
Prognostic value of desmoplastic stromal reaction, tumor budding and tumor-stroma ratio in stage II colorectal cancer
Introduction
Colorectal cancer (CRC) is the third most prevalent cancer worldwide with a high mortality rate (1); in China, the incidence of CRC increased to the second most common in 2020 (2). Approximately 25% of CRC patients are in stage II. Surgical resection for curative purposes is the mainstay of treatment for patients with stage II CRC. However, 20% to 25% of these patients still experience tumor recurrence after surgery (3), and treatment strategies after surgical resection are controversial (4,5). Recently, the tumor microenvironment has been recognized to play an important role in patient prognosis. The discovery of biomarkers that can predict the tumor microenvironment may be the key to overcoming the current treatment status.
The tumor microenvironment is a bidirectional, dynamic and intricate interaction network between stromal cells and tumor cells that is closely related to tumor proliferation, spread, metastasis, recurrence and chemotherapy resistance (6,7). The tumor-stroma ratio (TSR) is an estimate of the proportion of epithelial and stromal cells. Numerous studies have considered the TSR as an independent prognostic biomarker in CRC, with a high amount of stroma (low TSR) being predictive of an adverse prognosis (8-12). Desmoplastic reaction (DR) is the proliferation of myofibroblasts in the stroma of invasive cancer (13,14). Similar to TSR, DR is an important part of the tumor microenvironment and has been identified as an independent factor for poor prognosis in stage II CRC (15). Furthermore, tumor budding (TBd) is an acknowledged prognostic factor in CRC and other carcinomas. The presence of TBd has been associated with worse prognoses in CRCs (16,17). The International Tumor Budding Consensus Conference (ITBCC) (17) has proposed to include the presence of TBd in CRC staging systems and recommended using a three-tier system based on the quantification of tumor buds.
Recent studies revealed that immature DR, high TBd and low TSR were significantly associated with several high-risk clinicopathological factors and poor overall survival (OS) in gall bladder carcinoma (18), but the prognostic role of these parameters in CRC, especially in the Chinese population, remains controversial (19,20). In addition, due to the differences in biological behavior in stages I to IV CRC, our study design included only stage II patients of the Chinese population to perform a meaningful relapse-free survival (RFS) analysis. Hence, this study sought to investigate the prognostic value of DR, TBd and TSR, and combined them to build a prognostic model for the prediction of RFS in stage II CRC patients. We present the following article in accordance with the TRIPOD reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-758/rc).
Methods
Patients
This single-center, retrospective study was approved by the Ethics Committee of Tianjin Medical University Cancer Institute & Hospital (TMUCIH ) (No. EK2022175), and a waiver of patient consent was granted. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
A total of 688 stage II CRC patients who underwent surgical resection at TMUCIH between January 2012 and August 2018 were enrolled in this study. The exclusion criteria were as follows: (I) synchronous multiple tumors (n=55); (II) received preoperative neoadjuvant therapy (n=78); (III) insufficient clinicopathology data (n=212) and (IV) lost to follow-up (n=136). In total, 207 patients with stage II CRC were included. Data on clinicopathological factors including age, gender, hypertension history, diabetes history, smoking history, family history of cancer, preoperative CEA, CA724, CA199, and CA242 levels, histological type, differentiation grade, T stage, presence or absence of lymphovascular invasion (LVI), perineural invasion (PNI), internal obstruction or perforation (IOP) status, number of lymph nodes examined (≥12 vs. <12), mismatch repair status, Ki-67 expression level and history of postoperative adjunctive chemotherapy (present vs. absent) were obtained from medical records and pathology reports. All patients were followed-up for at least 3 years from the date of surgical resection. The RFS as the primary endpoint was defined as the date from surgery to confirmed clinical recurrence, death or last available contact.
Assessment of DR categorization, TBd and TSR
Hematoxylin and eosin-stained slides of the resection specimens were re-reviewed by two pathologists. They were blinded to the clinical information while confirming the findings of TBd, TSR, and DR. If the two pathologists disagreed with the diagnosis, they would convene to discuss and decide the final diagnosis.
DR
DR categorization was performed according to the Ueno method (21-23), which classified DR into three types: immature, intermediate and mature. Immature DR was defined as the presence of myxoid stroma at the tumor invasive front involving at least one high microscopic field (×40); cases without myxoid stroma larger than the ×40 objective field and with the presence of keloid-like collagens at the tumor invasive front were classified as intermediate DR; and those without keloid-like collagen or myxoid stroma were classified as mature DR (Figure 1A-1C).
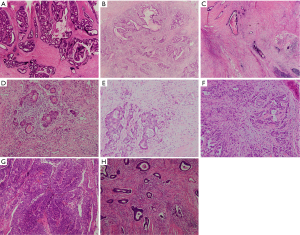
TBd
TBd was scored using the proposed three-tier approach by the ITBCC 2016 international criteria (17), which relied on the quantification of the buds at the hotspot (in a field measuring 0.785 mm2) and was divided into three categories: less than 5 (BD1), 5 to 9 (BD2) and greater than or equal to 10 (BD3) (Figure 1D-1F).
TSR
TSR, also known as the tumor stroma percentage, was defined as the proportion of stromal area at the most invasive region in a microscopic field with a ×10 objective magnification. Patients were divided into TSR high (less than or equal to 50% stroma) and TSR low (greater than 50% stroma) groups using a cutoff value of 50% (24) (Figure 1G,1H).
Nomogram development and performance evaluation
Kaplan–Meier curve analysis and the log-rank test were applied to examine RFS in subgroups. The Cox proportional hazards model was used for univariate and multivariate analyses. To provide an individual predictive graphical presentation, we subsequently constructed a nomogram on the basis of multivariate Cox analysis in the cohort.
To evaluate the performance of the prediction model, a calibration curve with bootstrapping was constructed by measuring the agreement between the predicted and actual 3-year RFS. Furthermore, a receiver operating characteristic (ROC) curve was produced to further verify the prediction performance of the model by calculating the area under the curve (AUC) as well as accuracy, sensitivity and specificity.
