
Efficacy and safety of camrelizumab plus chemotherapy versus chemotherapy alone in patients with untreated, HER2-negative, unresectable locally advanced, or metastatic gastric cancer or gastroesophageal junction cancer: a retrospective comparative cohort study
Highlight box
Key findings
• Compared with chemotherapy alone, camrelizumab combined with chemotherapy has achieved encouraging efficacy and tolerable toxicity in the first-line treatment of HER2-negative, unresectable locally advanced, or metastatic gastric cancer (GC).
What is known and what is new?
• Oxaliplatin combined with fluoropyrimidine is the main first-line regimen for GC. Nivolumab combined with chemotherapy has also become one of options;
• Compared with chemotherapy alone, camrelizumab combined with chemotherapy showed numerical advantages in terms of ORR, DCR, and PFS, with a tolerable safety profile.
What is the implication, and what should change now?
• This provides a new option for first-line treatment of GC/GEJ and offers the possibility of screening the beneficiary population according to NLR.
Introduction
Gastric cancer (GC) remains prevalent worldwide, of which there were over 1 million new cases in 2020 and an estimated 769,000 deaths (equating to one in every 13 deaths globally), ranking fifth for incidence and fourth for mortality globally (1). Over 70% of GC patients are diagnosed at an advanced stage and have a low 5-year survival rate (2). In inoperable advanced GC, the median overall survival (OS) for patients receiving chemotherapy alone is less than a year (3). Patients with advanced GC are often weaker and find it challenging to tolerate multiple rounds of treatment due to impaired digestive and absorption functions. Therefore, first-line treatment is an opportunity to achieve a better outcome, which emphasizes its importance.
The main first-line treatment option for human epidermal growth factor receptor 2 (HER-2) negative unresectable, locally advanced, recurrent, or metastatic GC is oxaliplatin plus fluoropyrimidine (fluorouracil or capecitabine) (4). As the clinical benefit was shown to be more significant, nivolumab in combination with chemotherapy is recommended for patients with programmed cell death ligand 1 (PD-L1) combined positive score (CPS) (5). Camrelizumab, a humanized, selective IgG4-κ monoclonal antibody against programmed cell death 1 (PD-1), exerts an antitumor effect in many tumors (6,7). It is currently believed that the main structure of the various PD-1 inhibitors is similar, with the main difference being in the site of drug binding site, resulting in phenotypic differences in the clinical efficacy and safety of the different PD-1 inhibitors. Although there is no indication for camrelizumab in GC, camrelizumab in combination with chemotherapy in first-line therapy of GC followed by camrelizumab plus apatinib maintenance therapy achieved favorable results in phase II study (7). There has also been recent clinical study (8) of neoadjuvant camrelizumab plus concurrent chemoradiotherapy exhibits promising pathological response in patients with locally advanced gastric adenocarcinoma, with an acceptable safety profile.
Successful clinical outcomes of the addition of immune checkpoint inhibitors (ICIs) to chemotherapy have been reported in many cancers, such as non-small cell lung cancer (NSCLC) (9), small cell lung cancer (10,11), head and neck tumor (12), and GC (13,14), among others. Potential mechanisms underlying the synergistic effects of the combination regimen include induction of immunogenic tumor cell death, anti-angiogenesis, selective depletion of myeloid immunosuppressive cells, and lymphocytopenia, particularly reduction of regulatory T cells to make room for effector T cell proliferation (15). However, whether different PD-1 inhibitors can all be used in combination with chemotherapy to treat GC is still in an exploratory phase.
Biomarkers for predicting the efficacy of immunotherapy in GC include microsatellite instability (MSI) (16,17), PD-L1 (18), tumor mutational burden (TMB) (19), Epstein-Barr virus (EBV) (20), and other tests, which are now being applied in the clinic to facilitate screening for beneficiary patients. Although research on molecular typing of GC has shown that the immune microenvironment of a proportion of GC patients may be well suitable for immunotherapy, such as patients with MSI type and EBV infection type (21), the proportion of these patients is limited. Identifying more patients who would benefit from immunotherapy is an important challenge for GC treatment. During the treatment with ICIs, dynamic changes in systemic inflammation and immune status occur. A higher baseline neutrophil-lymphocyte ratio (NLR) has been associated with lower OS in patients with malignant melanoma or NSCLC (18-22). It has been reported that elevated pretreatment NLR is significantly associated with inferior progression-free survival (PFS) and OS in patients with metastatic GC who received PD-1 inhibitors (22). Still, there is no uniformity regarding the cut-off values and calculation methods for NLR.
