
Phosphatase regenerating liver 3 participates in Integrinβ1/FAK-Src/MAPK signaling pathway and contributes to the regulation of malignant behaviors in hepatocellular carcinoma cells
Highlight box
Key findings
• The mechanism of PRL-3 took part in proliferation, invasive and metastasis of HCC relied on Integrinβ1/FAK-Src/RasMAPK signaling pathway.
What is known and what is new?
• PRL-3 was an independent predictor for OS and PFS of HCC patients.
• PRL-3 played a critical role in HCC malignant behaviors.
What is the implication, and what should change now?
• PRL-3 maybe a new therapeutic target for HCC.
Introduction
Hepatocellular carcinoma (HCC), associated with a high incidence of hepatitis B or C and following cirrhosis, is one of the major health burdens worldwide, especially in eastern Asia. According to Globocan 2020, it is the third most common cause of fatality from cancer worldwide (1). Despite the advancement of the diagnosis and surgical techniques of HCC, only around 30% of HCC patients could be benefitted from curative therapy, such as resection or local ablation. The long-term survival is not satisfactory for HCC patients post curative therapy owing to the high postoperative recurrence (2,3). The systemic therapies are the preferred alternatives for advanced HCC patients; however, multiple pathways-mediated complex signal network resulting in metastasis (4-7) and drug resistance influences their efficacies (3,4,8). The molecular target therapy also reflects a low response rate for HCC patients due to multiple genes and pathways-mediated complex signal network leading to carcinogenesis and metastasis (4,7,9).
Understanding of HCC biology, its critical molecular players in tumor metastasis, could be targeted to achieve optimal therapies with improvement in patients’ long-term survival. Both phosphatases and protein kinases regulate the homeostasis of multiple physiological processes. Nonetheless, phosphatases have not gained much more attention as protein kinases (10). It has been reported that PRL-3 is co-localized with Integrinβ1, one of the important cell surface receptors, mediating cell adhesion and causing tyrosine phosphorylation of FAK at tyrosine 397 and ultimately to mitogen activated protein (MAP) kinase activation. Autophosphorylation of FAK leads to the binding of Src-homology 2 (SH2) domain of Src, then phosphorylates other tyrosine residues in FAK and thereby maximizes its kinase activity to play a crucial role in cell adhesion and cell signals transduction and activation (11-13).
Phosphatase of regenerating liver 3 (PRL-3), implicated in cancer metastasis, was known to be overexpressed in a variety of malignant tumors and associated with malignant behaviors in glioblastoma, gastric cancer, mammary cancer, colon cancer, ovarian cancer, and so on (14-18). Recently, PRL-3 was proposed as a potential biomarker for tumor aggressiveness (19,20). Previous research had demonstrated that PRL-3 associated with the worse prognosis of HCC patients (21), however, the role of PRL-3 in HCC development remains largely unidentified. In the present study, we worked on the hypothesis that PRL-3 is critical in the mechanisms of HCC patients, as it is in mice. Based on this, the PRL-3 expression was validated to be higher in HCC tissues. We also performed logistic regression to verify whether or not PRL-3 can predict death. Moreover, we provided relevant new insight into the underlying molecular mechanism of PRL-3 in HCC invasion and metastasis. We present the following article in accordance with the ARRIVE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-976/rc).
Methods
Patients and specimen
Overall, 114 HCC patients who underwent curative hepatectomy were enrolled in the study between May and November in 2010 from the Liver Cancer Institute of Zhongshan Hospital, Fudan University. Liver cancer tissues collected from these patients were fixed in 10% formaldehyde solutions and embedded in paraffin, prepared for tissue microarray (TMA). All patients were followed up with their serum a-fetoprotein (AFP) assay and liver image examination by ultrasonography, computed tomography (CT), and magnetic resonance imaging every three months. The last follow-up time was on 20th October 2015. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The human test subjects have been approved by the Ethics Committee of Zhongshan Hospital, Fudan University (approval No. B2020–138/pre-review No. Y2014–125). Written informed consent was obtained from the patients.
Immunohistochemical analysis
TMA construction and immunohistochemistry were conducted following a two-step method as detailed previously (21). Briefly, the primary antibody (polyclonal rabbit anti-human PRL-3, ab50276, ABCAM, Cambridge, UK) was diluted 1:400 in phosphate-buffered saline (Hyclone, Logan, Utah, USA) containing 1% bovine serum albumin (Gibco, USA).
