
Low-dose apatinib combined with camrelizumab and the SOX regimen in the neoadjuvant treatment of locally advanced gastric/gastroesophageal junction adenocarcinoma (SPACE-neo): a protocol for an open-label, single-arm, clinical trial
Introduction
According to the latest global statistics, gastric cancer ranks fifth in terms of incidence and fourth in terms of mortality among all malignancies worldwide (1). In China, approximately 80% of newly diagnosed gastric cancers are in the advanced stage (2). Neoadjuvant chemotherapy has shown promising results in terms of pathological response rate (pRR) and survival rate in patients with locally advanced gastric cancer (LAGC) (3-8). In Europe, the FLOT4 trial confirmed that FLOT (docetaxel, oxaliplatin, leucovorin, and fluorouracil) is superior to ECF (epirubicin, cisplatin, and fluorouracil), and has become the standard perioperative treatment of LAGC. In contrast, the S-1 plus oxaliplatin (SOX) regimen has been recommended based on the results of the RESOLVE and RESONANCE trials in China (9). Although significant efforts have been made to improve the efficacy of perioperative chemotherapy, the pathological complete regression (pCR) rate is still only 16% (6).
Apatinib, an oral small molecule antagonist to vascular endothelial growth factor receptor (VEGFR)-2, was formally approved for the third and later-line treatment of advanced gastric cancer (AGC) (10). A phase II clinical study explored the addition of apatinib to the SOX regimen in a neoadjuvant setting in patients with LAGC (11). In this study, the pRR was 54.2%, the major pathological response (MPR) was 25%, and the pCR was 6.3%. Also, postoperative complications were observed in 18.4% of patients. Another similar study using chemotherapy plus apatinib as neoadjuvant therapy achieved a pRR of 89.7%, an objective response rate (ORR) of 79.3%, and a pCR of 13.8%, with 42.9% of patients experiencing surgery-related complications (12). These trials preliminarily demonstrated the efficacy and safety of the neoadjuvant combination of apatinib and the SOX regimen in LAGC.
The application of immunotherapy combined with chemotherapy has provided considerable progress in the treatment of AGC. Based on the CheckMate 649 trial, nivolumab combined with chemotherapy has been approved for a first-line all-population indication. This phase III trial demonstrated that nivolumab combined with FOLFOX (leucovorin, fluorouracil, and oxaliplatin) or CAPOX (capecitabine plus oxaliplatin) showed significantly longer overall survival (OS) in all patients (13.8 vs. 11.6 months, P=0.0002) as well as in patients with a programmed cell death-ligand-1 (PD-L1) combined positive score (CPS) of five or more compared with chemotherapy alone (14.4 vs. 11.1 months, P<0.0001) (13). The ORRs of the combined therapy and control groups were 60% and 45%, respectively. The ATTRACTION-4 trial also demonstrated the benefit of adding nivolumab to SOX or CAPOX as a first-line treatment; the ORRs in the experimental and control groups were 57.5% and 47.8% (P=0.0088), and the progression-free survival (PFS) was 10.45 and 8.34 months (P=0.0007), respectively. Based on these results, immune checkpoint inhibitors plus chemotherapy were tested in the neoadjuvant setting. A small-scale clinical study showed a pCR of 17.6%, MPR of 58.8%, and ORR of 82.4% after treatment with sintilimab combined with the FLOT regimen in LAGC (14,15). Similarly, neoadjuvant therapy with sintilimab and CAPOX showed a pCR of 23.1% and an MPR of 53.8% (16).
In addition, anti-angiogenic therapy can improve local hypoxia and chemotherapy resistance, and exert immunomodulatory properties to further enhance immunotherapy simultaneously. Anti-VEGFR and immune checkpoint inhibitor treatment exhibited a synergistic effect. This combination has been widely tested and proved effective in lung cancer, hepatic carcinoma, kidney cancer, melanoma, and so on (17,18). Another study assessed camrelizumab combined with CAPOX followed by camrelizumab plus apatinib as a first-line combination regimen for advanced gastric/gastroesophageal junction cancer (AGC/GEJC). The results showed encouraging antitumor activity and manageable toxicity (19).
Our team then carried out the SPACE study to assess the efficacy and safety of low-dose apatinib combined with camrelizumab and the SOX regimen as a first-line treatment of AGC/GEJC (20). The preliminary findings of four-drug combination showed an ORR of 94.7%, a disease control rate (DCR) of 100%, and a median PFS (mPFS) of 8.3 months. The incidence of grade 3–4 AEs was 45% and no new security signal was found, mainly including rash, elevated concentration of aminotransferase, and decreased leucocytes. Nevertheless, the SPACE study was conducted in patients with AGC, but the efficacy of LAGC patients is not yet known.
And considering the high ORR and the acceptable safety achieved in the SPACE study, we designed the SPACE-neo study with the expectation of longer survival rates and higher pCR.
It is hoped that through the four-drug combination, the tumor will shrink significantly, thereby improving the long-term efficacy after surgery. It is estimated that the study can achieve a higher MPR. It will also provide evidence regarding the efficacy and safety of low-dose apatinib combined with camrelizumab and the SOX regimen in the neoadjuvant treatment of LAGC/GEJC, which may be used as a candidate standard perioperative treatment. Moreover, the genomic, transcriptome, pathological, and immune microenvironment changes of tumor tissues and body fluids before and after treatment will also be carefully observed. Furthermore, molecular markers will also be screened to explore the mechanism of their influence on combination therapy. Thus, this study will further expand the proportion of patients who will obtain a advantage of neoadjuvant therapy and provide a foundation for large-scale, randomized, controlled, phase III clinical trials in the future.