Here we conducted a retrospective study to compare the efficacy and safety of camrelizumab plus chemotherapy with chemotherapy alone as first-line therapy for patients with HER2-negative, unresectable advanced or metastatic GC. Furthermore, we analyzed biomarkers such as PD-L1 CPS and NLR to screen for a beneficiary population. We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1229/rc).
Methods
Study design and patients
This was a single-center retrospective observational cohort study performed in the Department of Oncology, The Affiliated Yantai Yuhuangding Hospital of Qingdao University, Shandong, China. Patients were included if they met the inclusion criteria: HER-2 negative, unresectable locally advanced, recurrent or metastatic gastric or gastroesophageal junction cancer; Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 1; measurable lesions according to Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 criteria; treated with the first-line treatment of camrelizumab plus S-1 plus oxaliplatin (SOX)/capecitabine plus oxaliplatin (CapeOX) or SOX/CapeOX followed by S-1/capecitabine plus camrelizumab or S-1/capecitabine maintenance therapy from December 2020 to April 2022. Clinical baseline factors assessed included: age, gender, ECOG score, target lesion location, stage, number of metastases, previous treatment, EBV, PD-L1 expression, MSI, NLR and combined chemotherapy regimen. The above clinical information is mainly collected through medical records and followed up by telephone. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of The Affiliated Yantai Yuhuangding Hospital of Qingdao University (No. 2022-400) and individual consent for this retrospective analysis was waived.
Treatment
Among 103 patients, 49 received camrelizumab plus SOX/CapOX, and 54 received SOX/CapOX. After 6 cycles of first-line treatment, patients who had not progressed in 2 groups continued maintenance treatment of S-1 plus camrelizumab or S-1/capecitabine until disease progression, death, or intolerable toxicity. Camrelizumab was given intravenously at 200 mg every 3 weeks. Oxaliplatin 130 mg/m2 was given intravenously for 2 hours once daily, followed by 20 days off. Capecitabine 1,000 mg/m2 was administered twice daily (bid) by continuous oral method for 14 days, followed by a recovery period of 7 days. S-1 40–60 mg was administered bid orally for 14 days, followed by 7 days off.
Efficacy and safety assessments
Tumor imaging assessment and safety assessment were done every 6 weeks. According to RECIST v1.1, the objective response rate (ORR) was defined as the proportion of patients who achieved complete response (CR) and partial response (PR). The disease control rate (DCR) was defined as the proportion of patients who achieved CR, PR, or stable disease (SD). PFS was defined as the duration from the start of treatment to the last follow-up in patients with progressive disease (PD) or death from any cause, whichever occurred first. OS was defined as the duration from the beginning of treatment until death due to any cause. The toxicity was recorded based on the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0 (CTCAE 5.0). Patients were followed up until death or the data cutoff date of 31 July 2022.
Analysis for baseline NLR
Data on baseline blood cell tests were collected from 49 patients treated with the combination treatment. The NLR was defined as the neutrophil count divided by the lymphocyte count. Baseline blood tests were required within 1 week before the initiation of camrelizumab combined with chemotherapy.
Statistical analysis
We performed ORR and DCR comparisons with Pearson’s chi-square test. The PFS and OS were estimated using Kaplan-Meier methods and compared between subgroups using the log-rank test (two-sided). The corresponding 95% confidence intervals (CIs) were estimated using the Cox proportional regression model. Two-sided P value <0.05 was considered statistically significant. Youden index was applied to determine the cutoff value of NLR. Statistical analyses were conducted using SPSS 26.0 (IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
Among 103 patients included, 70 were male, and 33 were female. The median age of patients was 62 years (range, 43–83 years). A total of 49 patients received camrelizumab plus SOX/CapOX (32 patients on SOX regimen and 17 on CapOX), and 54 received SOX/CapOX (35 on SOX and 19 on CapOX). A total of 4 patients had MSI status, 2 were EBV positive, and 17 had PD-L1 CPS ≥1 in the combination therapy group, whereas 2 had MSI status, none were EBV positive, and 3 had PD-L1 CPS ≥1 in the chemotherapy group. Because of more unknown, the baseline characteristics beyond EBV status and PD-L1 CPS were similar between the combination therapy group and the chemotherapy group. There was no difference between combination group and chemotherapy group among patients in different age (P=0.856), different sex (0.582), different ECOG (P=0.652), different disease stage (P=0.459), and different chemotherapy regimen (P=0.958), etc. (Table 1).