Other statistical analyses
All statistical calculations were performed with SPSS statistical software (version 20.0) or R software (version 6.1). The data were presented as numbers (%) for categorical variables. Normally distributed continuous variables were expressed as the mean [standard deviation (SD)], while non-normally distributed continuous variables were expressed as the median and range. The chi-square test or Fisher’s exact test was used for association analysis between categorical variables where appropriate. R packages of “rms,” “survival,” “calibrate,” “pROC,” and “OptimalCutpoints” were used to construct the prognostic nomogram, plot calibration curves, ROC curves and cutoff value. The optimal cutoff point was estimated by Youden’s J index (25). A two-sided P value less than 0.05 in the statistical test was statistically significant. All confidence intervals (CIs) were stated at the 95% confidence level.
Results
Patient characteristics
The baseline characteristics of the 207 patients are summarized in Table 1. Briefly, there were 126 males and 81 females of whom 51 patients older than 65 years. The mean age at the time of surgery was 58.31±10.43 years. Fifty (24.15%) patients of the entire population had disease recurrence, and all of them experienced recurrence within 3 years. A total of 17 patients were confirmed by surgery and histopathology, and the other patients were diagnosed by radiologic features. The mean RFS was 1,039.33±475.09 days (range, 33–1,802 days).
Table 1
| Characteristics | All patients (N=207) |
|---|---|
| Gender, n (%) | |
| Male | 126 (60.9) |
| Female | 81 (39.1) |
| Age, n (%) | |
| >65 years | 51 (24.6) |
| ≤65 years | 156 (75.4) |
| Histologic grade, n (%) | |
| Well | 30 (14.5) |
| Moderate | 116 (56.0) |
| Poor | 61 (29.5) |
| Location, n (%) | |
| Ascending colon | 23 (11.1) |
| Transverse colon | 4 (1.9) |
| Descending colon | 8 (3.9) |
| Sigmoid colon | 36 (17.4) |
| Rectum | 136 (75.7) |
| Smoking, n (%) | |
| No | 164 (79.2) |
| Yes | 43 (20.8) |
| Hypertension, n (%) | |
| No | 152 (73.4) |
| Yes | 55 (26.6) |
| Family history of cancer, n (%) | |
| No | 178 (86.0) |
| Yes | 29 (14.0) |
| Diabetes, n (%) | |
| No | 187 (90.3) |
| Yes | 20 (9.7) |
| CEA level, n (%) | |
| Normal | 115 (55.6) |
| Abnormal | 92 (44.4) |
| CA242, n (%) | |
| Normal | 164 (79.2) |
| Abnormal | 43 (20.8) |
| CA724, n (%) | |
| Normal | 164 (76.9) |
| Abnormal | 43 (23.1) |
| CA199, n (%) | |
| Normal | 176 (85.0) |
| Abnormal | 31 (15.0) |
| Ki-67 level, n (%) | |
| Low | 9 (4.3) |
| High | 198 (95.7) |
| T stage, n (%) | |
| T3 | 132 (63.8) |
| T4 | 75 (36.2) |
| Lymphovascular invasion, n (%) | |
| Absent | 160 (77.3) |
| Present | 47 (22.7) |
| Perineural invasion, n (%) | |
| Absent | 189 (91.3) |
| Present | 18 (8.7) |
| IOP status, n (%) | |
| No | 188 (90.8) |
| Yes | 19 (9.2) |
| Number of nodes examined, n (%) | |
| 12 or more | 175 (86.3) |
| Less than 12 | 32 (13.7) |
| Mismatch repair status, n (%) | |
| MSI-L | 160 (77.3) |
| MSI-H | 47 (22.7) |
| DR, n (%) | |
| Mature | 101 (48.8) |
| Intermediate | 62 (30.0) |
| Immature | 44 (21.2) |
| TBd, n (%) | |
| <5 (BD1) | 108 (52.2) |
| 5–9 (BD2) | 45 (21.7) |
| ≥10 (BD3) | 54 (26.1) |
| TSR, n (%) | |
| Low (stroma-high) | 93 (44.9) |
| High (stroma-low) | 114 (55.1) |
| Adjuvant therapy, n (%) | |
| No | 72 (34.8) |
| Yes | 135 (65.2) |
MSI, microsatellite instability; CEA, carcinoembryonic antigen; CA242, carbohydrate antigen 242; CA724, carbohydrate antigen 724; CA199, carbohydrate antigen199; IOP status, internal obstruction or perforation; DR, desmoplastic reaction; TBd, tumour budding; TSR, tumor-stroma ratio.
Association among the investigated parameters
Tables 2-4 show the correlations between DR, TBd, and TSR and the clinicopathological parameters of patients.
Table 2
| Characteristics | DR | P value | ||
|---|---|---|---|---|
| Immature (N=44) | Intermediate (N=62) | Mature (N=101) | ||
| Gender, n (%) | 0.526 | |||
| Male | 30 (68.2) | 36 (58.1) | 60 (59.4) | |
| Female | 14 (31.8) | 26 (41.9) | 41 (40.6) | |
| Age, n (%) | 0.714 | |||
| >65 years | 9 (20.5) | 17 (27.4) | 25 (24.8) | |
| ≤65 years | 35 (79.5) | 45 (72.6) | 76 (75.2) | |
| Histologic grade, n (%) | 0.158 | |||
| Well | 5 (11.4) | 5 (8.1) | 20 (19.8) | |
| Moderate | 22 (50.0) | 39 (62.9) | 55 (54.5) | |
| Poor | 17 (38.6) | 18 (29.0) | 26 (25.7) | |
| Location, n (%) | 0.815 | |||
| Ascending colon | 4 (9.1) | 10 (16.2) | 9 (8.9) | |
| Transverse colon | 1 (2.3) | 0 (0.0) | 3 (3.0) | |
| Descending colon | 3 (6.8) | 2 (3.2) | 3 (3.0) | |
| Sigmoid colon | 7 (15.9) | 9 (14.5) | 20 (19.8) | |
