
Expression and clinical significance of miR-17-5p in tumor tissues of patients with colorectal cancer
Highlight box
Key findings
• miR17-5 was found to be highly expressed in colorectal cancer
What is known and what is new?
• Aberrant miRNA expression has important functions in colorectal cancer development and metastasis, and can act as tumor suppressors and oncogenes to regulate mRNA expression.
• miR17-5P among miRNAs can regulate proliferation, invasion, apoptosis and metastasis of colorectal cancer cells.
What is the implication, and what should change now?
• To investigate the specific molecular mechanisms by which miR17-5P regulates the proliferation, invasion, apoptosis and metastasis of colorectal cancer cells.
Introduction
Colorectal cancer (CRC) is the third most common malignant tumor and the second leading cause of mortality worldwide, with approximately 1.9 million new cases and 940,000 deaths each year (1). CRC morbidity and mortality in China have remained high over the past decades (2). There is a gradual progression between the development of benign lesions to colorectal malignancy, and up to 90% of deaths can be avoided if detected in a timely manner. Early symptoms of CRC are atypical and are often misdiagnosed (3). Therefore, it is critical to investigate the mechanisms of CRC development, as well as to identify the clinical diagnostic indicators and molecular therapeutic targets of early CRC.
MicroRNAs (miRNAs) are a type of non-coding single-stranded RNA molecule that ranges in length from 19 to 25 nucleotides and are now classified as tumor suppressor genes and oncogenes. MiRNAs are involved in a variety of processes, including cell differentiation, cell cycle regulation, and apoptosis, and are commonly dysregulated in different cancers (4). For example, high expression of microRNA promotes the proliferation and invasion of bladder tumor tissue (5). MiR-17-5p is overexpressed in a variety of tumor tissues, including gastric (6), lung (7), breast (8), and cervical cancer (9), and it promotes tumor cell proliferation and migration. It has also been suggested that miRNAs can inhibit the proliferation and invasive ability of colorectal cancer cells through target genes (10). The present study examined the expression of miR-17-5p in colorectal tumors and paracancerous tissues obtained from patients and using data downloaded from TCGA and GEO databases. The expression of miR-17-5p in colorectal cancer tissues was found to be significantly higher than that in normal tissues (Figure S1). Therefore, this experiment mainly verified the expression of miR-17-5p in colorectal tumor tissues and paraneoplastic tissues, and investigated the effects of miR-17-5p on the proliferation, invasion, migration and apoptosis of colorectal cancer cells HCT-116 through in vitro cellular assays, in order to explore whether miR-17-5p can be used as an early diagnostic indicator and therapeutic target for colorectal cancer. We present the following article in accordance with the MDAR reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1185/rc).
Methods
Cell lines, tissue specimens, and major reagents
The human HCT-116 CRC cell line was purchased from the Cell Bank of Type Culture Collection of the Chinese Academy of Sciences Committee (Shanghai, China). Tumor tissues and corresponding paracancerous tissues were collected from 30 patients with CRC who underwent surgical resection in the Department of General Surgery V of the First Affiliated Hospital of Gannan Medical College from December 2019 to January 2021. The clinical characteristics, including gender, age, tumor location, tumor size, TNM stage (according to the International Union Against Cancer 8th edition), and pathological and CEA data were collated. Written informed consent was obtained from all participants. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Research Ethics Committee of The First Affiliated Hospital of Gannan Medical College (Ethics No. 20190507). All sample tissues were removed and transported in liquid nitrogen to −80 ℃ for storage in the refrigerator or used immediately for subsequent experiments. The following inclusion criteria were applied: (I) patients who underwent CRC surgery and were diagnosed with CRC based on post-surgical pathology; (II) preoperative detection and review of serum tumor markers was performed; and (III) complete clinical data was available. Patients with the following conditions were excluded: (I) inflammatory bowel disease; (II) rheumatoid arthritis; (III) multiple primary tumors; or (IV) combined heart, liver, lung, and kidney disease that was severely intolerant of surgery.