Methods
Trial design
The SPACE-neo trial is a prospective, open-label, single-arm study. The study protocol was developed according to the Transparent Reporting of Evaluations with Nonrandomized Designs (TREND) (21). A flowchart showing the screening, interventions, assessments, and treatment follow-up procedures is shown in Table 1. The study protocol has been approved by the Ethics Committee at Pukou Branch Hospital of Jiangsu Province Hospital (Nanjing Pukou Central Hospital) (approval number: No. 2021-SR-034). The study will be performed in accordance with the Declaration of Helsinki (as revised in 2013), Good Clinical Practice, and related laws.
Table 1
| Procedures | Screening [V1 (-d14 to d0)] | C1 (V2) | C2 (V3) | C3 (V4) | Surgery (V5) | Postoperative treatment (Vn) | Follow-up of survival 1 (V101) | Follow-up of survival n (Vn01) |
|---|---|---|---|---|---|---|---|---|
| Enrolment | ||||||||
| Inclusion criteria | X | |||||||
| Exclusion criteria | X | |||||||
| Informed consent | X | |||||||
| Demographics | X | |||||||
| Prior cancer therapies | X | |||||||
| Surgery history of primary lesion | X | |||||||
| Tumor history | X | |||||||
| Comorbidities | X | |||||||
| Drug allergy | X | |||||||
| Pre-transfusion testing | X | |||||||
| Pregnancy test A | X | |||||||
| Interventions and assessments | ||||||||
| Vital signs | X | X | X | X | X | X | ||
| Physical examination | X | X | X | X | X | X | ||
| ECOG PS | X | X | X | X | X | X | ||
| QOL | X | X | X | X | X | X | ||
| Blood routine | X | X | X | X | X | X | ||
| Blood biochemistry | X | X | X | X | X | X | ||
| Urine routine B | X | X | X | X | X | X | ||
| Stool routine | X | X | X | X | X | X | ||
| Tumor markers | X | X | X | X | X | X | ||
| Thyroid function | X | X | X | X | X | X | ||
| Coagulation function | X | X | X | X | X | X | ||
| ECG C | X | X | X | X | X | |||
| Thoracoabdominal enhanced CT D | X | X | X | |||||
| Ultrasound gastroscopy | X | X | ||||||
| Concomitant medication | X | X | X | X | X | |||
| AEs | X | X | X | X | ||||
| SAEs | X | X | X | X | ||||
| Follow up | ||||||||
| Research summary | X | |||||||
| Follow-up of survival | X | X | ||||||
(I) ECOG PS, physical examination, QOL, blood routine, blood biochemistry, urine routine, stool routine, coagulation function, pregnancy test, ECG and other examinations must be collected within 1 week before medication. (II): (i) Pregnancy tests are required for woman of bearing age; (ii) Female patients do not need routine urine test during the menstrual period; (iii) ECG should be performed three times consecutively, with an interval of about 5 minutes each time, and the QTc interval should be marked. ECG should be performed before administration during the screening visit and after administration during treatment visit. Ultrasound cardiogram (UCG) should be supplemented for clinically significant ECG abnormalities. If patients feel precordial pain, palpitations or other symptoms, myocardial enzyme should be detected immediately; (iv) Thoracoabdominal enhanced CT and ultrasound gastroscopy should be performed during the screening period. Pelvic CT or MRI should be performed when pelvic metastases are suspected. Brain MRI should be performed when central nervous system metastases are suspected. Bone scan ECT should be performed when bone metastases are suspected. At the end of the second cycle of treatment and before the third cycle of treatment, thoracoabdominal enhanced CT and ultrasound gastroscopy will be performed for the efficacy evaluation and surgical judgment, and additional imaging scan will be performed when there exist clinical indications. C, the cycle of cancer treatment; V, visit; ECOG PS, Eastern Cooperative Oncology Group performance status; QOL, quality of life score; ECG, electrocardiograph; CT, computed tomography; AEs, adverse event; SAEs, severe adverse events; MRI, magnetic resonance imaging; ECT, emission computed tomography.
Trial setting and recruitment
We plan to conduct this trial at the First Affiliated Hospital of Nanjing Medical University (Jiangsu Province Hospital) in China from September 2021 to November 2024 and the study is ongoing.
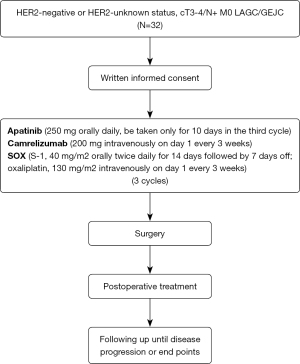
A total of 32 patients clinically staged as M0 and either T3 to T4 or N+ assessed by ultrasound endoscopy and thoracoabdominal enhanced computed tomography (CT) or magnetic resonance imaging (MRI) with HER2-negative or HER2-unknown LAGC/GEJC will be publicly recruited, mainly through online advertising by utilizing the advantages of audio-visual media. Each patient enrolled in this clinical study will be selected based on the inclusion and exclusion criteria. The first six patients are to be included in the safety induction period, and the remaining patients will be extended for treatment after the safety is confirmed. The overview of the trial is presented in Figure 1.