After incubating with the appropriate biotin-conjugated secondary antibody, the sections were stained with DAB Horseradish Peroxidase Color Development Kit (Beyotime, China). The intensity of the cytoplasmic and nuclear membrane immunostaining for PRL-3 was graded as follows: 0, no immunostaining; 1, immunostaining detected in 25% of tumor cells; 2, moderate immunostaining marked in 25–50% of tumor cells; and 3, strong and diffuse immunostaining observed in over 50% of tumor cells. Subsequently, a score of 0 to 3 was considered negative, weakly positive, moderately positive, and strongly positive. Two independent pathologists evaluated the immunohistochemical staining under a light microscope.
HCC cells and cell culture
MHCC97H cells established in the Liver Cancer Institute, Fudan University (22), were cultured in Dulbecco’s modified Eagle medium (DMEM, Gibco, USA) supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin (100 units/mL, each) in the condition of 37 ℃ in a humidified atmosphere containing 5% CO2.
Generation of stable cell lines
The recombinant PRL-3-shRNA (TargetSeq: CTACAAACACATGC GCTTCCT), PRL-3-expression sequence lentivirus, and the negative control lentivirus (both carrying GFP; Jiman Co. Shanghai, China) were developed and titered to 109 TU/mL (transfection unit). To obtain a stable PRL-3 knockdown/overexpressing cell line, MHCC97H cells were seeded in six-well dishes at a density of 2×105 cells per well. The cells were then infected with the same titer virus with 8 µg/mL polybrene on the following day. Approximately 72 h after a viral infection, GFP expression was confirmed under a fluorescence microscope, and the culture medium was replaced with a selection medium containing 4 µg/mL puromycin (Thermo Fisher Scientific, USA). The cells were, thereafter, cultured for at least 14 days. The puromycin-resistant cell clones were isolated, amplified in a medium containing 2 µg/mL puromycin for seven to nine days, and transferred to a medium without puromycin. The clones were designated PRL-3 knocking down (97H-SH4), overexpressing (97H-OE), or negative control (97H-NC) cells and finally validated by polymerase chain reaction (PCR) and Western-blot.
Cell invasion assays
The invasion assay was conducted using Transwells with filters coated with Matrigel (BD Bioscience, USA) on the upper surface. The experiments were performed according to the manufacturer’s protocol. HCC cells (1×105) were added to the upper chamber in serum-free medium, and the number of invaded HCC cells in the lower chamber was counted after incubating for 48 h at 37 ℃ in a humidified atmosphere with 5% CO2.
Wound healing assays
Different PTP4A3/PRL-3 expressing MHCC97H cells were seeded in 6-well plates (2×105 cells/2 mL in each well, Corning, USA). A straight scratch was made using a yellow pipette tip (200 µL, Axygen, USA) when all the cultures were confluent. The cells were then cultured in a serum-free medium, and the wounds were observed under 10× microscope (Leica MZ6/MZ205FA, Germany) at 0, 24, 48, and 72 h. The 97-OE cells were also treated with MEK1/2 inhibitor (U0126, Beyond time, China) in 10 µM in 1 h (23) or Src inhibitor Saracatinib (AZD0530, provided by AstraZeneca, UK) in 6 µM in 2 h (24).
Clone formation assays
Different PRL-3 expressing MHCC97H cells were trypsinized into single cells and resuspended in serum-free DMEM medium (Gibco, USA). They were then transferred into a 6-well plate with low attachment (500 cells/well, Corning, USA). The cells were photographed employing a phase-contrast microscope after 14 days of culture.
Establishment of orthotopic HCC model in nude mice
In this study, the experiments were conducted on BALB/c nu/nu male nude mice (Shanghai Institute of Materia Medica, Chinese Academy of Sciences, China) at six to eight weeks of age. First, different tumor tissues (97H-WT, 9H-NC, 97H-SH4, 97H-OE) were obtained from subcutaneous tumor implantation after three weeks (1×107 cells in each mice) (25). The cancerous tissues were divided into small pieces about 2 mm in diameter and transplanted to the liver of the nude mouse under specific-pathogen-free conditions, as described previously (26). Because of the greater metastasis potential of MHCC97H cells (described as 100%), all the nude mice were sacrificed on the 30th day, earlier than those under the experiment with the previously described methods (22,26), to observe the earlier lung metastasis in the four different groups, with six nude mice in each group. Experiments were performed under a project license (No. 2016-013) granted by the Animal Ethics Committee of Zhongshan Hospital, Fudan University, in compliance with institutional guidelines for the care and use of animals.