Table 1
| Variables | Camrelizumab + SOX/CapOX (n=49) | SOX/CapOX (n=54) | P value |
|---|---|---|---|
| Age (years) | 0.856 | ||
| <65 | 30 | 34 | |
| ≥65 | 19 | 20 | |
| Sex | 0.582 | ||
| Male | 32 | 38 | |
| Female | 17 | 16 | |
| ECOG | 0.652 | ||
| 0 | 10 | 9 | |
| 1 | 39 | 45 | |
| Primary tumor location | 0.242 | ||
| Gastric cancer | 46 | 47 | |
| Gastroesophageal junction cancer | 3 | 7 | |
| Disease stage | 0.459 | ||
| III | 11 | 9 | |
| IV | 38 | 45 | |
| Number of metastatic sites | 0.899 | ||
| 1 | 17 | 21 | |
| 2 | 14 | 15 | |
| ≥3 | 18 | 18 | |
| Previous treatment | 0.623 | ||
| Neoadjuvant | 5 | 3 | |
| Gastric surgery | 15 | 18 | |
| Adjuvant chemotherapy | 9 | 12 | |
| Radiotherapy | 2 | 3 | |
| EBV | <0.01 | ||
| Negative | 38 | 9 | |
| Positive | 2 | 0 | |
| Unknown | 9 | 45 | |
| PD-L1 expression | <0.01 | ||
| CPS <1 | 13 | 5 | |
| CPS ≥1 | 17 | 3 | |
| Unknown | 19 | 46 | |
| MSI | 0.661 | ||
| dMMR | 4 | 2 | |
| pMMR | 38 | 43 | |
| Unknown | 7 | 9 | |
| Chemotherapy regimen | 0.958 | ||
| SOX | 32 | 35 | |
| CapOX | 17 | 19 | |
SOX, S-1 plus oxaliplatin; CapOX, capecitabine plus oxaliplatin; ECOG, Eastern Cooperative Oncology Group; CPS, combined positive score; PD-L1, programmed death-ligand 1; EBV, Epstein-Barr Virus; MSI, microsatellite instability; dMMR, deficient mismatch repair; pMMR, proficient mismatch repair.
Treatment efficacy
At the cut-off time for data analyses of 31 July 2022, the median follow-up duration was 14.2 months (range, 3.67–19.99 months), and 15 patients (31%) in the combination therapy group and 9 (17%) in the chemotherapy group were still in maintenance therapy. The ORR was 59.18% in the combination therapy group and 38.89% in the chemotherapy group (P=0.048). The DCRs were 83.67% and 62.96%, respectively (P=0.018, Table 2). The median PFS in the combination therapy group was 10.03 months (95% CI: 7.468–12.592) compared to 6.24 months (95% CI: 4.727–7.753) in the chemotherapy group [hazard ratio (HR) 0.603, 95% CI: 0.368–0.989, P=0.045, Figure 1A]. The median OS in the combination therapy group was 14.70 months (95% CI: 11.238–18.162) and 11.58 months (95% CI: 10.494–12.666) in the chemotherapy group (Figure 1B), which is currently immature and will be updated subsequently.
Table 2
| Best response | Camrelizumab + SOX/CapOX (n=49), n (%) | SOX/CapOX (n=54), n (%) | P value |
|---|---|---|---|
| CR | 2 (4.08) | 1 (1.85) | – |
| PR | 27 (55.10) | 20 (37.04) | – |
| SD | 12 (24.49) | 13 (24.07) | – |
| PD | 8 (16.33) | 20 (37.07) | – |
| ORR | 29 (59.18) | 21 (38.89) | 0.048† |
| DCR | 41 (83.67) | 34 (62.96) | 0.018† |
†, the ORR and DCR comparisons between the two groups were analyzed with Pearson’s chi-square. SOX, S-1 plus oxaliplatin; CapOX, capecitabine plus oxaliplatin; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; DCR, disease control rate; ORR, objective response rate.