| Rectum | 29 (65.9) | 41 (66.1) | 66 (65.3) | |
| Smoking, n (%) | 0.712 | |||
| No | 36 (81.8) | 47 (75.8) | 81 (80.2) | |
| Yes | 8 (18.2) | 15 (24.2) | 20 (19.8) | |
| Hypertension, n (%) | 0.768 | |||
| No | 34 (77.3) | 44 (71.0) | 74 (73.4) | |
| Yes | 10 (22.7) | 18 (29.0) | 27 (26.7) | |
| Family history of cancer, n (%) | 0.318 | |||
| No | 38 (86.4) | 50 (80.6) | 90 (89.1) | |
| Yes | 6 (13.6) | 12 (19.4) | 11 (10.9) | |
| Diabetes, n (%) | 0.615 | |||
| No | 38 (86.4) | 57 (92.0) | 92 (91.1) | |
| Yes | 6 (13.6) | 5 (8.0) | 9 (8.9) | |
| CEA level, n (%) | 0.035* | |||
| Normal | 17 (38.6) | 36 (58.1) | 62 (61.4) | |
| Abnormal | 27 (61.4) | 26 (41.9) | 39 (38.6) | |
| CA242, n (%) | 0.567 | |||
| Normal | 33 (75.0) | 48 (77.4) | 83 (82.2) | |
| Abnormal | 11 (25.0) | 14 (22.6) | 18 (17.8) | |
| CA724, n (%) | 0.743 | |||
| Normal | 35 (79.5) | 51 (82.3) | 78 (77.2) | |
| Abnormal | 9 (20.5) | 11 (17.7) | 23 (22.8) | |
| CA199, n (%) | 0.271 | |||
| Normal | 36 (81.8) | 50 (80.6) | 90 (89.1) | |
| Abnormal | 8 (18.2) | 12 (19.4) | 11 (10.9) | |
| Ki-67 level, n (%) | 0.190 | |||
| Low | 4 (9.1) | 1 (1.6) | 4 (4.0) | |
| High | 40 (90.9) | 61 (98.4) | 97 (96.0) | |
| T stage, n (%) | 0.156 | |||
| T3 | 26 (59.1) | 35 (56.5) | 71 (70.3) | |
| T4 | 18 (40.9) | 27 (43.5) | 30 (29.7) | |
| Lymphovascular invasion, n (%) | 0.420 | |||
| Absent | 32 (72.7) | 46 (74.2) | 82 (81.2) | |
| Present | 12 (27.3) | 16 (25.8) | 19 (18.8) | |
| Perineural invasion, n (%) | 0.024* | |||
| Absent | 36 (81.8) | 56 (90.3) | 97 (96.0) | |
| Present | 8 (18.2) | 6 (9.7) | 4 (4.0) | |
| IOP status, n (%) | 0.030* | |||
| No | 37 (84.1) | 54 (87.1) | 97 (96.0) | |
| Yes | 7 (15.9) | 8 (12.9) | 4 (4.0) | |
| Nodes examined, n (%) | 0.502 | |||
| 12 or more | 35 (79.5) | 52 (83.9) | 88 (87.1) | |
| Less than 12 | 9 (20.5) | 10 (16.1) | 13 (12.9) | |
| Mismatch repair status, n (%) | 0.909 | |||
| MSI-L | 35 (79.5) | 48 (77.4) | 77 (76.2) | |
| MSI-H | 9 (20.5) | 14 (22.6) | 24 (23.8) | |
| TBd, n (%) | 0.000* | |||
| <5 (BD1) | 5 (11.4) | 28 (45.2) | 75 (74.3) | |
| 5–9 (BD2) | 13 (29.5) | 21 (33.9) | 11 (10.9) | |
| ≥10 (BD3) | 26 (59.1) | 13 (20.9) | 15 (14.8) | |
| TSR, n (%) | 0.000* | |||
| Low (stroma-high) | 36 (81.8) | 30 (48.4) | 27 (26.7) | |
| High (stroma-low) | 8 (18.2) | 32 (51.6) | 74 (73.3) | |
| Adjuvant therapy, n (%) | 0.015* | |||
| No | 12 (27.3) | 15 (24.2) | 45 (44.6) | |
| Yes | 32 (72.7) | 47 (75.8) | 56 (55.4) | |
*P<0.05. MSI, microsatellite instability; CEA, carcinoembryonic antigen; CA242, carbohydrate antigen 242; CA724, carbohydrate antigen 724; CA199, carbohydrate antigen199; IOP status, internal obstruction or perforation; DR, desmoplastic reaction; TBd, tumour budding; TSR, tumor-stroma ratio.
Table 3
| Characteristics | TBd | P value | ||
|---|---|---|---|---|
| BD1 (N=108) | BD2 (N=45) | BD3 (N=54) | ||
| Gender, n (%) | 0.852 | |||
| Male | 65 (60.2) | 29 (64.4) | 32 (59.3) | |
| Female | 43 (39.8) | 16 (35.6) | 22 (40.7) | |
| Age, n (%) | 0.217 | |||
| >65 years | 24 (22.2) | 9 (20.0) | 18 (33.3) | |
| ≤65 years | 84 (77.8) | 36 (80.0) | 36 (66.7) | |
| Histologic grade, n (%) | 0.046* | |||
| Well | 20 (18.5) | 6 (13.3) | 4 (7.4) | |
| Moderate | 65 (60.2) | 24 (53.3) | 27 (50.0) | |
| Poor | 23 (21.3) | 15 (33.4) | 23 (42.6) | |
| Location, n (%) | 0.382 | |||
| Ascending colon | 16 (14.8) | 2 (4.4) | 5 (9.3) | |
| Transverse colon | 2 (1.9) | 1 (2.2) | 1 (1.9) | |
| Descending colon | 4 (3.7) | 2 (4.4) | 2 (3.7) | |
| Sigmoid colon | 16 (14.8) | 8 (17.8) | 12 (22.2) | |
| Rectum | 70 (64.8) | 32 (71.1) | 34 (62.9) | |
| Smoking, n (%) | 0.886 | |||
| No | 85 (78.7) | 35 (77.8) | 44 (81.5) | |
| Yes | 23 (21.3) | 10 (22.2) | 10 (18.5) | |
| Hypertension, n (%) | 0.004* | |||
| No | 70 (64.8) | 34 (75.6) | 48 (88.9) | |
| Yes | 38 (35.2) | 11 (24.4) | 6 (11.1) | |
| Family history of cancer, n (%) | 0.504 | |||
| No | 91 (84.3) | 38 (84.4) | 49 (90.7) | |
| Yes | 17 (15.7) | 7 (15.6) | 5 (9.3) | |
| Diabetes, n (%) | 0.106 | |||
| No | 102 (94.4) | 39 (86.7) | 46 (85.2) | |
| Yes | 6 (5.6) | 6 (13.3) | 8 (14.8) | |
| CEA level, n (%) | 0.396 | |||
| Normal | 63 (58.3) | 21 (46.7) | 31 (57.4) | |
| Abnormal | 45 (41.7) | 24 (53.3) | 23 (42.6) | |
| CA242, n (%) | 0.431 | |||
| Normal | 89 (82.4) | 33 (73.3) | 42 (77.8) | |
| Abnormal | 19 (17.6) | 12 (26.7) | 12 (22.2) | |
| CA724, n (%) | 0.852 | |||
| Normal | 84 (77.8) | 36 (80.0) | 44 (81.5) | |
| Abnormal | 24 (22.2) | 9 (20.0) | 10 (18.5) | |
| CA199, n (%) | 0.114 | |||
| Normal | 97 (89.9) | 35 (77.8) | 44 (81.5) | |
| Abnormal | 11 (10.1) | 10 (22.2) | 10 (18.5) | |
| Ki-67 level, n (%) | 0.962 | |||