Statistical analysis
Statistical analysis software SPSS (Statistical Package for Social Sciences) 18.0 (SPSS Inc, Chicago, IL, USA) was exploited to perform the Poisson distribution events test and Chi-square test. Overall survival (OS) and progression-free survival (PFS) curves were obtained by the Kaplan-Meier method and compared with the log-rank test. Univariate and multivariate analyses were executed by the Cox proportional hazard models. A P value less than 0.05 was considered statistically significant.
Results
Overexpression of PRL-3 predicted poor prognosis in HCC patients
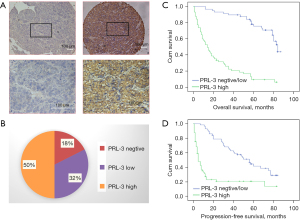
The enrolled 114 patients who underwent curative resection were clinically diagnosed as HCC between May and November in 2008. The clinic-pathological features of the tumors were detailed in Table S1; the middle age of these HCC patients was 52 years old (from 19 to 79 years old). Among the study population, 83.3% (95/114) were male, 82.5% (94/114) were positive for Hepatitis B Virus (HBV) surface antigen, 0.9% (1/114) was positive for Hepatitis C Virus (HCV), and 16.6% (19/114) were negative for both. Furthermore, two patients had received TACE before the resection, and the others had not received any treatment before. The liver function of all patients had been diagnosed as Child-Pugh A before the resection. During follow-up, 79 HCC patients showed tumors recurrence or metastasis, and 73 patients died. As illustrated in Figure 1A, the expression of PRL-3 was mainly distributed at the cytoplasm and nuclear membrane of the cancer cells, but rarely at the nucleus. Higher expressions of PRL-3 were documented in 57 cases (50%) (Figure 1B). HCC patients with PRL-3-overexpression demonstrated a significantly poorer survival as compared to those with PRL-3-low expression; their median OS was 14±2.696 months (95% CI: 8.716–19.284) and 84±3.271 months (95% CI: 77.588–90.412) (P<0.001), respectively (Figure 1C). The difference of PFS between the two groups was also statistically significant, and their median PFS was found to be 5±1.208 months (95% CI: 2.633–7.367) and 56±5.761 (95% CI: 44.708–67.292), (P<0.001), respectively (Figure 1D). Moreover, the expression of PRL-3 was correlated with AFP level (P<0.001), tumor diameter (P<0.001), vascular invasion (P<0.001), and pathological differentiation (P=0.009) (Table S2). Further multivariate analysis revealed that overexpression of PRL-3 was an independent predictor for OS and PFS of HCC patients (Tables S3,S4).
PRL-3 overexpression promoted the malignant behaviors of HCC cells
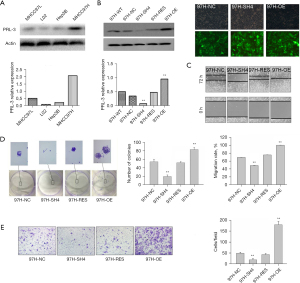
To investigate the roles of PRL-3 in HCC cell migration, invasiveness, and metastasis, we demonstrated overexpression of PRL-3 in highly metastatic MHCC97H cells compared with that in poorly metastatic Hep3B cells (Figure 2A). We employed LV-mediated RNAi and overexpressing sequences to create stable differential PRL-3 expressing cells. First, the silencing efficiency of PRL-3 was validated (Figure 2B), then the migration, invasive, and colony-forming capacities of the treated HCC cells was assessed. Significant suppression in the migration (P<0.01), invasiveness (P<0.01), and colony formation abilities of HCC cells were attributed to PRL-3 knockdown. However, the migration and invasion abilities of HCC cells could be regained by recovering PRL-3 expression (P<0.01) (Figure 2C-2E).