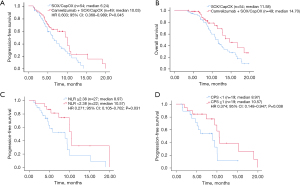
To assess the value of NLR in predicting the prognosis of patients treated with combination treatment, we calculated the cut-off value of NLR and divided patients into 2 groups based on the cut-off value. In this study, AUC values were calculated separately using 2- to 12-month PFS as a cut-off (Figure S1A). May and June PFS had the highest AUC values of 0.681 and 0.712, respectively. Figure S1B shows the receiver operating characteristic (ROC) curves for NLR bounded by 5- and 6-month PFS, representing the predictive power of NLR for 5-month/6-month PFS. The AUC for NLR of 5- and 6-month PFS were 0.681 (P=0.036) and 0.712 (P=0.012), respectively, and the best cut-off value was 2.38 for both. The sensitivity of NLR =2.38 for 6-month PFS was 76%, and specificity was 73.9%. Therefore, the optimal cut-off value for NLR in this study was defined as 2.38. There were 22 patients with NLR <2.38 and 27 with NLR ≥2.38 in the combination therapy group. As shown in Figure 1C, PFS in the low NLR group (NLR <2.38) was 10.57 months, and in the high NLR group (NLR ≥2.38) was 8.97 months (HR 0.271, 95% CI: 0.105–0.702, P=0.031). Moreover, PFS was longer in patients with CPS ≥1 than in patients with CPS <1 (10.57 vs. 8.97 months, HR 0.374, 95% CI: 0.148–0.947, P=0.038) (Figure 1D). Univariate analysis revealed that NLR was significantly associated with PFS (P=0.007), whereas age, sex, stage, or chemotherapy regimen were not associated with PFS. Multivariate analysis by including variables with P<0.1 showed that NLR was an independent prognosis biomarker for PFS (P=0.006) (Figure 2).
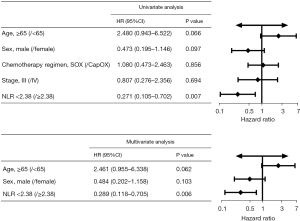
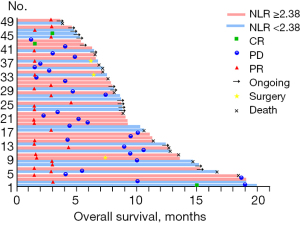
Figure 3 displays treatment response and survival in the combination therapy group on a per-patient level. A total of 3 patients achieved CR during the study. Among 27 patients with PR, 3 had successful translational therapy and ultimately underwent radical surgery for GC.
Safety
In this study, 103 patients were included in the safety analysis. Almost all patients (101, 98.1%) experienced adverse events (AEs). The most treatment-related AEs (TRAEs) were grade 1/2. The most common grade 3–4 TRAEs were granulocytopenia [28 (57%) in the combination therapy group vs. 29 (54%) in the chemotherapy group], anemia [19 (39%) vs. 18 (33%), respectively], and thrombocytopenia [19 (39%) vs. 18 (33%), respectively]. The proportion of reactive capillary endothelial proliferation (RCCEP; 73% vs. 0%, respectively), elevated aspartate aminotransferase (AST; 45% vs. 28%, respectively), elevated alanine aminotransferase (ALT; 33% vs. 22%), and increased thyroid stimulating hormone (TSH; 24% vs. 2%) was higher in the combination therapy group; all were grade 1–2 (Table 3). The proportion of serious AEs in the combination and chemotherapy group was 22% (11 patients) vs. 15% (8 patients), and AEs leading to treatment interruption (27% vs. 17%), delay, or dose reduction (43% vs. 35%), were generally similar between the 2 groups. The dose of capecitabine/S-1 was reduced in 29 patients because of myelosuppression or hand-foot syndrome. In the combination therapy group, 3 cases of interstitial pneumonia and one case of severe immune enterocolitis were observed. There were 3 and 1 patient who developed gastric bleeding in the combination therapy and chemotherapy groups, respectively, which were considered related to the primary disease and possibly unrelated to the treatment. No treatment-related deaths were observed in the 2 groups.