| Low | 5 (4.6) | 2 (4.4) | 2 (3.7) | |
| High | 103 (95.4) | 43 (95.6) | 52 (96.3) | |
| T stage, n (%) | 0.203 | |||
| T3 | 74 (68.5) | 24 (53.3) | 34 (63.0) | |
| T4 | 34 (31.5) | 21 (46.7) | 20 (37.0) | |
| Lymphovascular invasion, n (%) | 0.503 | |||
| Absent | 87 (80.6) | 33 (73.3) | 40 (74.1) | |
| Present | 21 (19.4) | 12 (26.7) | 14 (25.9) | |
| Perineural invasion, n (%) | 0.738 | |||
| Absent | 100 (92.6) | 41 (91.1) | 48 (88.9) | |
| Present | 8 (7.4) | 4 (8.9) | 6 (11.1) | |
| IOP status, n (%) | 0.164 | |||
| No | 102 (94.4) | 39 (86.7) | 47 (87.0) | |
| Yes | 6 (5.6) | 6 (13.3) | 7 (13.0) | |
| Nodes examined, n (%) | 0.470 | |||
| 12 or more | 94 (87.0) | 38 (84.4) | 43 (79.6) | |
| Less than 12 | 14 (13.0) | 7 (15.6) | 11 (20.4) | |
| Mismatch repair status, n (%) | 0.712 | |||
| MSI-L | 81 (75.0) | 36 (80.0) | 43 (79.6) | |
| MSI-H | 27 (25.0) | 9 (20.0) | 11 (20.4) | |
| DR, n (%) | 0.000* | |||
| Mature | 75 (69.4) | 11 (24.4) | 16 (29.6) | |
| Intermediate | 28 (25.9) | 21 (46.7) | 13 (24.1) | |
| Immature | 5 (4.7) | 13 (28.9) | 25 (46.3) | |
| TSR, n (%) | 0.000* | |||
| Low (stroma-high) | 28 (25.9) | 23 (51.1) | 42 (77.8) | |
| High (stroma-low) | 80 (74.1) | 22 (48.9) | 12 (22.2) | |
| Adjuvant therapy, n (%) | 0.000* | |||
| No | 47 (43.5) | 11 (24.4) | 14 (25.9) | |
| Yes | 61 (56.5) | 34 (75.6) | 40 (74.1) | |
*P<0.05. MSI, microsatellite instability; CEA, carcinoembryonic antigen; CA242, carbohydrate antigen 242; CA724, carbohydrate antigen 724; CA199, carbohydrate antigen199; IOP status, internal obstruction or perforation; DR, desmoplastic reaction; TBd, tumour budding; TSR, tumor-stroma ratio.
Table 4
| Characteristics | TSR | P value | |
|---|---|---|---|
| Low (stroma-high) (N=93) | High (stroma-low) (N=114) | ||
| Gender, n (%) | 0.123 | ||
| Male | 62 (66.7) | 64 (56.1) | |
| Female | 31 (33.3) | 50 (43.9) | |
| Age, n (%) | 0.048* | ||
| >65 years | 29 (31.2) | 22 (19.3) | |
| ≤65 years | 64 (68.9) | 92 (80.7) | |
| Histologic grade, n (%) | 0.221 | ||
| Well | 13 (14.0) | 17 (14.9) | |
| Moderate | 47 (50.5) | 69 (60.5) | |
| Poor | 33 (35.5) | 28 (24.6) | |
| Location, n (%) | 0.300 | ||
| Ascending colon | 7 (7.5) | 16 (14.0) | |
| Transverse colon | 2 (2.2) | 2 (1.8) | |
| Descending colon | 4 (4.3) | 4 (3.5) | |
| Sigmoid colon | 21 (22.6) | 15 (13.2) | |
| Rectum | 59 (63.4) | 77 (67.5) | |
| Smoking, n (%) | 0.356 | ||
| No | 71 (76.3) | 93 (81.6) | |
| Yes | 22 (23.7) | 21 (18.4) | |
| Hypertension, n (%) | 0.241 | ||
| No | 72 (77.4) | 80 (70.2) | |
| Yes | 21 (22.6) | 34 (29.8) | |
| Family history of cancer, n (%) | 0.696 | ||
| No | 79 (84.9) | 99 (86.8) | |
| Yes | 14 (15.1) | 15 (13.2) | |
| Diabetes, n (%) | 0.995 | ||
| No | 84 (90.3) | 103 (90.4) | |
| Yes | 9 (9.7) | 11 (9.6) | |
| CEA level, n (%) | 0.925 | ||
| Normal | 52 (55.9) | 63 (55.3) | |
| Abnormal | 41 (44.1) | 51 (44.7) | |
| CA242, n (%) | 0.356 | ||
| Normal | 71 (76.3) | 93 (81.6) | |
| Abnormal | 22 (23.7) | 21 (18.4) | |
| CA724, n (%) | 0.815 | ||
| Normal | 73 (78.5) | 91 (79.8) | |
| Abnormal | 20 (21.5) | 23 (20.2) | |
| CA199, n (%) | 0.111 | ||
| Normal | 75 (80.6) | 101 (88.6) | |
| Abnormal | 18 (19.4) | 13 (11.4) | |
| Ki-67 level, n (%) | 0.318 | ||
| Low | 6 (6.5) | 3 (2.6) | |
| High | 87 (93.5) | 111 (97.4) | |
| T stage, n(%) | 0.930 | ||
| T3 | 59 (63.4) | 73 (64.0) | |
| T4 | 34 (36.6) | 41 (36.0) | |
| Lymphovascular invasion, n (%) | 0.710 | ||
| Absent | 73 (78.5) | 87 (76.3) | |
| Present | 20 (21.5) | 27 (23.7) | |
| Perineural invasion, n (%) | 0.015* | ||
| Absent | 80 (86.0) | 109 (95.6) | |
| Present | 13 (14.0) | 5 (4.4) | |
| IOP status, n (%) | 0.822 | ||
| No | 84 (90.3) | 104 (91.2) | |
| Yes | 9 (9.7) | 10 (8.8) | |
| Nodes examined, n (%) | 0.530 | ||
| 12 or more | 77 (82.8) | 98 (86.0) | |
| Less than 12 | 16 (17.2) | 16 (14.0) | |
| Mismatch repair status, n (%) | 0.05 | ||
| MSI-L | 66 (71.0) | 94 (82.5) | |
| MSI-H | 27 (29.0) | 20 (17.5) | |
| DR, n (%) | 0.000* | ||
| Mature | 27 (29.0) | 74 (64.9) | |
| Intermediate | 30 (32.3) | 32 (28.1) | |
| Immature | 36 (38.7) | 8 (7.0) | |
| TBd, n (%) | 0.000* | ||
| <5 (BD1) | 28 (30.1) | 80 (70.2) | |
| 5–9 (BD2) | 23 (24.7) | 22 (19.3) | |
| ≥10 (BD3) | 42 (45.2) | 12 (10.5) | |
| Adjuvant therapy, n (%) | 0.117 | ||
| No | 27 (29.0) | 45 (39.5) | |
| Yes | 66 (71.0) | 69 (60.5) | |
*P<0.05. MSI, microsatellite instability; CEA, carcinoembryonic antigen; CA242, carbohydrate antigen 242; CA724, carbohydrate antigen 724; CA199, carbohydrate antigen199; IOP status, internal obstruction or perforation; DR, desmoplastic reaction; TBd, tumour budding; TSR, tumor-stroma ratio.