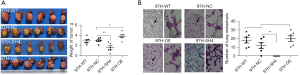
The significant role of PRL-3 in motility and invasion of HCC cells in vitro motivated further investigation of the PRL-3-mediated effects on metastasis in the orthotopic HCC model of nude mice (26). We explored tumor growth and lung metastasis in orthotopic HCC model of nude mice derived from PRL-3 overexpression or knockdown. The average wet weight of HCC tumor was 1.60330±0.35081, 3.80330±0.29388, 2.91330±0.27260, and 2.97330±0.20718 g in the groups of PTP4A3/PRL-3 knockdown (97H-SH4), PTP4A3/PRL-3 over-expression (97H-OE), the wild-type (97H-WT), and the negative-control (97H-NC), respectively (Figure 3A). A significant difference in average wet weight was prominent between the PRL-3 down-regulating group (97H-SH4) and the wild-type group (97H-WT) or the negative control group (97H-NC) (P=0.007). Lung metastasis was reported in six mice in the 97H-WT group (6/6,100%) and five mice in the 97H-NC group (5/6, 83%). The average number of lung metastasis was 18.1667±3.16667 and 12±3.4737 in the 97H-WT and 97H-NC groups. All the mice in the 97H-OE group exhibited lung metastasis (6/6, 100%), and the average lung metastasis lesion number was 20±2.26599. The lung metastasis was more easily witnessed in the 97H-OE group, although there was no statistical difference between 97H-OE and 97H-WT or 97H-NC groups (Figure 3B). The average size of xenograft tumor in the liver of the 97H-SH4 group was 1.60330±0.35081 g (P=0.007), and there was no detectable lung metastasis lesion in the 97H-SH4 group (0/6, 0%) (P=0.006). These findings indicated that the downregulation of PRL-3 could suppress tumor growth and inhibit lung metastasis of HCC in nude mice (Figure 3B).
PRL-3 participated in the integrin β1/FAK-Src/MAPK signaling pathway and contributed to the regulation of malignant behaviors in HCC cells
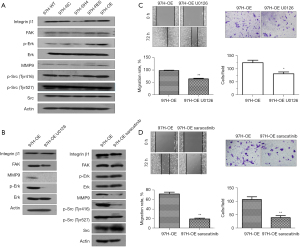
MHCC97H cells with PRL-3 overexpression exhibited enhanced migration, invasiveness, and metastasis; in contrast, suppression of PRL-3 attenuated the malignant behaviors of HCC cells. PRL-3 is co-localized with Integrin β1, one of the important cell surface receptors, playing a crucial role in cell adhesion and subsequent cell signals transduction and activation (12). In this study, knockdown of PRL-3 suppressed the expression of Integrin β1 in MHCC97H cells as well as reduced the phosphorylation levels of its downstream signal molecule c-Src or ERK. The two sites of tyrosine phosphorylation of Src, Tyr416 and Tyr527, with an opposing effect, determined the Src activity. Phosphorylation at Tyr416 in the activation loop of kinase domain results in upregulation of enzyme activity, whereas phosphorylation at Tyr527 in the carboxyterminal tail by Csk renders the enzyme less active (27,28). Marked inhibition of the phosphorylation of p-Src (Tyr416) and p-ERK (Thr202/Tyr204) was evident with PRL-3 knockdown in MHCC97H (97H-SH4). On the contrary, PRL-3 overexpression (97H-OE) induced the expression of Integrin β1 and increased the p-Src (Tyr416) and p-Erk (Thr202/Tyr204) levels in HCC cells. The above results clearly implicated that the overexpression of PRL-3 induced and activated the Integrinβ1/FAK-Src/ERK signaling pathway (Figure 4A). MMP9, one of the zinc-MMPs families, involved in the degradation of the Extracellular matrix (ECM), thereby facilitating invasiveness and metastasis of HCC (29,30). Research findings also confirmed integrin and ERK signaling activated MMPs (31,32). Present study also revealed increased production of MMP9 medicated by PRL-3 overexpression, whereas knockdown of PRL-3 resulted in a sharp decline in MMP9 in HCC (Figure 4A). HCC cells were treated with MEK1/2 inhibitor U0126 at a concentration of 10 µM for 1 h. The results authenticated that the phosphorylation of ERK and the expression of MMP9 was inhibited. Meanwhile, PRL-3-induced migration and invasion were attenuated after the above treatment. However, the Integrinβ1 and FAK expressions exhibited no obvious changes (Figure 4B,4C). We further used Src (Tyr416) inhibitor Saracatinib at a concentration of 6 µM to treat the 97H-OE cells for 2 h. This inhibitor repressed the phosphorylation of Src (Tyr416) as well as the expression of MMP9, simultaneously reversed PRL-3-induced migration and invasiveness (Figure 4D). But no significant changes could be detected in the expressions of Integrinβ1 and FAK, as well as the phosphorylation of ERK or Src (Tyr527) (Figure 4B).