Table 3
| Adverse events | All grade, n [%] | Grade 3/4, n [%] | |||
|---|---|---|---|---|---|
| Camrelizumab + SOX/CapOX | SOX/CapOX | Camrelizumab + SOX/CapOX | SOX/CapOX | ||
| All events | 48 [98] | 53 [98] | 31 [63] | 32 [59] | |
| Serious events | 11 [22] | 8 [15] | 8 [16] | 6 [11] | |
| Events leading to discontinuation | 13 [27] | 9 [17] | 6 [12] | 5 [9] | |
| Events leading to dose delay or reduction | 21 [43] | 19 [35] | 11 [22] | 12 [22] | |
| TRAEs (≥10%) | |||||
| Granulocytopenia | 39 [80] | 42 [78] | 28 [57] | 29 [54] | |
| RCCEP | 36 [73] | 0 | 0 | 0 | |
| Fatigue | 31 [63] | 29 [54] | 2 [4] | 2 [4] | |
| Anemia | 29 [59] | 29 [54] | 19 [39] | 18 [33] | |
| Thrombocytopenia | 28 [57] | 30 [56] | 11[20] | 12 [22] | |
| Sensory neuropathy | 27 [55] | 29 [54] | 2 [4] | 3 [6] | |
| Nausea | 26 [53] | 27 [50] | 1 [2] | 0 | |
| Decreased appetite | 23 [47] | 25 [46] | 2 [4] | 2[4] | |
| Aspartate aminotransferase increased | 22 [45] | 15 [28] | 3 [4] | 1[2] | |
| Alanine aminotransferase increased | 16 [33] | 12 [22] | 2 [6] | 1 [2] | |
| Diarrhea | 16 [33] | 17 [31] | 2 [4] | 3 [6] | |
| Vomiting | 14 [29] | 15 [28] | 1 [2] | 0 | |
| Thyroid-stimulating hormone increased | 12 [24] | 1 [2] | 0 | 0 | |
| Blood bilirubin increased | 9 [18] | 5 [9] | 1 [2] | 1 [2] | |
| Hand-foot syndrome | 8 [16] | 10 [19] | 2 [4] | 3 [6] | |
| Bilirubin conjugated increased | 7 [14] | 4 [7] | 1 [2] | 0 | |
| Rash | 6 [12] | 3 [6] | 0 | 1 [2] | |
| Gamma-glutamyltransferase increased | 5 [10] | 6 [11] | 1 [2] | 2 [4] | |
SOX, S-1 plus oxaliplatin; CapOX, capecitabine plus oxaliplatin; TRAEs, treatment-related grade adverse events; RCCEP, reactive cutaneous capillary endothelial proliferation.
Discussion
This retrospective study revealed that camrelizumab combined with SOX/CapOX was superior to chemotherapy alone in terms of ORR, DCR, PFS, and OS in patients with untreated, HER2-negative, unresectable advanced or recurrent gastric/gastroesophageal junction cancer. The OS was not mature at the time of the OS analysis, with 40% of patients having died. As a result, the OS could have potentially been underestimated. We further found that NLR predicted PFS of combination therapy. Patients with NLR <2.38 had longer PFS than those with NLR ≥2.38. Lastly, patients with CPS ≥1 had longer PFS than those with CPS <1.
In this study, we obtained an ORR of 59.18% in the combination therapy group, which was in accordance with the ORR of CheckMate 649 (13) and ATTRACTION-4 (14). The DCR of 83.67% did not reach 93.8% in the previous phase II clinical trial (7) but was close to that of ATTRACTION-4 and Keynote 059 (23), probably because of the higher DCR obtained with sequential camrelizumab plus apatinib for anti-tumor angiogenesis. The PFS was numerically higher than CheckMate 649 and close to ATTRACTION-4. Although immature, the median OS reached 14.2 months and will be updated with more extended follow-up visits. It is observed that although PD-1 inhibitors target the same site, the efficacy of different drugs for GC varies. Therefore, the choice of different PD-1 inhibitors should be made clinically based on the findings of evidence-based medical studies.
In the subgroup analysis of the combination therapy group, we found that PFS was longer with CPS <1 and NLR ≥2.38 than in the SOX/CapOX group, suggesting that camrelizumab combined with chemotherapy in first-line treatment of GC can be beneficial regardless of PD-L1 status and NLR value. Nivolumab in combination with chemotherapy in first-line treatment of GC also demonstrated a high PD-L1 CPS population as the superior population for immune benefit (13), which was consistent with this study. Unfortunately, this was a retrospective study, and data on PD-L1 CPS were limited. A total of 19 of the included patients had unknown CPS, resulting in CPS not being entered into the Cox regression analysis along with NLR. Most importantly, this study identified NLR as a biomarker for combination therapy in first-line treatment of GC, as was found in studies of nivolumab (24) and other PD-1 inhibitors (22) for GC with different cut-off values, which may be related to different testing machines, dosing regimens, and so on. However, this was the first time analyzing the relationship between NLR and PFS in camrelizumab combined with SOX/CapOX. The NLR is more accessible to obtain than TMB and PD-L1 CPS because every patient is tested for baseline blood tests before first-line treatment. In addition, review articles on NLR in tumor immunotherapy have shown that cut-off values varied among studies (25-27). The mechanism by which camrelizumab plus SOX/CapOX affects NLR needs further exploration.