Eligibility criteria
Patients with histologically or cytologically confirmed HER2-negative or HER2-unknown and clinically staged as M0 and either T3 to T4 or N+ LAGC/GEJC are eligible for inclusion in this study. The main exclusion criteria are as follows: (I) patients diagnosed with other malignant tumors; or (II) those who had received previous systematic treatment. The inclusion and exclusion criteria are detailed in Table 2. Written consent from all of the participants will be obtained prior to study commencement. The informed consent is displayed in Appendix 1.
Table 2
| Criteria | Details |
|---|---|
| Inclusion criteria | |
| 1 | Aged 18–75 years (including 18 and 75) |
| 2 | Understand the research procedures and content, and voluntarily sign a written informed consent |
| 3 | HER2-negative or HER2-unknown LAGC/GEJC confirmed by histopathology or cytology and clinically staged M0 and either T3 to T4 or N+ assessed by ultrasound endoscopy and CT or MRI |
| 4 | No systematic treatment in the past |
| 5 | ECOG PS: 0–1 |
| 6 | Life expectancy ≥3 months |
| 7 | Adequate organ functions within 14 days before enrollment with the following criteria |
| (I) Hemoglobin level ≥80 g/L (no transfusions within 14 days) | |
| (II) Neutrophil count >1.5×109/L | |
| (III) Platelet count ≥100×109/L | |
| (IV) Total bilirubin ≤1.5× ULN | |
| (V) ALT or AST ≤2.5× ULN; if with liver metastasis, ALT or AST ≤5× ULN | |
| (VI) Endogenous creatinine clearance rate ≥60 mL/min (using the Cockcroft-Gault equation) | |
| (VII) Two-dimensional echocardiography: LVEF ≥50% | |
| 8 | Thyroid function index: TSH and FT3/FT4 within the normal range or shown slight alterations without clinical significance |
| 9 | Weight ≥40 kg, or BMI >18.5 |
| Exclusion criteria | |
| 1 | Other malignant tumors in the past or at the same time, not including early staged cancers having been cured (e.g., basal cell carcinoma of the skin and carcinoma in situ of the cervix), and early tumors (e.g., stage I lung cancer, stage I colorectal cancer) that have been judged by the investigator to have no impact on the patient's life in the short term after radical treatment |
| 2 | Participating in other clinical trials within 1 month |
| 3 | Factors influencing the oral administration of drugs (e.g., inability to swallow, chronic diarrhoea and bowel obstruction) |
| 4 | Bleeding events ≥ grade 3 using the CTCAE 5.0 criteria within 4 weeks prior to screening |
| 5 | With or with a history of CNS metastases. Performing CT or MRI examination for patients with clinically suspected CNS metastasis within 28 days before enrollment to exclude CNS metastasis |
| 6 | Hypertension cannot be well controlled by single antihypertensive therapy (systolic >140 mmHg, diastolic >90 mmHg); history of unstable angina pectoris; angor pectoris diagnosed within 3 months or myocardial infarction occurred within 6 months; arhythmia requiring long-term antiarrhythmic drugs therapy and NYHA ≥ grade II (including QTcF: male ≥450 ms, female ≥470 ms) |
| 7 | Long-term unhealed wounds or malunion of fracture |
| 8 | Invading the important blood vessel or at high risk of invading the important blood vessel based on imaging to cause fatal hemorrhage during treatment |
| 9 | Coagulation disorders or bleeding tendency (within 14 days prior to randomization, INR must be within the normal range without the use of anticoagulant); treated with anticoagulants or vitamin K antagonists such as warfarin, heparin, or analogues; permitting prophylactic use of low-dose warfarin (1 mg, po, qd) or low-dose aspirin (≤100 mg, po, qd) on the condition that the INR of PT ≤1.5 |
| 10 | Arteriovenous thrombosis events, such as cerebrovascular accident (including transient ischemic attack), deep venous thrombosis (except those who have recovered from venous thrombosis caused by intravenous catheterization during previous chemotherapy) and pulmonary embolism within 6 months |
| 11 | Urine protein ≥++, 24-hour urinary protein quantification >1.0 g |
| 12 | Prior immunotherapy and targeted therapy |
| 13 | History of acquired or congenital immunodeficiency disease, or organ transplantation (not including non-severe immune diseases like dermatitis, arthritis, psoriasis, etc.) |
| 14 | Infectious pneumonia, noninfectious pneumonia, interstitial pneumonia, and others requiring corticosteroids |
| 15 | History of severe chronic autoimmune diseases (e.g., SLE), inflammatory bowel diseases (e.g., UC, Crohn Disease), chronic diarrheal diseases (e.g., IBS), sarcoidosis or tuberculosis, active hepatitis B, hepatitis C or HIV (not including hepatitis B virus titer <2,000 copy/mL) |
| 16 | Allergy history to the human or mouse monoclonal antibodies |
| 17 | History of psychotropic drug abuse and cannot quitting or suffering from mental disturbance |
| 18 | Pleural or abdominal effusion with clinical symptoms requiring clinical intervention |
| 19 | Not following the doctor’s advice, not taking medicine as prescribed, or having incomplete information that will affect the judgment of efficacy or safety |
| 20 | Comorbidities seriously endangering patients’ safety or preventing patients from completing the study as judged by the investigator |
HER2, human epidermal growth factor receptor 2; LAGC, locally advanced gastric cancer; GEJC, gastroesophageal junction adenocarcinoma; CT computed tomography; MRI, magnetic resonance imaging; ECOG PS, Eastern Cooperative Oncology Group performance status; ULN, upper limit of normal; ALT, alanine aminotransferase; AST, aspartate aminotransferase; LVEF, left ventricular ejection fraction; TSH, thyroid-stimulating hormone; FT3, free triiodothyronine; FT4, free thyroxine; BMI, body mass index; CTCAE 5.0, Common Terminology Criteria for Adverse Events version 5.0; CNS, central nervous system; QTcF, interval corrected by Fridericia’s formula; INR, international normalized ratio; PT, prothrombin time; SLE, systemic lupus erythematosus; UC, ulcerative colitis; IBS, irritable bowel syndrome; HIV, human immunodeficiency virus.