DR was significantly associated with CEA level (P=0.035), PNI (P=0.024), IOP status (P=0.030), TBd (P<0.001), TSR (P<0.001) and adjuvant therapy (P=0.015). TBd was significantly associated with histological grade (P=0.046), hypertension (P=0.004), DR (P<0.001), TSR (P<0.001) and adjuvant therapy (P<0.001); and TSR was significantly associated with age (P=0.048), PNI (P=0.015), DR (P<0.001) and TBd (P<0.001).
Patients with immature DR had a significantly higher proportion of BD3 and low TSR; in contrast, participants with mature and intermediate DR had higher proportions of BD1 and high TSR, respectively. Patients with a low TSR more commonly presented with BD3, whereas patients with a TSR >50% more commonly presented with BD1.
Survival analysis
Overall, our results showed abnormal CA242, CEA, T4 stage, presence of hypertension, LVI, PNI, IOP, number of nodes examined less than 12, MSI-L, higher Ki-67 and immature DR to be associated with a lower RFS (Table S1). Kaplan–Meier curves with the log-rank test for RFS according to the presence of DR, TSR and TBd are presented in Figure 2. Patients with higher TB (BD3) and low TSR (stroma-high tumors) tended to have a worse RFS; however, the difference was not statistically significant (P=0.13 and 0.15, respectively). In addition, it is worth noting that the findings from Kaplan–Meier curves showed similar survival curves for the DR-intermediate and DR-mature groups, but only the DR-immature group had a significantly worse prognosis compared to the other two groups. According to this, in the univariate and multivariate analyses that followed, DR was categorized into 2 groups: immature DR and other DR types, as previously reported (15,26).

The univariate and multivariate Cox proportional hazards regression analyses for RFS in CRC are detailed in Table 5. In the univariate analyses of RFS, pT stage (P=0.040), LVI (P<0.001), PNI (P<0.001), IOP status (P=0.001), number of nodes examined (P<0.001), mismatch repair status (P=0.019), CEA (P=0.001), CA242 (P=0.024), DR (P<0.001) and adjuvant chemotherapy (P=0.001) were associated with RFS. When subjected to multivariate analysis, DR remained prognostic for RFS (HR =2.111; 95% CI: 1.184–3.766; P=0.011). LVI (HR 1.919; 95% CI 1.004-3.669; P=0.049) and PNI (HR =2.724; 95% CI: 1.362–5.448; P=0.005) were the only other parameters significantly associated with RFS.
Table 5
| Variable | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Gender | 0.839 | ||||||
| Male | Referent | ||||||
| Female | 0.942 | 0.532–1.668 | |||||
| Age | 0.111 | ||||||
| ≤65 years | Referent | ||||||
| >65 years | 1.023 | 0.995–1.052 | |||||
| Histologic grade | 0.328 | ||||||
| Well | Referent | ||||||
| Moderate | 1.408 | 0.542–3.657 | |||||
| Poor | 1.969 | 0.731–5.303 | |||||
| Location | 0.111 | ||||||
| Ascending colon | Referent | ||||||
| Transverse colon | 1.392 | 0.156–12.453 | |||||
| Descending colon | 2.582 | 0.578–11.544 | |||||
| Sigmoid colon | 2.598 | 0.855–7.896 | |||||
| Rectum | 1.166 | 0.409–3.324 | |||||
| Smoking | 0.726 | ||||||
| No | Referent | ||||||
| Yes | 1.127 | 0.577–2.201 | |||||
| Hypertension | 0.054 | ||||||
| No | Referent | ||||||
| Yes | 0.475 | 0.223–1.013 | |||||
| Family history of cancer | 0.656 | ||||||
| No | Referent | ||||||
| Yes | 1.188 | 0.558–2.530 | |||||
| Diabetes | 0.823 | ||||||
| No | Referent | ||||||
| Yes | 1.111 | 0.441–2.799 | |||||
| CEA | 0.001* | 0.190 | |||||
| Normal | Referent | ||||||
| Abnormal | 2.640 | 1.481–4.707 | 1.514 | 0.815–2.813 | |||
| CA724 | 0.109 | ||||||
| Normal | Referent | ||||||
| Abnormal | 1.657 | 0.894–3.074 | |||||
| CA242 | 0.024* | 0.091 | |||||
| Normal | Referent | ||||||
| Abnormal | 1.986 | 1.096–3.601 | 1.717 | 0.918–3.212 | |||
| CA199 | 0.075 | ||||||
| Normal | Referent | ||||||
| Abnormal | 1.837 | 0.940–3.589 | |||||
| Ki-67 level | 0.311 | ||||||
| Low | Referent | ||||||
| High | 1.01 | 0.990–1.031 | |||||
| T stage | 0.040* | 0.269 | |||||
| T3 | Referent | ||||||
| T4 | 1.787 | 1.026–3.114 | 1.416 | 0.764–2.623 | |||
| Lymphovascular invasion | <0.001* | 0.049* | |||||
| Absent | Referent | ||||||
| Present | 3.100 | 1.767–5.439 | 1.919 | 1.004–3.669 | |||
| Perineural invasion | <0.001* | 0.005* | |||||
| Absent | Referent | ||||||
| Present | 5.696 | 3.051–10.633 | 2.724 | 1.362–5.448 | |||
| IOP status | 0.001* | 0.240 | |||||
| Absent | Referent | ||||||
| Present | 2.976 | 1.522–5.817 | 1.571 | 0.740–3.334 | |||
| Number of nodes examined | <0.001* | 0.085 | |||||
| 12 or more | Referent | ||||||
| Less than 12 | 3.301 | 1.834–5.940 | 1.777 | 0.925–3.417 | |||
| Mismatch repair status | 0.019* | 0.267 | |||||
| MSI-L | Referent | ||||||
| MSI-H | 0.331 | 0.132–0.835 | 0.574 | 0.215–1.530 | |||
| DR | <0.001* | ||||||
| Mature + intermediate | Referent | ||||||
| Immature | 3.021 | 1.715–5.323 | 2.111 | 1.184–3.766 | 0.011* | ||
| TBd | 0.135 | ||||||
| <5 (BD1) | Referent | ||||||
| 5–9 (BD2) | 1.632 | 0.812–3.281 | |||||
| ≥10 (BD3) | 1.872 | 0.981–3.574 | |||||
| TSR | 0.158 | ||||||
| High (stroma-low) | Referent | ||||||
| Low (stroma-high) | 1.493 | 0.856–2.604 | |||||
| Adjuvant therapy | 0.001* | 0.400 | |||||
| No | Referent | ||||||
| Yes | 3.713 | 1.670–8.256 | 1.482 | 0.593–3.704 | |||
*, P<0.05. MSI, microsatellite instability; CEA, carcinoembryonic antigen; CA242, carbohydrate antigen 242; CA724, carbohydrate antigen 724; CA199, carbohydrate antigen199; IOP status, internal obstruction or perforation; DR, desmoplastic reaction; TBd, tumour budding; TSR, tumor-stroma ratio.