Dulbecco's Modified Eagle Medium (DMEM), fetal bovine serum, trypsin, penicillin, and streptomycin were purchased from Gibco. The miR-17-5p overexpression mimics, inhibitor, and corresponding controls, hairpin miRNAs quantification kits, and U6 calibration real-time fluorescence quantitative PCR (RT-qPCR) kits were purchased from Genepharma Co. (Shanghai, China). Trizol (Invitrogen), SYBR Green (Bio-rad), Annexin V-FITC/PI apoptosis detection kit, RIPA lysate, PMSF, Western blocking solution, and BeyoColor color pre-stained protein molecular standards (10-170KD) were purchased from Beyotime Co. (Shanghai, China). The following antibodies were purchased from Beyotime Co. (Shanghai, China): CD44 (AF0105), MMP2 (AF1420), BCL2 (AF0060), BAX (AF0054), β-actin (AF003), and E-cadherin (AF0138).
Cell culture and transfection
The HCT-116 cell line was cultured in DMEM medium supplemented with 1% penicillin-streptomycin and 10% fetal bovine serum and cultured at 37 ℃ in a humidified atmosphere containing 5% CO2. The cells were digested with 0.25% trypsin for passaging when cell confluence reached 80%.
HCT-116 cells at the logarithmic growth stage were digested, inoculated at 1.5×105 cells/well in a 6-well culture plate overnight. The next day, miR-17-5p mimic, negative control (NC), miR-17-5p inhibitor, and inhibitor negative control (in-NC) were transfected into the cells using LipofectamineTM 2000 according to the manufacturer’s instructions, and the efficiencies were detected by fluorescence microscopy and RT-qPCR at 24 hours.
RT-qPCR
Total RNA was extracted from CRC tissues and transfected cells using Trizol reagent, and reverse transcription was performed using the SYBR Green kit with β-actin as the internal reference. The reaction conditions were 40 cycles at 95 ℃ for 5 minutes, 95 ℃ for 12 seconds, 60 ℃ for 30 seconds, and 72 ℃ for 30 seconds. The 2–ΔΔCt method was used for quantification.
Clone formation assay
HCT-116 cells were inoculated at 1.5×105 cells per well in 6-well plates and divided into four groups: the inhibitor group, the in-NC group, the mimics group, and the NC group. After transfection, the culture process would continue for 24 hours. Following digestion, 150 cells from each group were inoculated and culture for 1–2 weeks in new 6-well plates. After staining with 1% crystal violet for 20 minutes, the number of colonies in each well was observed under light microscopy (Zeiss) and recorded.
Scratch assay
After cell inoculation and transfection, when the cell confluence reached 80–90% the following day, a horizontal marker line was drawn at the bottom of the six-well plate. A 10 μL gun tip thaner straightedge was used to draw a line perpendicular to the scratch behind. The cell culture medium was discarded, and the cells were washed once with PBS, followed by culture in fresh complete medium culture. Photographs (at 4× magnification) were taken at 0, 24, and 48 hours, and the healing area was statistically analyzed.
Transwell assay
Transwell chambers were pre-populated with 100 µL matrigel (1:8 dilution with medium) and incubated for 4 hours at 37 ℃ until the liquid solidified. Complete medium (10%) was added to the lower layer of the Transwell chambers. A total of 1×105 transfected cells were resuspend in 200 µL of 1% bovine serum albumin (BSA)-free culture medium and seeded into the Transwell chambers. After incubation for 12 hours, the upper cells of the chambers were removed with cotton swabs, fixed in methanol for 15 minutes, stained with crystal violet, and placed under a 400× microscope for observation and counting. Since cell adhesion may lead to inaccurate counts under the microscope, the crystalline violet was again dissolved with sodium acetate and the OD value was measured at 570 nm wavelength to indirectly reflect the number of cells.
Flow cytometry
After cell inoculation and transfection, cells were removed at 24 and 48 hours and resuspended in phosphate buffered saline (PBS) at a density of 1×105 cells/mL, centrifuged, and the supernatant was discarded. Annexin V-FITC conjugate (195 µL) was added to the centrifuge tube, resuspended, and mixed with 10 µL propidium iodide staining solution (PI). The cell suspension was then incubated for 10 minutes at 20–25 ℃, protected from light. Subsequently, 200 µL of the mixture was transferred to a Falcon tube and mixed with 1× binding buff (400 µL). Flow cytometry (BD) was performed to detect the apoptosis rate of each group of cells.