Intervention
Patients will receive three cycles of apatinib (250 mg orally daily) (Jiangsu Hengrui Pharmaceuticals Co., Ltd., Shanghai, China) combined with camrelizumab (200 mg intravenously on day 1 every 3 weeks) (Jiangsu Hengrui Pharmaceuticals Co., Ltd, Shanghai, China) plus SOX (S-1, 40 mg/m2 orally twice daily for 14 days followed by 7 days off; oxaliplatin, 130 mg/m2 intravenously on day 1 every 3 weeks) (Jiangsu Hengrui Pharmaceuticals Co., Ltd., Shanghai, China) as neoadjuvant treatment. Intravenous medication will be administered by the paramedic at the First Affiliated Hospital of Nanjing Medical University (Jiangsu Province Hospital). Postoperative treatment will be selected based on the pathological results and current treatment guidelines. Notably, oral apatinib will be used only for 10 days during the last cycle and discontinued at least 10 days before surgery to reduce the influence of apatinib on the surgery.
Imaging and ultrasound gastroscopy will be performed at baseline and during the second or third cycle of neoadjuvant therapy for safety and efficacy evaluation. Based on the multi-disciplinary treatment (MDT)-directed discussions, the patient will continue the regimen for another 1–2 cycles after evaluation, considering the low possibility of radical surgical resection and the likelihood of sustained shrinkage of lesions compared with the baseline. During the trial, drugs can be suspended or terminated based on the incidence of drug-related toxic and side effects. In the event of significant clinical adverse events (AEs), investigational drugs should be suspended and patients should be actively treated.
Outcomes
The primary endpoints of the study are MPR and safety. (I) MPR is defined as the proportion of patients whose residual tumor cells make up less than 10% of the primary tumor from among the total cohort of resectable gastric cancer patients after neoadjuvant therapy, including Becker tumor regression grading (TRG) 1a and 1b. Grade 1a is defined as 0% residual tumor and Grade 1b is defined as <10% residual tumor per tumor bed (22). (II) AEs refer to adverse medical events occurring in patients after receiving a drug or regimen, which are not necessarily caused by the treatment. Severe adverse events (SAEs) refer to medical events that occur during treatment, which require hospitalization or prolong the hospitalization time of patients, affect their ability to work, or threaten their lives, resulting in congenital malformations, disability, or death. AEs shall be evaluated in each patient according to the Common Terminology Criteria for Adverse Events version 5.0 (CTCAE 5.0). The total number of patients with AEs will be taken as the numerator when calculating the incidence of AEs, and the total number of patients used to evaluate safety will be taken as the denominator. The proportion of patients whose surgery would be delayed more than 30 days or cannot be performed altogether due to treatment-related AEs will also be calculated.
The secondary endpoints are the R0 resection rate, disease-free survival (DFS), and OS. (I) The R0 resection rate refers to the proportion of patients with surgically resectable gastric cancer and margin-negative resection after three treatment cycles using the regimen from among the total patients enrolled. (II) DFS is defined as the time from randomization to recurrence or death due to recurrence. (III) OS is defined as the duration from the date of enrollment to death due to any cause or to the last follow-up appointment for surviving patients.
The exploratory study endpoints are as follows: (I) genetic mutations in the baseline and postoperative tumor tissue and blood; (II) paired pre- versus post-treatment single-cell RNA transcriptome (scRNA-seq); (III) tumor mutational burden (TMB); (IV) metabonomics; (V) circulating tumor deoxyribonucleic acid (ctDNA); (VI) changes in the immune microenvironment; and (VII) the relationship between specific T-cell subsets and the therapeutic effect.
Data collection and management
All data from screening, treatment, and follow-up visits will be recorded in the electronic Case Report Form (eCRF). The eCRF will be updated in real-time using an online sharing mode for authorized personnel. To minimize the drop out of subjects, patients will be recruited from among the outpatients and inpatients. In addition, we will collect the phone numbers and WeChat IDs of patients and their family members and keep regular contact with them.
Screening visit
Screening visits will be conducted within 2 weeks before the first cycle of treatment. The contents and details of data collection will include demographic data, tumor history, primary lesion surgical history, comorbidity history, history of drug allergy, vital signs, physical examination, blood routine, blood biochemistry, Eastern Cooperative Oncology Group Performance Status (ECOG PS) score, quality of life (QOL), and other examinations, as well as the imaging data at baseline, the pathological and immunohistochemical results of ultrasonic gastroscopy, and genetic test results (see Table 2).