Construction and validation of a nomogram
A nomogram was developed based on the above prediction model (Figure 3A), providing the predicted 3-year disease recurrence probability for each patient. The calibration curve of the nomogram for the probability of disease recurrence showed a good agreement between the predicted and actual survival (Figure 3B). Moreover, an ROC curve was plotted for the evaluation of the model performance (Figure 4). An optimal cut-off value that maximized the sum of sensitivity and specificity in the ROC curve was identified. The best cut-off point was 0.235. The prediction model yielded an AUC of 0.826 (95% CI: 0.758–0.895; accuracy 0.749; sensitivity 0.780; specificity 0.739) in our cohort.


Discussion
In this retrospective study, two important clinical findings were discovered. First, TBd, TSR, and DR were significantly associated with conventional clinicopathological prognostic factors in stage II CRC. Second, DR was an independent prognostic factor in addition to LVI and PNI in the multivariate analysis of RFS. Furthermore, we developed an easy-to-use nomogram incorporating the above variables for the preoperative individualized prediction of disease recurrence in patients with stage II CRC.
Adjuvant chemotherapy (fluorouracil plus oxaliplatin regimen) was recommended only for stage II CRC patients with high risk factors for recurrence, defined by the NCCN guidelines (27), including T4 tumor stage, poor histologic grade, obstruction or perforation, LVI, PNI, a small number (<12) of lymph nodes, and positive margins. However, the methodology of identifying patients with high recurrence risk remains controversial (28). Several studies have found that according to these clinicopathological factors with a high recurrence risk, many stage II CRC patients defined as having a high recurrence risk have no recurrence and have long-term survival, while some patients without high recurrence risk factors experience recurrence (29,30). Hence, the high recurrence risk factors, as judged only by “macroscopic” and “submicroscopic” indicators such as anatomy and histopathology alone, cannot always correctly predict the prognosis of patients. Recently, an increasing number of people have realized that in addition to the heterogeneity of the tumor itself, the heterogeneity of the tumor microenvironment is also an important factor.
According to our study, we showed an association between TBd and TSR. Consistent with our study results, van Wyk (31) reported that TBd was positively correlated with tumor stroma. However, the current study showed that both TBd and TSR failed to predict RFS outcomes. To the best of our knowledge, the prognostic value of TSR and TBd in CRC has been extensively studied, but the results remain controversial; for example, Mesker et al. (32) was the first study to show that a high TSR associated with both longer DFS and OS is an independent prognostic variable for colon cancer. Other reports have reached the opposite conclusion (8,9,20,33). Similarly, a wealth of studies has identified the independent prognostic value of TBd for the prediction of patient clinical outcomes in CRC (34-37), but this is contradictory to other studies that have not found any significant correlation between TBd and OS or RFS (19,38). We speculated that these findings may be attributable to the differences in the study populations. In our cohort, we found that only 55.1% of tumors had a high TSR, which is lower than that reported by others (70.8% [9], 70.51% [19], and 73.00% [32]). Additionally, our study included patients with stage II CRC, of which rectal cancer constituted the majority (65.7%), whereas the study by Koelzer et al. (39) contained only upper rectal cancers. Furthermore, the heterogeneity of stage II tumors is inevitable, and thus these might explain the divergence in the results obtained, although larger study sample sizes are needed before widespread implementation in clinical practice.
DR refers to the presence of excess extracellular matrix at the tumor invasive front (40). Previous studies performed by Ueno et al. have shown that DR is associated with adverse clinicopathological features (advanced T stage and lymphatic or vascular invasion) in CRC (23,40). Meanwhile, DR is an independent factor for poor prognosis in stage II CRC (15). In addition, Ueno et al. (23) showed that compared with T stage, MSI and TBd, DR is the best prognostic factor for stage II colon cancer. In our study, the DR categorization was the mature type in 101 patients (48.8%), the intermediate type in 62 patients (30.0%), and the immature type in 44 patients (21.2%). Moreover, this study found that DR was associated with CEA level, PNI, IOP, TSR and TBd. Additionally, our results revealed that DR categorization could stratify the risk of recurrence in stage II CRC patients. Compared with patients with mature stroma, patients with immature stroma had the most unfavorable prognosis. Notably, compared to TSR and TBd, DR was the only independent factor for RFS, as demonstrated by multivariate analysis in the current study. Based on the current results, we showed that the prognostic significance of DR outperformed TBd and TSR and recommended adding DR as a biomarker in routine pathological reports.