Western blot
Total protein was extracted 48 hours after cell transfection and quantified using the BCA protein quantification kit. 5× protein loading buffer at 4:1 volume was added to the samples and incubated in boiling water at 100 ℃ for 5 minutes. Follow protein electrophoresis and electrotransformation, samples were incubated with primary and secondary antibodies. Human β-actin protein was used as an internal reference for analysis. The relative gray value of each band was analyzed using the Image J software.
Statistical analysis
For clinical and database samples, Wilcoxon analysis was used to assess the correlation between miR-17-5p expression levels and clinicopathological characteristics. For cellular experimental data, normally distributed measures were analyzed by mean ± standard deviation. The Student’s t-test was used for comparison between two groups, and one-way analysis of variance (ANOVA) was used for comparison between multiple groups. A P value <0.05 or <0.01 was considered statistically significant. Data was processed using SPSS 22.0, Adobe Illustrator 2020, and GraphPad 6.0 software.
Results
The expression of miR-17-5p in CRC tumor tissues and paracancer tissues
The expression of miR-17-5p mRNA in 30 pairs of clinical CRC tumor tissues and paracancerous tissues was assessed by RT-qPCR. MiR-17-5p was found to be more abundant in 25 pairs of CRC cancer tissues compared to the paired paracancerous tissues, but less abundant in 5 pairs of CRC cancer tissues compared to the paracancerous tissues (Figure 1A). Statistical analysis showed that the expression of miR-17-5p was significantly higher in CRC than in paracancerous tissues (P<0.01, Figure 1B).

The relationship between miR-17-5p and clinicopathological characteristics
To investigate the relationship between miR-17-5p expression and clinicopathological features, patients were divided into a high expression group (relative expression >1.3) and a low expression group (relative expression <1.3), based on the expression of miR-17-5p in individual tumor and paracancerous tissues. As shown in Table 1, the expression of miR-17-5p was only related to differentiation degree, TNM stage, tumor size, and lymph node metastasis of patients (P<0.05), but not to age, gender, nor site of occurrence (P>0.05).
Table 1
| Clinicopathological characteristics |
Cases | MiR-17-5p expression | P | |
|---|---|---|---|---|
| Low-expression | High-expression | |||
| Gender | 0.632 | |||
| Male | 16 | 5 | 11 | |
| Female | 14 | 3 | 11 | |
| Age (years) | 0.461 | |||
| ≥60 | 17 | 4 | 13 | |
| <60 | 13 | 4 | 9 | |
| Differentiation | 0.02 | |||
| High | 3 | 1 | 2 | |
| Moderate | 19 | 5 | 14 | |
| Low | 8 | 2 | 6 | |
| Lymph node metastasis | <0.001 | |||
| Yes | 27 | 8 | 19 | |
| No | 3 | 0 | 3 | |
| Tumor size (cm) | 0.012 | |||
| <5 | 14 | 6 | 8 | |
| ≥5 | 16 | 2 | 14 | |
| TNM stage | 0.018 | |||
| I, II | 17 | 5 | 12 | |
| III, IV | 13 | 3 | 10 | |
| T stage | 0.008 | |||
| T1, T2 | 2 | 1 | 1 | |
| T3, T4 | 28 | 7 | 21 | |
| Distant metastasis | 0.657 | |||
| Yes | 24 | 3 | 21 | |
| No | 6 | 5 | 1 | |
| Site of occurrence | 0.652 | |||
| Colon | 7 | 4 | 3 | |
| Rectal | 23 | 4 | 19 | |
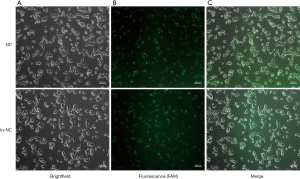
Fluorescence microscope observation of transfection efficiency
Untransfected cells were seeded into 6-well plates at a density of 1.5×105 cells/well and cultured for 16 hours. When the cell density reached 50–60%, transfection was performed. Fluorescent FAM-labeled NC and in-NC were used to determine the transfection efficiency and the results were observed by fluorescence microscopy to determine the amount of liposomes with miRNA. The liposome dosage was 5 µL, and the miRNA amount was at 100 pmol/L, and the transfection efficiency was greater than 90%. All subsequent experiments were performed according to this ratio and amount (Figure 2).