Treatment visit
Patients will underwent repeat vital signs, physical examination, ECOG PS, QOL, blood routine, blood biochemistry and other examinations before every cycle. All treatments and medications used in combination within 30 days prior to initiation of treatment and during the study should be documented in the eCRF. The same imaging recognition scanner will be used for the same patient over time and all imaging data will be retained. Imaging data after treatment, pathological and immunohistochemical results of ultrasonic gastroscopy and surgical specimens should be collected. Blood and stool specimens will be collected at baseline and after treatment for the detection of exploratory indicators (see “The exploratory study endpoints” in Outcomes).
The planning to collect AEs-related data is always performed as follows. Follow-up for safety will be performed once a month (plus or minus seven days) for three consecutive times. The first time must be performed in the study center, and the second and third times can be followed by telephone to track the remission of AEs. Safety-related data will be collected from the time when the patient signs the informed consent to 30 days after surgery or 28 days after the last treatment. For assessment of safety, all AEs and SAEs during the clinical study, including abnormal clinical symptoms and vital signs, abnormal laboratory examinations, and clinical features, severity, occurrence time, duration, treatment, prognosis of them will be recorded in the eCRF. Abnormal laboratory tests will be repeated weekly until the parameters revert back to normal range. Surgery-related AEs include bleeding, infection, delayed wound healing, anastomotic fistula and so on. The correlation between AEs and drugs will also be determined.
Follow-up visit
Patients will enter post-treatment follow-up period after the last treatment. Follow-up will be performed every 3 months (plus or minus fourteen days) at the follow-up period. AEs, SAEs and date of disease progression or death occurred in the study should be recorded during follow-up. Patients out of the group due to disease progression should be followed for survival-related data. Survival information could be collected through telephone, including date and cause of death and information after the end of study treatment until death or loss to follow-up.
Quality assurance
All researchers will receive training programs arranged by the clinical trial institution and work under the guidance of senior professionals. The investigators will follow the standard operating procedures and monitors will check the eCRF and supervise the conduct of the clinical trial to ensure that all data are recorded and reported correctly. ECRF will be filled truthfully, correctly, and consistently with the original data. In accordance with local law, it is recommended that researchers should preserve documents for at least 5 years after completion or suspension of the study. Information about patients involved in the study is confidential and shall not be disclosed to any third party, and the protocol content is used only to conduct the study.
Statistical analysis
Sample size
Based on previous data, it is assumed that the MPR rate used as the main evaluation criterion of intervention is 40%. To achieve such accuracy, the significance level for the confidence interval (CI) is set to 0.9 and the CI width is set to 0.3. A sample size of 29 patients is expected to be needed. Considering the 10% shedding rate, a total of 32 patients are required. The sample size calculation was conducted using PASS 15 Power Analysis and Sample Size Software 2017 (NCSS, LLC. Kaysville, Utah, USA, ncss.com/software/pass).
Basic characteristics of the included patients
Measurement data such as age will be presented as the mean, standard deviation, median, minimum, maximum, and interquartile range (IQR). Qualitative data such as gender and ECOG PS will be presented as the frequency and percentage.
Efficiency analysis
MPR is the main curative effect index and the Clopper-Pearson method will be used to estimate the 95% CI of the MPR. The Kaplan-Meier method will be applied to draw DFS and OS curves and estimate the DFS and OS as well as their 95% CIs. Multivariate hierarchical survival analysis will be conducted via the COX regression model. No confirmatory statistical analyses will be performed. Multivariate survival analysis will be performed using the COX regression model to analyze multiple factors associated with efficacy, including gender, age, clinical stage, pathological classification, etc. The objective is to study the relationship between these factors and efficacy and prognosis. We will impute missing primary endpoints with random hot deck multiple imputation method using a regression based approach. Clopper-Pearson method by hot deck imputation method will be used to estimate the 95% CI of the MPR. The Kaplan-Meier method by hot deck imputation method will be used to estimate the 95% CI of the DFS and OS. All statistical tests will be evaluated using two-tailed 95% CI and tests with a P value <0.05 will be considered statistically significant.
Safety evaluation
Safety data will be presented in terms of descriptive statistical analysis, which lists the incidence, severity, association, risk, and outcome of AEs in the study. We will describe the normal pre-test and abnormal post-test laboratory results and their relationship to the experimental drug.
Single-cell RNA-sequencing data analysis
Data will be analyzed using Seurat version 3 (http://satijalab.org/seurat/). Single-cell copy number variation (CNV) analysis will be performed using inferCNV (23). Trajectory inference analysis will be conducted with SlingShot (24). TradeSeq (25) will be used to calculate the differentially expressed genes between the trajectories inferred by SlingShot. We will use CellRanger version 7 (https://www.10xgenomics.com/products), the velocyto.py package, and the scVelo package for the RNA velocity analysis. The CellPhoneDB algorithm will be used to infer the cell-to-cell interactions. Gene set variation analysis (GSVA) will be used to calculate gene set scores per cell, and limma will be applied to identify significantly enriched gene sets. The hallmark (referred to as “H”) and Gene Ontology (GO: “C5”) gene sets will be used from the MSigDB and exported using GSEABase version 1.60.0 (26). Receiver operating characteristic (ROC) curve analyses will be carried out using the R package Proc.