Several studies have focused on PD-1 inhibitors in combination with chemotherapy for GC, gradually rising from the back-line therapy to the front-line. A phase II study found that camrelizumab combined with chemotherapy sequential camrelizumab plus apatinib achieved encouraging clinical outcomes in the first-line treatment of GC (7). In contrast, this study used sequential camrelizumab plus capecitabine/S-1. This regimen did not increase the toxicity of the anti-angiogenic drugs, and the side effects were tolerable. The phase 3 ATTRACTION-4 trial compared the efficacy of nivolumab combined with SOX/CapOX versus SOX/CapOX chemotherapy (14). However, patients in the ATTRACTION-4 trial were treated on the original regimen until progression without a reduction in maintenance therapy. The side effects of peripheral neurotoxicity and bone marrow suppression were relatively significant (14). After 6 cycles of effective combination therapy, the side effects would be better tolerated by removing oxaliplatin and applying camrelizumab plus capecitabine/S-1 or capecitabine/S-1 maintenance therapy. The safety of camrelizumab in combination with chemotherapy in this study was generally consistent with other immune combination chemotherapy research (13,14), and previous camrelizumab-related research (7). The grade ≥3 AEs seen in the study were mainly granulocytopenia, anemia, and thrombocytopenia, broadly in line with the chemotherapy alone group. No new safety signals emerged.
This study had several limitations. First, this was a retrospective single-center design, and the sample size was not large. Second, more patients who only received chemotherapy were enrolled in the early stage of this study. Then, the number of patients who received combination therapy increased, which was influenced by treatment guidelines. No more than half of the deaths occurred in the combination therapy group, resulting in an immature OS, whereas the median OS was achieved in the chemotherapy group. Therefore, no NLR and PD-L1 CPS subgroup analyses were performed for OS in the combination group. The KEYNOTE-158 study confirmed a significant clinical benefit of pembrolizumab in patients with high TMB (28). However, this study did not analyze the association between TMB and efficacy, as TMB was assessed in only 4 patients.
Conclusions
Together, this study suggested the superior efficacy of camrelizumab plus SOX/CapOX over SOX/CapOX in the first-line treatment of HER2-negative, unresectable advanced, or metastatic gastric/gastroesophageal junction cancer. This combination treatment modality increased tumor control in GC, and the sequential immuno-combination with single-agent maintenance reduced cumulative toxicity while delaying disease progression. Meanwhile, we observed that lower NLR was associated with longer PFS, suggesting that NLR is an important predictor of the efficacy of immunotherapy in GC. Further validation in a prospective, randomized controlled trial is needed.
Acknowledgments
We thank the patients, their families, and colleagues who contributed to this study.
Funding: This work was supported by the Wu Jieping Medical Foundation of China (No. 320.6750.2020-02-45).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1229/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1229/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1229/coif). All authors report that this work was supported by the Wu Jieping Medical Foundation of China (No. 320.6750.2020-02-45). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of The Affiliated Yantai Yuhuangding Hospital of Qingdao University (No. 2022-400). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Song Z, Wu Y, Yang J, et al. Progress in the treatment of advanced gastric cancer. Tumour Biol 2017;39:1010428317714626. [Crossref] [PubMed]
- Wang H, Guo W, Hu Y, et al. Superiority of the 8th edition of the TNM staging system for predicting overall survival in gastric cancer: Comparative analysis of the 7th and 8th editions in a monoinstitutional cohort. Mol Clin Oncol 2018;9:423-31.
