010 and 2018 recommendations were followed, 70 patients (46.7%) and 40 patients (26.7%), respectively, would not have undergone potentially curative treatment.
Palliative treatments for HCC can have a positive impact on patient survival. The overall survival of patients classified as BCLC B after chemoembolization was approximately 60%, 35%, and 15% at 1, 3, and 5 years, respectively (18,25,26). The median overall survival of patients with BCLC C treated with sorafenib, the systemic treatment recommended by the algorithm during the study period, is between 6 and 10 months (27-29). Comparative studies on HCC treatment are complex. In fact, only a few studies have compared palliative treatments with resection for intermediate-stage or even advanced HCC. Lu et al. recently showed that liver resection was superior to TACE for intermediate-stage HCC (30). The possibility of offering a potentially curative treatment for selected patients stimulated our group to adopt a more liberal approach for the indication of resection.
Despite an improvement in BCLC 2018 compared to 2010, a quarter of patients who are potentially suitable for resection would receive palliative therapy. Nevertheless, in our study, patients for whom the BCLC 2018 guidelines recommended palliative treatment had median OS and DFS of 32.5 and 12.2 months, respectively, following resection.
In contrast to the BCLC staging system, in our study, patients who had a single negative prognostic factor (among portal hypertension, portal vein invasion, and multiple nodules) had a median OS of 43.3 months, which justifies resection. However, although their OS were significantly lower than those of patients without risk factors, it still represents a good result, which is better than the outcomes of palliative treatments, as recommended by the BCLC guidelines for most of these patients.
The presence of these two negative factors had a significant negative impact on survival. Despite an unfavorable prognosis in this group, 2 patients (20%) from our series had 22 and 95 months of DFS, respectively, and three patients (30%) had an OS of >33 months. These findings highlight the possible benefits of resection in patients with advanced tumors.
Our study has some limitations including its retrospective nature, the limited number of patients, the small number of patients with two or three of the following criteria: multinodular disease, portal hypertension, and portal system invasion, and the absence of a comparative group of patients with HCC treated with other curative intent or palliative strategies.
Despite these limitations, our findings suggest that the presence of one risk factor should not be a contraindication to resection when liver function and status are preserved. For patients with two risk factors, resection should be considered in selected cases and favorable scenarios. The presence of all three factors is a contraindication for resection.
Conclusions
Our results showed that there is room for a more liberal indication of HCC resection, allowing potentially curative treatment for several patients who would receive palliative therapy or would be enrolled in a long waitlist for transplantation. The changes introduced in the BCLC guidelines in the past decade corroborate the more liberal indication of resection for HCC in accordance with many international expert groups, including ours (7-8,11-16,20,21). Although in its latest version (2022) the BCLC guidelines considers a more individualized decision, the guideline remains very restrictive for the indication of resection (20).
Acknowledgments
Funding: none.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-833/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-833/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-833/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-833/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics committee of Hospital das Clínicas, University of São Paulo School of Medicine, Brazil, with the number 05892819.4.0000.0068. Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chagas AL, Mattos AA, Carrilho FJ, et al. Brazilian society of hepatology updated recommendations for diagnosis and treatment of hepatocellular carcinoma. Arq Gastroenterol 2020;57:1-20. [Crossref] [PubMed]
- Yang JD, Hainaut P, Gores GJ, et al. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol 2019;16:589-604. [Crossref] [PubMed]
- Kim HS, El-Serag HB. The Epidemiology of Hepatocellular Carcinoma in the USA. Curr Gastroenterol Rep 2019;21:17. [Crossref] [PubMed]
- Zhang DY, Friedman SL. Fibrosis-dependent mechanisms of hepatocarcinogenesis. Hepatology 2012;56:769-75. [Crossref] [PubMed]
- Forner A, Gilabert M, Bruix J, et al. Treatment of intermediate-stage hepatocellular carcinoma. Nat Rev Clin Oncol 2014;11:525-35. [Crossref] [PubMed]