Harms
SAEs of special concern in the clinical trial should be reported within 24 hours to the study sponsor, research unit, ethics committee, China Food and Drug Administration (CFDA) and Jiangsu Medical Products Administration. SAEs include grade ≥3 infusion reaction, grade ≥2 diarrhea/colitis, uveitis and interstitial pneumonia, grade ≥3 other immune-related AEs, any events of abnormal liver enzymes (which lack evidence of other associated etiology, such as disease progression, acute viral hepatitis, cholestasis, combined drugs, or previous liver disease), grade ≥4 elevation in amylase or lipase. In principle, the suspension of camrelizumab should be taken as the main method according to the severity of AEs. When the SAEs are recovered to grade 1 or lower, the use of camrelizumab can be considered again. When severe grade 3 or life-threatening grade 4 AEs occur, camrelizumab should be permanently discontinued. Management of immune-related AEs should be in accordance with the institution's medical practice and guidelines.
Discussion
The clinical stage at the time of diagnosis directly determines the prognosis of patients with gastric cancer. Patients with localized, early-stage gastric cancer usually have a high 5-year OS rate (>60%), whereas the 5-year OS rate of patients with local and distant metastases dramatically decreases to 30% and 5%, respectively (27). Neoadjuvant therapy can degrade the tumor stage and improve the R0 resection rate and has been increasingly used in clinical practice.
The SPACE-neo study aims to evaluate the efficacy and safety of SOX with concomitant low-dose apatinib and camrelizumab as a neoadjuvant regimen for patients with LAGC/GEJC.
In 2020, our team carried out a dose escalation and extension phase Ib clinical trial (SPACE) on the first-line treatment of potentially resectable and initially unresectable AGC/GEJC with low-dose apatinib combined with camrelizumab and the SOX regimen (20). At first, all patients received this combined regimen (3 weeks/cycle) for a maximum of eight cycles, followed by apatinib and camrelizumab as maintenance therapy until discontinuation for any reason. The primary endpoint of the study was the maximum tolerated dose (MTD) and the ORR. By November 30, 2021, the safety and efficacy were assessed in 19 evaluable patients; the preliminary results demonstrated an ORR of 94.7%, a DCR of 100%, and an mPFS of 9.0 months. The incidence of grade 3–4 AEs was 45%, mainly including rash, elevated aminotransferase, and decreased leucocytes (28). According to the dose exploration in the study, we concluded that apatinib 250 mg combined with camrelizumab 200 mg plus oxaliplatin 130 mg/m2 and S-1 40 mg is an appropriate recommended dosing regimen. At these doses, the patients tolerated the treatment well and the adverse reactions were controllable. Considering the high ORR, DCR, and appropriate drug dose in the SPACE study, in addition to the safety and efficacy of SOX in combination with apatinib in AGC/GEJC, we will apply this dosing regimen to neoadjuvant therapy in the SPACE-neo study.
The primary endpoint of the study is the MPR. MPR, which is strongly associated with an improved DFS and treatment effect, is widely used in neoadjuvant studies. Using MPR as the primary endpoint rather than the survival index can reduce the impact of surgery and postoperative chemotherapy on the endpoint. Furthermore, it will also take less time to reach this primary endpoint. Therefore, the use of the MPR as a surrogate endpoint for survival in neoadjuvant therapy trials is supported (29). However, an important limitation of the use of the MPR is the inability to access the long-term effect of treatment-related AEs. In general, the MPR is an appropriate endpoint for a small-scale study. Based on previous study, we assumed that the MPR is 40% (30).
Another highlight of this study is the paired pre- versus post-treatment scRNA-seq. Currently, the efficacy markers of immunotherapy for gastric cancer under study include PD-L1, deficient mismatch repair/microsatellite instability-high (dMMR/MSI-H), tumor mutational burden-high (TMB-H), Epstein-Barr virus (EBV), ctDNA mutational burden scores, and so on. In general, dMMR/MSI-H is a recognized therapeutic marker for gastric cancer, while the other markers are uncertain and even PD-L1 is controversial (31). Single-cell sequencing can detect changes in the immune microenvironment after drug treatment from the perspective of single cells. This may help to reveal the mechanism of treatment effectiveness or drug resistance and lay the foundation for further improvement of the therapeutic efficacy. Therefore, tumor biopsies will be collected before and after neoadjuvant therapy. Using established protocols for scRNA-seq, high-quality data from the pre- and post-treatment biopsies of patients will be obtained to profile a blueprint of the heterogeneous tumor microenvironment (32-34).
In summary, the study aims to evaluate the safety and prognosis of the SOX regimen combined with low-dose apatinib and camrelizumab as a neoadjuvant regimen for patients with LAGC/GEJC to provide more optimized treatment strategies for these patients.
Trial status
The trial is registered in ChiCTR.gov.cn (registration number: ChiCTR2100049305, named SPACE-neo) (“Trial registration data” see “Table 3”). It is expected to last for 27 months. As of July 11, 2022, the study has recruited 19 patients. The first patient was enrolled in September 2021. The survival follow-up data is expected to be completed by December 2023 and the time of database lock is scheduled before November 2024.