- Ajani JA, D'Amico TA, Bentrem DJ, et al. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2022;20:167-92. [Crossref] [PubMed]
- Hindson J. Nivolumab plus chemotherapy for advanced gastric cancer and oesophageal adenocarcinoma. Nat Rev Gastroenterol Hepatol 2021;18:523. [Crossref] [PubMed]
- Zhou C, Chen G, Huang Y, et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): a randomised, open-label, multicentre, phase 3 trial. Lancet Respir Med 2021;9:305-14. [Crossref] [PubMed]
- Peng Z, Wei J, Wang F, et al. Camrelizumab Combined with Chemotherapy Followed by Camrelizumab plus Apatinib as First-line Therapy for Advanced Gastric or Gastroesophageal Junction Adenocarcinoma. Clin Cancer Res 2021;27:3069-78. [Crossref] [PubMed]
- Tang Z, Wang Y, Liu D, et al. The Neo-PLANET phase II trial of neoadjuvant camrelizumab plus concurrent chemoradiotherapy in locally advanced adenocarcinoma of stomach or gastroesophageal junction. Nat Commun 2022;13:6807. [Crossref] [PubMed]
- Sugawara S, Lee JS, Kang JH, et al. Nivolumab with carboplatin, paclitaxel, and bevacizumab for first-line treatment of advanced nonsquamous non-small-cell lung cancer. Ann Oncol 2021;32:1137-47. [Crossref] [PubMed]
- Horn L, Mansfield AS, Szczęsna A, et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med 2018;379:2220-9. [Crossref] [PubMed]
- Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet 2019;394:1929-39. [Crossref] [PubMed]
- Cohen EEW, Soulières D, Le Tourneau C, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet 2019;393:156-67. [Crossref] [PubMed]
- Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 2021;398:27-40. [Crossref] [PubMed]
- Kang YK, Chen LT, Ryu MH, et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2022;23:234-47. [Crossref] [PubMed]
- Salas-Benito D, Pérez-Gracia JL, Ponz-Sarvisé M, et al. Paradigms on Immunotherapy Combinations with Chemotherapy. Cancer Discov 2021;11:1353-67. [Crossref] [PubMed]
- Joshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer. CA Cancer J Clin 2021;71:264-79. [Crossref] [PubMed]
- Puliga E, Corso S, Pietrantonio F, et al. Microsatellite instability in Gastric Cancer: Between lights and shadows. Cancer Treat Rev 2021;95:102175. [Crossref] [PubMed]
- Gu L, Chen M, Guo D, et al. PD-L1 and gastric cancer prognosis: A systematic review and meta-analysis. PLoS One 2017;12:e0182692. [Crossref] [PubMed]
- Wang F, Wei XL, Wang FH, et al. Safety, efficacy and tumor mutational burden as a biomarker of overall survival benefit in chemo-refractory gastric cancer treated with toripalimab, a PD-1 antibody in phase Ib/II clinical trial NCT02915432. Ann Oncol 2019;30:1479-86. [Crossref] [PubMed]
- Naseem M, Barzi A, Brezden-Masley C, et al. Outlooks on Epstein-Barr virus associated gastric cancer. Cancer Treat Rev 2018;66:15-22. [Crossref] [PubMed]
- Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202-9. [Crossref] [PubMed]
- Gou M, Qu T, Wang Z, et al. Neutrophil-to-Lymphocyte Ratio (NLR) Predicts PD-1 Inhibitor Survival in Patients with Metastatic Gastric Cancer. J Immunol Res 2021;2021:2549295. [Crossref] [PubMed]
- Fuchs CS, Doi T, Jang RW, et al. Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol 2018;4:e180013. [Crossref] [PubMed]
- Yamada T, Hayashi T, Inokuchi Y, et al. Impact of the Neutrophil-to-Lymphocyte Ratio on the Survival of Patients with Gastric Cancer Treated with Nivolumab Monotherapy. Target Oncol 2020;15:317-25. [Crossref] [PubMed]
- Valero C, Lee M, Hoen D, et al. Pretreatment neutrophil-to-lymphocyte ratio and mutational burden as biomarkers of tumor response to immune checkpoint inhibitors. Nat Commun 2021;12:729. [Crossref] [PubMed]
- Bartlett EK, Flynn JR, Panageas KS, et al. High neutrophil-to-lymphocyte ratio (NLR) is associated with treatment failure and death in patients who have melanoma treated with PD-1 inhibitor monotherapy. Cancer 2020;126:76-85. [Crossref] [PubMed]
- Sacdalan DB, Lucero JA, Sacdalan DL. Prognostic utility of baseline neutrophil-to-lymphocyte ratio in patients receiving immune checkpoint inhibitors: a review and meta-analysis. Onco Targets Ther 2018;11:955-65. [Crossref] [PubMed]
- Marabelle A, Fakih M, Lopez J, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol 2020;21:1353-65. [Crossref] [PubMed]
(English Language Editor: J. Jones)