- Forner A, Reig ME, de Lope CR, et al. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis 2010;30:61-74. [Crossref] [PubMed]
- Guglielmi A, Ruzzenente A, Conci S, et al. Hepatocellular carcinoma: surgical perspectives beyond the barcelona clinic liver cancer recommendations. World J Gastroenterol 2014;20:7525-33. [Crossref] [PubMed]
- Herman P, Perini MV, Coelho FF, et al. Laparoscopic resection of hepatocellular carcinoma: when, why, and how? A single-center experience. J Laparoendosc Adv Surg Tech A 2014;24:223-8. [Crossref] [PubMed]
- Barman PM, Su GL. Limitations of the barcelona clinic liver cancer staging system with a focus on transarterial chemoembolization as a key modality for treatment of hepatocellular carcinoma. Clin Liver Dis (Hoboken) 2016;7:32-5. [Crossref] [PubMed]
- Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68:723-50. [Crossref] [PubMed]
- Chang YJ, Chung KP, Chang YJ, et al. Long-term survival of patients undergoing liver resection for very large hepatocellular carcinomas. Br J Surg 2016;103:1513-20. [Crossref] [PubMed]
- Glantzounis GK, Paliouras A, Stylianidi MC, et al. The role of liver resection in the management of intermediate and advanced stage hepatocellular carcinoma. A systematic review. Eur J Surg Oncol 2018;44:195-208. [Crossref] [PubMed]
- Guo H, Wu T, Lu Q, et al. Surgical resection improves long-term survival of patients with hepatocellular carcinoma across different Barcelona Clinic Liver Cancer stages. Cancer Manag Res 2018;10:361-9. [Crossref] [PubMed]
- Herman P, Lopes Fde L, Kruger JA, et al. Is resection of hepatocellular carcinoma in the era of liver transplantation worthwile? A single center experience. Arq Gastroenterol 2016;53:169-74. [Crossref] [PubMed]
- Liu PH, Lee YH, Hsia CY, et al. Surgical resection versus transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombosis: a propensity score analysis. Ann Surg Oncol 2014;21:1825-33. [Crossref] [PubMed]
- Glantzounis GK, Karampa A, Peristeri DV, et al. Recent advances in the surgical management of hepatocellular carcinoma. Ann Gastroenterol 2021;34:453-65. [Crossref] [PubMed]
- Choi JY, Lee JM, Sirlin CB. AMECT and MR imaging diagnosis and staging of hepatocellular carcinoma: part I. Development, growth, and spread: key pathologic and imaging aspects. Radiology 2014;272:635-54. [Crossref] [PubMed]
- Fonseca GM, de Mello ES, Faraj SF, et al. Prognostic significance of poorly differentiated clusters and tumor budding in colorectal liver metastases. J Surg Oncol 2018;117:1364-75. [Crossref] [PubMed]
- Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187-96. [Crossref] [PubMed]
- Yin L, Li H, Li AJ, et al. Partial hepatectomy vs. transcatheter arterial chemoembolization for resectable multiple hepatocellular carcinoma beyond Milan Criteria: a RCT. J Hepatol 2014;61:82-8. [Crossref] [PubMed]
- Ruzzenente A, Capra F, Pachera S, et al. Is liver resection justified in advanced hepatocellular carcinoma? Results of an observational study in 464 patients. J Gastrointest Surg 2009;13:1313-20. [Crossref] [PubMed]
- Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol 2022;76:681-93. [Crossref] [PubMed]
- Yokoo T, Patel AD, Lev-Cohain N, et al. Extrahepatic metastasis risk of hepatocel-lular carcinoma based on α-fetoprotein and tumor staging parameters at cross-sectional imaging. Cancer Manag Res. 2017;9:503-11. [Crossref] [PubMed]
- Ibrahim C, Parra N, Macedo FI, et al. Is hepatic resection better than transarterial chemoembolization in hepatocellular carcinoma with portal vein tumor thrombosis? J Gastrointest Oncol 2019;10:1064-72. [Crossref] [PubMed]
- Lin CW, Chen YS, Lo GH, et al. Comparison of overall survival on surgical resection versus transarterial chemoembolization with or without radiofrequency ablation in intermediate stage hepatocellular carcinoma: a propensity score matching analysis. BMC Gastroenterol 2020;20:99. [Crossref] [PubMed]
- Zhong JH, Xiang BD, Gong WF, et al. Comparison of long-term survival of patients with BCLC stage B hepatocellular carcinoma after liver resection or transarterial chemoembolization. PLoS One 2013;8:e68193. [Crossref] [PubMed]
- Hsiao P, Hsieh KC, Chen YS, et al. Sorafenib with concurrent multiple-line therapies improves overall survival in advanced stage hepatocellular carcinoma. Medicine (Baltimore) 2019;98:e16074. [Crossref] [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [Crossref] [PubMed]
- Choi GH, Shim JH, Kim MJ, et al. Sorafenib alone versus sorafenib combined with transarterial chemoembolization for advanced-stage hepatocellular carcinoma: results of propensity score analyses. Radiology 2013;269:603-11. [Crossref] [PubMed]
- Lu L, Zheng P, Wu Z, et al. Hepatic Resection Versus Transarterial Chemoembolization for Intermediate-Stage Hepatocellular Carcinoma: A Cohort Study. Front Oncol 2021;11:618937. [Crossref] [PubMed]