Since many patients who are diagnosed with stage II CRC have concerns about their outcome after surgery, there is an urgent need to build a scoring system for reference. To date, several potential biomarkers, such as FOXP3+ tumor-infiltrating lymphocytes (TILs), microRNAs and radiomics signatures, have been included in nomograms to substratify these patients and guide individualized treatment decisions (41-43). However, these biomarkers still require further validation and are not included in the routine checklist for CRC.
In the present study, we constructed a predictive nomogram that can generate an individual probability of having disease recurrence by integrating both the significant preoperative clinicopathologic variables and DR, which is easily evaluated using hematoxylin-eosin-stained specimens. Therefore, both physicians and patients could use this novel, easy-to-use and cost-effective scoring system to make preoperative individualized predictions of the disease recurrence risk, in line with the current trends in personalized medicine (44).
Although this is a preliminary study, it still has some limitations. First, as a retrospective study, there was an unavoidable selection bias. Second, this study included patients from 5 to 10 years ago, and the development of treatment for CRC during this period may have affected the prognosis. Finally, the data were based on a single cancer center and lacked external validation. For this reason, large multicenter studies are needed to further investigate the trends.
To conclude, our study highlighted the prognostic value of DR in stage II CRC. Moreover, the results of the study provided evidence that DR, as a novel histopathological predictor, could contribute to improve the prognostic performance for the prediction of RFS in stage II CRC after surgery. Therefore, DR should be considered in the routine pathological report. Furthermore, the significant correlation of DR with several conventional high-risk clinicopathological factors suggests that such a model with DR may have the potential to substratify the high-risk patients and predict patients who are more likely to benefit from postoperative adjuvant chemotherapy, although future multicentric and prospective studies are needed before widespread implementation in clinical practice.
Acknowledgments
Funding: This study was supported by the National Key Research and Development Program of China (Nos. 2021YFC2500400 and 2021YFC2500402) and Tianjin Key Medical Discipline (specialty) Construction Project (No. TJYXZDXK-009A).
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-758/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-758/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-758/prf
Conflicts of interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-758/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective study was approved by the Ethics Committee of Tianjin Medical University Cancer Institute & Hospital (No. EK2022175) and a waiver of patient consent was granted for the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Cao W, Chen HD, Yu YW, et al. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J (Engl) 2021;134:783-91. [Crossref] [PubMed]
- Milburn J, Richard MG, Elliot AA, et al. AJCC cancer staging manual. 8th ed. New York, NY: Springer, 2016;251-651.
- Schrag D, Rifas-Shiman S, Saltz L, et al. Adjuvant chemotherapy use for Medicare beneficiaries with stage II colon cancer. J Clin Oncol 2002;20:3999-4005. [Crossref] [PubMed]
- O'Connor ES, Greenblatt DY, LoConte NK, et al. Adjuvant chemotherapy for stage II colon cancer with poor prognostic features. J Clin Oncol 2011;29:3381-8. [Crossref] [PubMed]
- Di Maggio F, El-Shakankery KH. Desmoplasia and Biophysics in Pancreatic Ductal Adenocarcinoma: Can We Learn From Breast Cancer? Pancreas 2020;49:313-25. [Crossref] [PubMed]
- Domen A, Quatannens D, Zanivan S, et al. Cancer-Associated Fibroblasts as a Common Orchestrator of Therapy Resistance in Lung and Pancreatic Cancer. Cancers (Basel) 2021;13:987. [Crossref] [PubMed]
- Huijbers A, Tollenaar RA, V, Pelt GW, et al. The proportion of tumor-stroma as a strong prognosticator for stage II and III colon cancer patients: validation in the VIC-TOR trial. Ann Oncol 2013;24:179-85. [Crossref] [PubMed]
- Park JH, Richards CH, McMillan DC, et al. The relationship between tumour stroma percentage, the tumour microenvironment and survival in patients with primary operable colorectal cancer. Ann Oncol 2014;25:644-51. [Crossref] [PubMed]
- Park JH, McMillan DC, Powell AG, et al. Evaluation of a tumor microenvironment-based prognostic score in primary operable colorectal cancer. Clin Cancer Res 2015;21:882-8. [Crossref] [PubMed]
- Hansen TF, Kjaer-Frifeldt S, Lindebjerg J, et al. Tumor-stroma ratio predicts recurrence in patients with colon cancer treated with neoadjuvant chemotherapy. Acta Oncol 2018;57:528-33. [Crossref] [PubMed]
- van Pelt GW, Sandberg TP, Morreau H, et al. The tumour-stroma ratio in colon cancer: the biological role and its prognostic impact. Histopathology 2018;73:197-206. [Crossref] [PubMed]
- Angeli F, Koumakis G, Chen MC, et al. Role of stromal fibroblasts in cancer: Promoting or impeding? Tumour Biol 2009;30:109-20. [Crossref] [PubMed]
- Ohno K, Fujimori T, Okamoto Y, et al. Diagnosis of desmoplastic reaction by immunohistochemical analysis, in biopsy specimens of early colorectal carcinomas, is efficacious in estimating the depth of invasion. Int J Mol Sci 2013;14:13129-36. [Crossref] [PubMed]
- Nearchou IP, Kajiwara Y, Mochizuki S, et al. Novel Internationally Verified Method Reports Desmoplastic Reaction as the Most Significant Prognostic Feature for Dis-ease-specific Survival in Stage II Colorectal Cancer. Am J Surg Pathol 2019;43:1239-48. [Crossref] [PubMed]