Table 3
| Data category | Information |
|---|---|
| Primary registry and trial identifying number | ChiCTR.gov.cn; ChiCTR2100049305 |
| Date of registration in primary registry | 29 July, 2021 |
| Secondary identifying numbers | N/A |
| Source(s) of monetary or material support | Collaborative Innovation Center for Cancer Personalized Medicine (award number: N/A) |
| Primary sponsor | Jiangsu Province Hospital (the First Affiliated Hospital of Nanjing Medical University) |
| Contact for public queries | Xiaofeng Chen [chenxiaofengnjmu@163.com] |
| Contact for scientific queries | Xiaofeng Chen [chenxiaofengnjmu@163.com] |
| Public title | Space-neo study: a single arm study of apatinib combined with camrelizumab and SOX regimen in neoadjuvant treatment of local advanced gastric/gastroesophageal junction adenocarcinoma |
| Scientific title | Space-neo study: a single arm study of apatinib combined with camrelizumab and SOX regimen in neoadjuvant treatment of local advanced gastric/gastroesophageal junction adenocarcinoma |
| Countries of recruitment | China |
| Health condition(s) or problem(s) studied | Anticancer treatment |
| Intervention(s) | Apatinib (250 mg orally daily) combined with camrelizumab (200 mg intravenously on day 1 every 3 weeks) plus SOX (S-1, 40 mg/m2 orally twice daily for 14 days followed by 7 days off; oxaliplatin, 130 mg/m2 intravenously on day 1 every 3 weeks) |
| Key inclusion and exclusion criteria | Key inclusion criteria: aged 18–75 years (including 18 and 75); HER2-negative or HER2-unknown local advanced gastric/gastroesophageal junction adenocarcinoma confirmed by histopathology or cytology and clinically staged M0 and either T3 to T4 or N+ assessed by ultrasound endoscopy and CT or MRI |
| Key exclusion criteria: Other malignant tumors in the past or at the same time, not including early staged cancers having been cured; with or with a history of central nervous system metastases; prior immunotherapy and targeted therapy | |
| Study type | Interventional, open-label and single-arm study. Primary purpose: the SPACE-neo study aims to evaluate the safety and prognosis of the regimen |
| Date of first enrolment | September 2021 |
| Target sample size | The trial plans to enroll thirty-two patients in total |
| The trial has enrolled nineteen patients | |
| Recruitment status | Recruiting |
| Primary outcome(s) | Main pathological remission rate (MPR) and safety |
| Key secondary outcomes | The secondary endpoints are R0 resection rate, DFS, OS |
N/A, not applicable; SOX, S-1 plus oxaliplatin; CT, computed tomography; MRI, magnetic resonance imaging; DFS, disease-free survival; OS, overall survival.
Acknowledgments
Funding: The study was funded by the Collaborative Innovation Center for Cancer Personalized Medicine (Xiaofeng Chen), Pukou Branch Hospital of Jiangsu Province Hospital (Nanjing Pukou Central Hospital) General Project of Science and Technology Development Fund in 2021 (No. KJ2021-22 to Xiaofeng Chen), Pukou District Social Cause Science and Technology Development Project in 2020 (No. S2020-21 to Xiaofeng Chen), and Pukou Branch Hospital of Jiangsu Province Hospital (Nanjing Pukou Central Hospital) Major Project of Science and Technology Development Fund in 2021 (No. KJ2021-1 to Xiaofeng Chen).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1158/coif). XC report that this study was funded by the Collaborative Innovation Center for Cancer Personalized Medicine, Pukou District Social Cause Science and Technology Development Project in 2020 (No. S2020-21), Pukou Branch Hospital of Jiangsu Province Hospital (Nanjing Pukou Central Hospital) General Project of Science and Technology Development Fund in 2021 (No. KJ2021-22), and Pukou Branch Hospital of Jiangsu Province Hospital (Nanjing Pukou Central Hospital) Major Project of Science and Technology Development Fund in 2021 (No. KJ2021-1). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol has been approved by the Ethics Committee at Pukou Branch Hospital of Jiangsu Province Hospital (Nanjing Pukou Central Hospital) (approval number: No. 2021-SR-034). The study will be performed in accordance with the Declaration of Helsinki (as revised in 2013), Good Clinical Practice, and related laws. Written consent from all of the participants will be obtained prior to study commencement.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Yang L, Zheng R, Wang N, et al. Incidence and mortality of stomach cancer in China, 2014. Chin J Cancer Res 2018;30:291-8. [Crossref] [PubMed]
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 2011;29:1715-21. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Al-Batran SE, Hofheinz RD, Pauligk C, et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol 2016;17:1697-708. [Crossref] [PubMed]
- Zhang X, Liang H, Li Z, et al. Perioperative or postoperative adjuvant oxaliplatin with S-1 versus adjuvant oxaliplatin with capecitabine in patients with locally advanced gastric or gastro-oesophageal junction adenocarcinoma undergoing D2 gastrectomy (RESOLVE): an open-label, superiority and non-inferiority, phase 3 randomised controlled trial. Lancet Oncol 2021;22:1081-92. [Crossref] [PubMed]
- Wang X, Li S, Xie T, et al. Early results of the randomized, multicenter, controlled evaluation of S-1 and oxaliplatin as neoadjuvant chemotherapy for Chinese advanced gastric cancer patients (RESONANCE Trial). J Clin Oncol 2020;38:abstr 280.