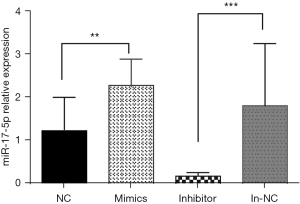
Detection of interference and overexpression effects by RT-qPCR
The effects of enhancing or inhibiting miR-17-5p expression on cell function were examined in HCT-116 cells. First, the effects of the synthesized miR-17-5p interference fragment (inhibitor) and the overexpression fragment (mimics) were analyzed. MiR-17-5p expression was decreased by 83% in the inhibitor group compared to the negative transfection group (in-NC group), while mimic expression was increased by 127% in the overexpression group compared to the negative transfection group (NC group) (Table 2 and Figure 3).
Table 2
| Calculation method | NC | Mimics | in-NC | Inhibitor |
|---|---|---|---|---|
| △CT miR-17-5p-U6 | 7.66±1.28 | 6.52±1.33 | 5.96±2.43 | 8.66±1.79 |
| 2−△△CT test group-control group | 1.23 | 2.27 | 1.8 | 0.17 |
NC, negative control; in-NC, inhibitor negative control; △CT:CT miR-17-5p-CT U6.
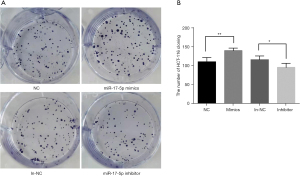
The effects of miR-17-5p on the proliferative capacity of HCT-116 cells
Cells transfected with the miR-17-5p mimics showed increased proliferation rates, while cells transfected with the inhibitor had the slowest proliferation rate (Figure 4).
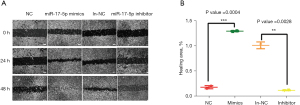
The effects of miR-17-5p on the migration ability of HCT-116 cells
HCT-116 cells were transfected with the miR-17-5p mimic or inhibitor and the effects on migration were observed at 0, 24, and 48 hours (Figure 5A), and statistical analysis of the healing area (Figure 5B). The cells in the overexpression mimics group had a significantly narrower scratch width at 24 hours compared to that at 0 hours, and the cells migrated significantly faster than cells in the NC group. At 48 hours, the scratch in the overexpression mimics group was no longer visible, whereas the scratch in the NC group remained visible. The scratch width in the inhibitor group at 24 hours was not different from that at 0 hours, becoming only slightly narrower at 48 hours. In contrast, the scratch width of the in-NC group became significantly narrower over 48 hours. Statistical analysis showed that the healing area of the mimics group was significantly larger than that of cells transfected with NC (P<0.001); and the healing area of the inhibitor group was significantly smaller than that of the in-NC group (P<0.01).
The effects of miR-17-5p on the invasive ability of HCT-116 cells
HCT-116 cells can secrete matrix metalloprotein (MMPs) to degrade Matrigel matrix gels and thus cross the vesicles. Figure 6A shows the microscopic observations (10×10) after crystalline violet staining. The number of membrane penetrating cells in Figure 6A was counted microscopically for the NC group (121.32±14.43), the mimics group (206.0±23.56), the in-NC group (117.28±11.21), and the inhibitor group (56±9.17). Since the cells were not homogeneous below the chambers, for more accurate comparison, crystalline violet was dissolved using 33% acetic acid and the OD value was measured. Higher OD value indicated higher number of perforated cells (see Figure 6B). By counting the results under the microscope and calculating the OD value, statistical analysis showed that the number of cells transfected with miR-17-5p mimics that lysed matrix gel and crossed the cells of the small chamber within the same time was significantly greater than that of cells transfected with negative NC group (P<0.01). The number of cells transfected with miR-17-5p inhibitor that lysed matrix gel and crossed the cells of the small chamber within the same time was significantly smaller than that of in- NC group (P<0.01). HCT-116 cells transfected with miR-17-5p crossed the cellular compartment significantly more than cells transfected with the miR-17-5p inhibitor, whereas cells transfected with miR-17-5p inhibitor showed the opposite trend.