Discussion
Enhanced expression of PRL-3 in MHCC97H cells with high metastasis potential identified previously by mass spectrometry indicated the relevance of PRL-3 in regulating malignant behaviors of HCC (the result has not been published). In this study, we defined the significance of PRL-3 in the migration, invasive, and metastasis of HCC cells. Overexpression of PRL-3 was in close correlation with vascular invasiveness, poor pathological differentiation, incomplete capsule, late clinical-stage, elevated AFP level, or huge tumors and was also associated with poor OS and PFS, even as an independent risk factor. Thus, it could be utilized as a promising predictor for postoperative survival and recurrence of HCC patients.
Being a critical potential target molecule for preventing the malignant biological behaviors of HCC, further investigations were conducted to elucidate the potential mechanism both in vivo and in vitro. Peng et al. reported the association and co-localization of endogenous Integrinβ1 with endogenous PRL-3 in LoVo cells of colon cancer. Although they failed to find the direct interaction between these two molecules, they hypothesized Integrinα1 mediated the PRL-3-Integrinβ1 interaction (12). In this study, the results revealed the PRL-3-induced regulation of the expression of Integrinβ1 and its crucial downstream protein FAK; however, the detailed mechanism of interaction with each other needs to be further dissected. Integrinβ1/FAK signaling, known as one of the key pathways, contributes to cancer cell apoptosis, proliferation, adhesion, migrating, invasion, and metastasis (33-36). FAK and c-Src can form a dual kinase complex and c-Src phosphorylated and activated FAK-Tyr576/Tyr577 (37,38). The FAK/c-Src complex, in turn, induces the phosphorylation of protein Paxillin and Cas (39), activating Ras/MAPK pathway and promoting the production of MMPs by the intermediary adaptor molecules Crk and Grb2 (40-42). Here we demonstrated activation of Integrinβ1/FAK-Src/MAPK signal axis, induction of the migrating and invasive behaviors of HCC cells, and the production of MMP9 mediated by PRL-3 overexpression. On the other hand, MEK1/2 inhibitor (u0126) reversed PRL-3 overexpression-mediated effects as described above. Src is one of the downstream factors in Integrinβ1 signaling, whereas, an upstream molecule of ERK activation (43,44).
Our results also confirmed that Saracatinib (AZD0530) could selectively inhibit the auto-phosphorylation of Src-Tyr416 and reverse PRL-3 overexpression-mediated migration and invasion in HCC cells, but did not affect the inhibitory phosphorylation site Tyr527 of Src and the upstream molecules Integrinβ1 and FAK. We presumed the contribution of PRL-3 in Integrinβ1/FAK-Src/RasMAPK signal and regulation of invasive and metastasis behaviors in HCC cells. PRL-3 could be a crucial upstream molecule of this pathway and a potential key regulator in HCC metastasis. However, other co-regulation pathways could not be excluded, and further validation of the conclusion in other HCC cells owing to the heterogeneous expression of PRL-3 is essential.
Besides, there are some limitations in present study. In the first of all, the only one cell line might not be optimal. However, in the study, we had used in vivo mouse model and human samples to further verify the results. These results also gave us sufficient evidence for our conclusion. Secondly, the clinical sample was not large enough, which might be the reason of PRL-3 seemed to be too strong as the predictive factor of HCC in this study. Finally, the binding sites of PRL-3 on the downstream genes for regulating the malignant biological behavior of HCC needs to be further study.
Conclusions
Overall, our data highlighted significant overexpression of PRL-3 in HCC patients, which, therefore, can be considered as an independent prognostic factor to predict the OS of HCC patients. Mechanically, these data substantiated the crucial role of PRL-3 in HCC invasive and metastasis via Integrinβ1/FAK-Src/RasMAPK signaling. Validation of PRL-3 as a clinical prediction marker in HCC warrants further investigation.