- Wang FH, Zhang XT, Li YF, et al. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun (Lond) 2021;41:747-95. [Crossref] [PubMed]
- Xu Z, Hu C, Yu J, et al. Efficacy of Conversion Surgery Following Apatinib Plus Paclitaxel/S1 for Advanced Gastric Cancer With Unresectable Factors: A Multicenter, Single-Arm, Phase II Trial. Front Pharmacol 2021;12:642511. [Crossref] [PubMed]
- Lin JX, Xu YC, Lin W, et al. Effectiveness and Safety of Apatinib Plus Chemotherapy as Neoadjuvant Treatment for Locally Advanced Gastric Cancer: A Nonrandomized Controlled Trial. JAMA Netw Open 2021;4:e2116240. [Crossref] [PubMed]
- Zheng Y, Yang X, Yan C, et al. Effect of apatinib plus neoadjuvant chemotherapy followed by resection on pathologic response in patients with locally advanced gastric adenocarcinoma: A single-arm, open-label, phase II trial. Eur J Cancer 2020;130:12-9. [Crossref] [PubMed]
- Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 2021;398:27-40. [Crossref] [PubMed]
- Li N, Li Z, Fu Q, et al. Phase II study of sintilimab combined with FLOT regimen for neoadjuvant treatment of gastric or gastroesophageal junction (GEJ) adenocarcinoma. Ann Oncol 2020;31:abstr 216.
- Kang YK, Chen LT, Ryu MH, et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2022;23:234-47. [Crossref] [PubMed]
- Jiang H, Yu X, Kong M, et al. Sintilimab plus oxaliplatin/capecitabine (CapeOx) as neoadjuvant therapy in patients with locally advanced, resectable gastric (G)/esophagogastric junction (GEJ) adenocarcinoma. J Clin Oncol 2021;39:abstr 211.
- Zhao S, Ren S, Jiang T, et al. Low-Dose Apatinib Optimizes Tumor Microenvironment and Potentiates Antitumor Effect of PD-1/PD-L1 Blockade in Lung Cancer. Cancer Immunol Res 2019;7:630-43. [Crossref] [PubMed]
- Liu J, Liu Q, Li Y, et al. Efficacy and safety of camrelizumab combined with apatinib in advanced triple-negative breast cancer: an open-label phase II trial. J Immunother Cancer 2020;8:e000696. [Crossref] [PubMed]
- Peng Z, Wei J, Wang F, et al. Camrelizumab Combined with Chemotherapy Followed by Camrelizumab plus Apatinib as First-line Therapy for Advanced Gastric or Gastroesophageal Junction Adenocarcinoma. Clin Cancer Res 2021;27:3069-78. [Crossref] [PubMed]
- Wang KX, Cui TY, Yang XD, et al. Study on Efficacy and Safety of Low-Dose Apatinib Combined with Camrelizumab and SOX Regimen as First-Line Treatment of Locally Advanced and Unresectable Gastric/Gastroesophageal Junction Cancer: A Protocol for an Open-Label, Dose Escalation and Extension Phase Ib Clinical Trial. Onco Targets Ther 2021;14:4859-65. [Crossref] [PubMed]
- Des Jarlais DC, Lyles C, Crepaz N, et al. Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: the TREND statement. Am J Public Health 2004;94:361-6. [Crossref] [PubMed]
- Becker K, Mueller JD, Schulmacher C, et al. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer 2003;98:1521-30. [Crossref] [PubMed]
- Patel AP, Tirosh I, Trombetta JJ, et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 2014;344:1396-401. [Crossref] [PubMed]
- Street K, Risso D, Fletcher RB, et al. Slingshot: cell lineage and pseudotime inference for single-cell transcriptomics. BMC Genomics 2018;19:477. [Crossref] [PubMed]
- Van den Berge K, Roux de Bézieux H, Street K, et al. Trajectory-based differential expression analysis for single-cell sequencing data. Nat Commun 2020;11:1201. [Crossref] [PubMed]
- Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 2005;102:15545-50. [Crossref] [PubMed]
- Thrift AP, El-Serag HB. Burden of Gastric Cancer. Clin Gastroenterol Hepatol 2020;18:534-42. [Crossref] [PubMed]
- Chen X, Ding Y, Wang D, et al. Camrelizumab plus low-dose apatinib and SOX in the first-line treatment of advanced gastric/gastroesophageal junction (G/GEJ) adenocarcinoma: A single-arm, dose-escalation and expansion study. J Clin Oncol 2022;40:e16053. [Crossref]
- Hellmann MD, Chaft JE, William WN Jr, et al. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol 2014;15:e42-50. [Crossref] [PubMed]
- Cunningham D, Stenning SP, Smyth EC, et al. Peri-operative chemotherapy with or without bevacizumab in operable oesophagogastric adenocarcinoma (UK Medical Research Council ST03): primary analysis results of a multicentre, open-label, randomised phase 2-3 trial. Lancet Oncol 2017;18:357-70. [Crossref] [PubMed]
- Fuchs CS, Doi T, Jang RW, et al. Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol 2018;4:e180013. Erratum in: JAMA Oncol 2019;5:579. [Crossref] [PubMed]
- Lambrechts D, Wauters E, Boeckx B, et al. Phenotype molding of stromal cells in the lung tumor microenvironment. Nat Med 2018;24:1277-89. [Crossref] [PubMed]
- Qian J, Olbrecht S, Boeckx B, et al. A pan-cancer blueprint of the heterogeneous tumor microenvironment revealed by single-cell profiling. Cell Res 2020;30:745-62. [Crossref] [PubMed]
- Wauters E, Van Mol P, Garg AD, et al. Discriminating mild from critical COVID-19 by innate and adaptive immune single-cell profiling of bronchoalveolar lavages. Cell Res 2021;31:272-90. [Crossref] [PubMed]
(English Language Editor: A. Kassem)