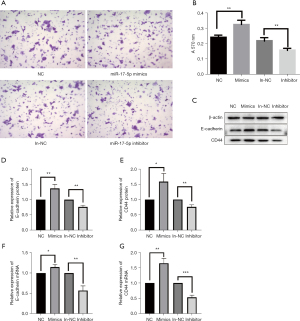
The relative mRNA and protein expression of migration-related genes, E-cadherin and CD44, were detected by Western blot and RT-qPCR in HCT-116 cells transfected with miR-17-5p mimics and inhibitors (Figures 6C-6G). The mRNA and protein expression followed a similar trend. When miR-17-5p expression was upregulated, E-cadherin and CD44 expression were both significantly upregulated. Conversely, when miR-17-5p expression was downregulated, E-cadherin and CD44 expression were both significantly decreased.
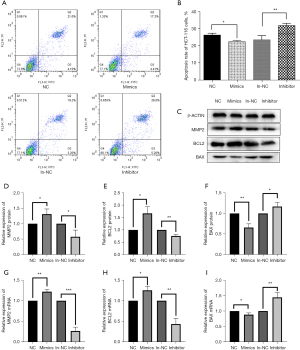
The effects of miR-17-5p on the apoptosis of HCT-116 cells
The cells of each group were collected 24 hours after successful transfection, AnnixinV-FITC/PI stained, and the effects of miR-17-5p expression on the apoptosis of HCT-116 cells were detected by flow cytometry. As shown in Figure 7A and 7B, the apoptosis of HCT-116 cells was significantly reduced after transfection with miR-17-5p mimics (P<0.05), while transfection with miR-17-5p inhibitor significantly increased the rate of apoptosis (P<0.01). The effects of miR-17-5p expression on the levels of MMP2, BCL2, and BAX were also determined by Western blot and RT-qPCR assays (Figure 7C-7I). When miR-17-5p expression was upregulated, MMP2 and BCL2 expression increased significantly, and BAX expression decreased significantly. Conversely, when miR-17-5p expression was downregulated, the expression of MMP2 and BCL2 decreased significantly, while BAX expression increased significantly. MMP2, a subtype of matrix metalloproteinases (MMPs), plays an important role in tumorigenesis and progression by affecting apoptosis, proliferation and promoting angiogenesis. And MMP2, BAX and BCL2 are the genes most associated with apoptosis. The increase of MMP2 in the mimics group compared with the inhibitor group suggests that miR17-5P is associated with apoptosis of cells.
Discussion
The incidence of CRC rises annually, and it is increasingly affecting the younger population. The pathogenesis of CRC remains unclear, but may be related to factors such as age, hyperlipidemia, genetics, and inflammatory bowel disease (11). CRC is primarily diagnosed through colonoscopy, but this is often performed only after the onset of symptoms, and screening the whole population is difficult. The symptoms associated with CRC are often subtle, and it is frequently only diagnosed at an advanced stage. As a result, early detection and treatment of CRC is critical for lowering patient mortality. Understanding the molecular mechanisms of CRC may contribute to the development of early detection methods and targeted treatment strategies.
In this study, the relative expression of miR-17-5p in cancer tissues was generally significantly higher than that in paracancerous tissues (P<0.01). This is consistent with the results of previous study (12). Furthermore, when the clinical data were compared, the level of miR-17-5p was not significantly correlated with age, gender, nor distant metastasis, but was significantly correlated with the TNM stage and tumor differentiation, diameter, and the presence of lymph node metastasis. The higher the TNM stage, the less differentiated the tumor is, and this is associated with greater malignancy and higher detection of tumor markers (13). High-grade tumors or poorly differentiated tissues have poor prognosis and low survival rates. Therefore, it may be possible to use miR-17-5p levels as a biomarker in CRC patients to better understand their clinicopathological characteristics, metastatic risk, progression, and treatment outcomes.