- Berg KB, Schaeffer DF. Tumor budding as a standardized parameter in gastrointestinal carcinomas: more than just the colon. Mod Pathol 2018;31:862-72. [Crossref] [PubMed]
- Lugli A, Kirsch R, Ajioka Y, et al. Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod Pathol 2017;30:1299-311. [Crossref] [PubMed]
- Goyal S, Banga P, Meena N, et al. Prognostic significance of tumour budding, tumour–stroma ratio and desmoplastic stromal reaction in gall bladder carcinoma. J Clin Pathol 2021; Epub ahead of print. [Crossref] [PubMed]
- Eriksen AC, Sørensen FB, Lindebjerg J, et al. The prognostic value of tumour stroma ratio and tumour budding in stage II colon cancer. A nationwide population-based study. Int J Colorectal Dis 2018;33:1115-24. [Crossref] [PubMed]
- Zhang Y, Liu Y, Qiu X, et al. Concurrent Comparison of the Prognostic Values of Tu-mor Budding, Tumor Stroma Ratio, Tumor Infiltrating Pattern and Lympho-cyte-to-Monocyte Ratio in Colorectal Cancer Patients. Technology in Cancer Research & Treatment 2021;20:1-11. [Crossref]
- Ueno H, Jones AM, Wilkinson KH, et al. Histological categorisation of fibrotic cancer stroma in advanced rectal cancer. Gut 2004;53:581-6. [Crossref] [PubMed]
- Ueno H, Shinto E, Shimazaki H, et al. Histologic categorization of desmoplastic reaction: its relevance to the colorectal cancer microenvironment and prognosis. Ann Surg Oncol 2015;22:1504-1512. [Crossref] [PubMed]
- Ueno H, Kajiwara Y, Shimazaki H, et al. New criteria for histologic grading of colorectal cancer. Am J Surg Pathol 2012;36:193-201. [Crossref] [PubMed]
- Mesker WE, Junggeburt JM, Szuhai K, et al. The carcinoma-stromal ratio of colon carcinoma is an independent factor for survival compared to lymph node status and tumor stage. Cell Oncol 2007;29:387-98. [PubMed]
- Youden WJ. Index for rating diagnostic tests. Cancer 1950;3:32-5. [Crossref] [PubMed]
- Ueno H, Kanemitsu Y, Sekine S, et al. A Multicenter Study of the Prognostic Value of Desmoplastic Reaction Categorization in Stage II Colorectal Cancer. Am J Surg Pathol 2019;43:1015-22. [Crossref] [PubMed]
- National Comprehensive Cancer Network. NCCN Guidelines Colon Cancer (Version 2. 2021). National Comprehensive Cancer Network. Available online: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf (accessed March 11, 2021).
- Tournigand C, Andre T, Bonnetain F, et al. Adjuvant therapy with fluorouracil and oxaliplatin in stage II and elderly patients (between ages 70 and 75 years) with colon cancer: subgroup analyzes of the multicenter international study of oxaliplatin, fluorouracil, and leucovorin in the adjuvant treatment of colon cancer trial. J Clin Oncol 2012;30:3353-60. [Crossref] [PubMed]
- Sargent DJ, André T, Grothey A. Further Evaluating the Benefit of Adjuvant Chemotherapy for Colon Cancer. J Clin Oncol 2016;34:3711-2. [Crossref] [PubMed]
- Påhlman LA, Hohenberger WM, Matzel K, et al. Should the Benefit of Adjuvant Chemotherapy in Colon Cancer Be Re-Evaluated? J Clin Oncol 2016;34:1297-9. [Crossref] [PubMed]
- van Wyk HC, Roseweir A, Alexander P, et al. The Relationship Between Tumor Budding, Tumor Microenvironment, and Survival in Patients with Primary Operable Colorectal Cancer. Ann Surg Oncol 2019;26:4397-404. [Crossref] [PubMed]
- Mesker WE, Liefers GJ, Junggeburt JM, et al. Presence of a High Amount of Stroma and Downregulation of SMAD4 Predict for Worse Survival for Stage I-II Colon Cancer Patients. Cell Oncol 2009;31:169-78. [PubMed]
- Fu M, Chen D, Luo F, et al. Association of the tumour stroma percentage in the pre-operative biopsies with lymph node metastasis in colorectal cancer. Br J Cancer 2020;122:388-96. [Crossref] [PubMed]
- Lang-Schwarz C, Melcher B, Haumaier F, et al. Budding and tumor-infiltrating lym-phocytes – combination of both parameters predict survival in colorectal cancer and leads to new prognostic subgroups. Hum Pathol 2018;79:160-7. [Crossref] [PubMed]
- Graham RP, Vierkant RA, Tillmans LS, et al. Tumor Budding in Colorectal Carcinoma: Confirmation of Prognostic Significance and Histologic Cutoff in a Population-based Cohort. Am J Surg Pathol 2015;39:1340-6. [Crossref] [PubMed]
- Wang LM, Kevans D, Mulcahy H, et al. Tumor budding is a strong and reproducible prognostic marker in T3N0 colorectal cancer. Am J Surg Pathol 2009;33:134-41. [Crossref] [PubMed]
- Ryan É, Khaw YL, Creavin B, et al. Tumor budding and PDC grade are stage independent predictors of clinical outcome in mismatch repair deficient colorectal cancer. Am J Surg Pathol 2018;42:60-8. [Crossref] [PubMed]
- Gilardoni E, Bernasconi DP, Poli S, et al. Surveillance for early stages of colon cancer: potentials for optimizing follow-up protocols. World J Surg Oncol 2015;13:260. [Crossref] [PubMed]
- Koelzer VH, Assarzadegan N, Dawson H, et al. Cytokeratin-based assessment of tumour budding in colorectal cancer: analysis in stage II patients and prospective diagnostic experience. J Pathol Clin Res 2017;3:171-8. [Crossref] [PubMed]
- Ueno H, Sekine S, Oshiro T, et al. Disentangling the prognostic heterogeneity of stage III colorectal cancer through histologic stromal categorization. Surgery 2018;163:777-83. [Crossref] [PubMed]
- Zhang JX, Song W, Chen ZH, et al. Prognostic and predictive value of a microRNA signature in stage II colon cancer: a microRNA expression analysis. Lancet Oncol 2013;14:1295-306. [Crossref] [PubMed]
- Feng Y, Li Y, Cai S, et al. Immunological nomograms predicting prognosis and guiding adjuvant chemotherapy in stage II colorectal cancer. Cancer Manag Res 2019;11:7279-94. [Crossref] [PubMed]
- Fan S, Cui X, Liu C, et al. CT-Based Radiomics Signature: A Potential Biomarker for Predicting Postoperative Recurrence Risk in Stage II Colorectal Cancer. Front Oncol 2021;11:644933. [Crossref] [PubMed]
- Balachandran VP, Gonen M, Smith JJ, et al. Nomograms in oncology: more than meets the eye. Lancet Oncol 2015;16:e173-80. [Crossref] [PubMed]