Acknowledgments
Funding: This study was sponsored by grants from Key Program of Xiamen Medical and Health (grant No. 3502Z20191105) and Shanghai Science and Technology Program (No. 16ZR1405300).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-976/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-976/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-976/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The human test subjects have been approved by the Ethics Committee of Zhongshan Hospital, Fudan University (approval No. B2020–138/pre-review No. Y2014–125). Written informed consent was obtained from the patients. Experiments were performed under a project license (No. 2016-013) granted by the Animal Ethics Committee of Zhongshan Hospital, Fudan University, in compliance with institutional guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Kanwal F, Singal AG. Surveillance for Hepatocellular Carcinoma: Current Best Practice and Future Direction. Gastroenterology 2019;157:54-64. [Crossref] [PubMed]
- Vogel A, Meyer T, Sapisochin G, et al. Hepatocellular carcinoma. Lancet 2022;400:1345-62. [Crossref] [PubMed]
- Yang C, Zhang H, Zhang L, et al. Evolving therapeutic landscape of advanced hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2023;20:203-22. [Crossref] [PubMed]
- Llovet JM, Montal R, Sia D, et al. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol 2018;15:599-616. [Crossref] [PubMed]
- Llovet JM, Villanueva A, Lachenmayer A, et al. Advances in targeted therapies for hepatocellular carcinoma in the genomic era. Nat Rev Clin Oncol 2015;12:436. [Crossref] [PubMed]
- Pinyol R, Nault JC, Quetglas IM, et al. Molecular profiling of liver tumors: classification and clinical translation for decision making. Semin Liver Dis 2014;34:363-75. [Crossref] [PubMed]
- Doycheva I, Thuluvath PJ. Systemic Therapy for Advanced Hepatocellular Carcinoma: An Update of a Rapidly Evolving Field. J Clin Exp Hepatol 2019;9:588-96. [Crossref] [PubMed]
- El-Kafrawy SA, El-Daly MM, Bajrai LH, et al. Genomic profiling and network-level understanding uncover the potential genes and the pathways in hepatocellular carcinoma. Front Genet 2022;13:880440. [Crossref] [PubMed]
- Pulido R, Hooft van Huijsduijnen R. Protein tyrosine phosphatases: dual-specificity phosphatases in health and disease. FEBS J 2008;275:848-66. [Crossref] [PubMed]
- Parkin A, Man J, Timpson P, et al. Targeting the complexity of Src signalling in the tumour microenvironment of pancreatic cancer: from mechanism to therapy. FEBS J 2019;286:3510-39. [Crossref] [PubMed]
- Peng L, Xing X, Li W, et al. PRL-3 promotes the motility, invasion, and metastasis of LoVo colon cancer cells through PRL-3-integrin beta1-ERK1/2 and-MMP2 signaling. Mol Cancer 2009;8:110. [Crossref] [PubMed]
- Huveneers S, Danen EH. Adhesion signaling - crosstalk between integrins, Src and Rho. J Cell Sci 2009;122:1059-69. [Crossref] [PubMed]
- Mu N, Gu J, Liu N, et al. PRL-3 is a potential glioblastoma prognostic marker and promotes glioblastoma progression by enhancing MMP7 through the ERK and JNK pathways. Theranostics 2018;8:1527-39. [Crossref] [PubMed]
- Tao Y, You W. The Deubiquitinating Enzyme USP4 Functions as an Oncoprotein in Gastric Cancer and Mediates NF-kappaB Signaling by Regulating PRL-3 Expression. Front Biosci (Landmark Ed) 2022;27:286. [Crossref] [PubMed]
- Xie H, Wang H. PRL-3 promotes breast cancer progression by downregulating p14(ARF)-mediated p53 expression. Oncol Lett 2018;15:2795-800. [PubMed]
- Hong XC, Liang QL, Chen M, et al. PRL-3 and MMP9 Expression and Epithelial-Mesenchymal Transition Markers in Circulating Tumor Cells From Patients With Colorectal Cancer: Potential Value in Clinical Practice. Front Oncol 2022;12:878639. [Crossref] [PubMed]
- Zhang M, Wei Y, Liu Y, et al. Metastatic Phosphatase PRL-3 Induces Ovarian Cancer Stem Cell Sub-population through Phosphatase-Independent Deacetylation Modulations. iScience 2020;23:100766. [Crossref] [PubMed]