The HCT-116 cell cloning assay demonstrated that the cell proliferation rate was significantly higher in the mimics group compared to the control group, while that of the inhibitor group was significantly lower than that of the control group. This suggested that overexpression of miR-17-5p may promote CRC cell proliferation, while low expression of miR-17-5p inhibited CRC cell proliferation. Transwell experiments showed that the number of cells after transfection with miR-17-5p mimics that lysed stromal gel and crossed the cells in the small chamber in the same time was significantly greater than that in the NC group (P<0.01). The number of cells transfected with miR-17-5p inhibitor that lysed stromal gel and crossed the cells in the small chamber in the same time was significantly smaller than that in the in-NC group (P<0.01). The increased expression of miR-17-5p promoted cell invasion, while the decreased expression of miR-17-5p inhibited its invasive effect. The crystalline violet cell proliferation assay confirmed the effect on cell proliferation ability, and the scratch assay and Transwell assay confirmed that overexpression of miR-17-5p promoted metastasis and migration of CRC cells, whereas low expression of miR-17-5p inhibited metastasis and migration of CRC cells. Flow cytometry was used to detect apoptosis in the miR-17-5p mimics and inhibitor groups, as well as their corresponding control groups. The results revealed that the apoptosis rate of the mimics group was significantly lower than that of the control group and the inhibitor group, suggesting that that overexpression of miR-17-5p inhibited apoptosis in CRC cells, while low expression of miR-17-5p promoted apoptosis. These results further confirmed that miR-17-5p is associated with the development of CRC. To investigate the potential mechanisms of miR-17-5p in CRC, we examined the expression of several proteins that are associated with invasive migration mechanisms, including E-cadherin (14), CD44 (15), MMP2 (16), as well as proteins involved in apoptosis, including BCL-2 (17) and BAX (18). The RT-qPCR and Western blot results revealed that miR-17-5p upregulation increased E-cadherin, CD44, MMP2, and BCL2 expression while decreasing BAX expression, whereas miR-17-5p downregulation had the opposite effect. Tano et al. (19) noted that the immune system of cancer patients was dramatically decreased, and that CD44, BCL2, and BAX were associated with cancer cell growth. They further demonstrated that patients with head and neck tumors who had a BCL2/BAX ratio >1.2 had a longer survival time. Indeed, regulation of CD44, BCL2, BAX, E-cadherin, and MMP2 expression may be the mechanism by which miR-17-5p regulates colorectal cells.
Conclusions
Studies on the expression of miRNAs in the development of CRC and their regulatory mechanisms have yielded some significant results in recent years. MiRNAs are differentially expressed in tumor tissues, and can either upregulate or downregulate target genes as proto-oncogenes or oncogenes (20,21) we found that miR-17-5p expression was upregulated in colorectal cancer tissues and was significantly correlated with TNM stage and tumor differentiation, tumor diameter and the presence of lymph node metastasis, and had an effect on proliferation, metastasis and invasion and apoptosis of colorectal cancer cells. In addition, we also identified CD44, BCL2, BAX, E-Cadherin, and MMP2 genes as the mechanisms of miR-17-5p regulation in colorectal cancer. These experimental results suggest that miR-17-5p may become a molecular marker for early diagnosis of colorectal cancer and a targeted therapeutic site in the future, but more scientific studies are needed to further prove this conclusion. This study only examined its expression in tumor tissues; and it remains unclear whether its expression is different in blood and stool, or which tissues are more convenient and accurate as early predictors. Therefore, additional research is required to better understand the mechanisms of miR-17-5p regulation of CRC.
Acknowledgments
Funding: The study was supported by the Ganzhou City Key Laboratory of Colorectal and Anal Diseases Research (No. 20220101-25), the project of Jiangxi Provincial Education Department (No. GJJ180810), and Jiangxi Provincial Health Commission (No. 202210908).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1185/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1185/dss
Conflicts of interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1185/coif). All authors report funding support from the Ganzhou City Key Laboratory of Colorectal and Anal Diseases Research (No. 20220101-25), the project of Jiangxi Provincial Education Department (No. GJJ180810), and Jiangxi Provincial Health Commission (No. 202210908). The authors have no other conflicts of interest to declare.
Ethical statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Research Ethics Committee of The First Affiliated Hospital of Gannan Medical College (Ethics No. 20190507). Written informed consent was obtained from all participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Li N, Lu B, Luo C, et al. Incidence, mortality, survival, risk factor and screening of colorectal cancer: A comparison among China, Europe, and northern America. Cancer Lett 2021;522:255-68. [Crossref] [PubMed]
- Yau TO, Tang CM, Harriss EK, et al. Faecal microRNAs as a non-invasive tool in the diagnosis of colonic adenomas and colorectal cancer: A meta-analysis. Sci Rep 2019;9:9491. [Crossref] [PubMed]
- Kent OA, Mendell JT. A small piece in the cancer puzzle: microRNAs as tumor suppressors and oncogenes. Oncogene 2006;25:6188-96. [Crossref] [PubMed]
- Tsai KW, Kuo WT, Jeng SY. microRNA-324 plays an oncogenic role in bladder cancer cell growth and motility. Transl Cancer Res 2020;9:707-16. [Crossref] [PubMed]
- Song J, Liu Y, Wang T, et al. MiR-17-5p promotes cellular proliferation and invasiveness by targeting RUNX3 in gastric cancer. Biomed Pharmacother 2020;128:110246. [Crossref] [PubMed]
- Li Y, Liang M, Zhang Y, et al. miR-93, miR-373, and miR-17-5p Negatively Regulate the Expression of TBP2 in Lung Cancer. Front Oncol 2020;10:526. [Crossref] [PubMed]
- Wang Y, Xu W, Wang Y, et al. miR-17-5p promotes migration and invasion in breast cancer cells by repressing netrin 4. Int J Clin Exp Pathol 2019;12:1649-57. [PubMed]
- Zou M, Zhang Q. miR-17-5p accelerates cervical cancer cells migration and invasion via the TIMP2/MMPs signaling cascade. Cytotechnology 2021;73:619-27. [Crossref] [PubMed]
- Chen Z, Zhong T, Zhong J, et al. MicroRNA‑129 inhibits colorectal cancer cell proliferation, invasion and epithelial‑to‑mesenchymal transition by targeting SOX4. Oncol Rep 2021;45:61. [Crossref] [PubMed]
- Mauri G, Sartore-Bianchi A, Russo AG, et al. Early-onset colorectal cancer in young individuals. Mol Oncol 2019;13:109-31. [Crossref] [PubMed]
- Ling XK, Xie WH, Zhang Z, et al. Mechanism of miR-17-5p in promoting proliferation and metastasis of colorectal cancer cells by down-regulating MYLIP. The Journal of Practical Medicine 2020;36:3338-43.
- Shao P, Sun D, Wang L, et al. Deep sequencing and comprehensive expression analysis identifies several molecules potentially related to human poorly differentiated hepatocellular carcinoma. FEBS Open Bio 2017;7:1696-706. [Crossref] [PubMed]
- Onder TT, Gupta PB, Mani SA, et al. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res 2008;68:3645-54. [Crossref] [PubMed]
- Hassn Mesrati M, Syafruddin SE, Mohtar MA, et al. CD44: A Multifunctional Mediator of Cancer Progression. Biomolecules 2021;11:1850. [Crossref] [PubMed]
- Cui N, Hu M, Khalil RA. Biochemical and Biological Attributes of Matrix Metalloproteinases. Prog Mol Biol Transl Sci 2017;147:1-73. [Crossref] [PubMed]
- Jian X, Qu L, Wang Y, et al. Trichostatin A-induced miR-30a-5p regulates apoptosis and proliferation of keloid fibroblasts via targeting BCL2. Mol Med Rep 2019;19:5251-62. [Crossref] [PubMed]
- Yuan Y, Wang YY, Liu X, et al. KPC1 alleviates hypoxia/reoxygenation-induced apoptosis in rat cardiomyocyte cells though BAX degradation. J Cell Physiol 2019;234:22921-34. [Crossref] [PubMed]
- Tano T, Okamoto M, Kan S, et al. Prognostic impact of expression of Bcl-2 and Bax genes in circulating immune cells derived from patients with head and neck carcinoma. Neoplasia 2013;15:305-14. [Crossref] [PubMed]
- To KK, Tong CW, Wu M, et al. MicroRNAs in the prognosis and therapy of colorectal cancer: From bench to bedside. World J Gastroenterol 2018;24:2949-73. [Crossref] [PubMed]
- Liu H, Li J, Zhao H, et al. DNAJC2 is reversely regulated by miR-627-3p, promoting the proliferation of colorectal cancer. Mol Med Rep 2021;24:589. [Crossref] [PubMed]
(English Language Editor: J. Teoh)