- Ge Z, Gu T, Zhang L, et al. The phosphatase of regenerating liver-3 protein (PRL-3) promotes glioma cell invasiveness by interacting with beta3 -tubulin. Bioengineered 2022;13:4112-21. [Crossref] [PubMed]
- Su P, Lai W, Liu L, et al. Mesenchymal and Phosphatase of Regenerating Liver-3 Status in Circulating Tumor Cells May Serve as a Crucial Prognostic Marker for Assessing Relapse or Metastasis in Postoperative Patients With Colorectal Cancer. Clin Transl Gastroenterol 2020;11:e00265. [Crossref] [PubMed]
- Mayinuer A, Yasen M, Mogushi K, et al. Upregulation of protein tyrosine phosphatase type IVA member 3 (PTP4A3/PRL-3) is associated with tumor differentiation and a poor prognosis in human hepatocellular carcinoma. Ann Surg Oncol 2013;20:305-17. [Crossref] [PubMed]
- Tang ZY, Ye SL, Liu YK, et al. A decade's studies on metastasis of hepatocellular carcinoma. J Cancer Res Clin Oncol 2004;130:187-96. [Crossref] [PubMed]
- de Boussac H, Orbán TI, Várady G, et al. Stimulus-induced expression of the ABCG2 multidrug transporter in HepG2 hepatocarcinoma model cells involves the ERK1/2 cascade and alternative promoters. Biochem Biophys Res Commun 2012;426:172-6. [Crossref] [PubMed]
- Simpkins F, Hevia-Paez P, Sun J, et al. Src Inhibition with saracatinib reverses fulvestrant resistance in ER-positive ovarian cancer models in vitro and in vivo. Clin Cancer Res 2012;18:5911-23. [Crossref] [PubMed]
- Fidler IJ. Rationale and methods for the use of nude mice to study the biology and therapy of human cancer metastasis. Cancer Metastasis Rev 1986;5:29-49. [Crossref] [PubMed]
- Sun FX, Tang ZY, Lui KD, et al. Establishment of a metastatic model of human hepatocellular carcinoma in nude mice via orthotopic implantation of histologically intact tissues. Int J Cancer 1996;66:239-43. [Crossref] [PubMed]
- Chen ML, Chai CY, Yeh KT, et al. Crosstalk between activated and inactivated c-Src in hepatocellular carcinoma. Dis Markers 2011;30:325-33. [Crossref] [PubMed]
- Bräuninger A, Karn T, Strebhardt K, et al. Characterization of the human CSK locus. Oncogene 1993;8:1365-9. [PubMed]
- Han S, Han L, Yao Y, et al. Activated hepatic stellate cells promote hepatocellular carcinoma cell migration and invasion via the activation of FAK-MMP9 signaling. Oncol Rep 2014;31:641-8. [Crossref] [PubMed]
- Wang YH, Dong YY, Wang WM, et al. Vascular endothelial cells facilitated HCC invasion and metastasis through the Akt and NF-kappaB pathways induced by paracrine cytokines. J Exp Clin Cancer Res 2013;32:51. [Crossref] [PubMed]
- Missan DS, Mitchell K, Subbaram S, et al. Integrin alpha3beta1 signaling through MEK/ERK determines alternative polyadenylation of the MMP-9 mRNA transcript in immortalized mouse keratinocytes. PLoS One 2015;10:e0119539. [Crossref] [PubMed]
- Gao H, Peng C, Liang B, et al. beta6 integrin induces the expression of metalloproteinase-3 and metalloproteinase-9 in colon cancer cells via ERK-ETS1 pathway. Cancer Lett 2014;354:427-37. [Crossref] [PubMed]
- Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol 2004;5:816-26. [Crossref] [PubMed]
- Pantazaka E, Papadimitriou E. Chondroitin sulfate-cell membrane effectors as regulators of growth factor-mediated vascular and cancer cell migration. Biochim Biophys Acta 2014;1840:2643-50. [Crossref] [PubMed]
- Kuonen F, Secondini C, Rüegg C. Molecular pathways: emerging pathways mediating growth, invasion, and metastasis of tumors progressing in an irradiated microenvironment. Clin Cancer Res 2012;18:5196-202. [Crossref] [PubMed]
- Worthington JJ, Klementowicz JE, Travis MA. TGFbeta: a sleeping giant awoken by integrins. Trends Biochem Sci 2011;36:47-54. [Crossref] [PubMed]
- Bouchard V, Harnois C, Demers MJ, et al. B1 integrin/Fak/Src signaling in intestinal epithelial crypt cell survival: integration of complex regulatory mechanisms. Apoptosis 2008;13:531-42. [Crossref] [PubMed]
- Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol 2006;18:516-23. [Crossref] [PubMed]
- Webb DJ, Donais K, Whitmore LA, et al. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol 2004;6:154-61. [Crossref] [PubMed]
- Yee KL, Weaver VM, Hammer DA. Integrin-mediated signalling through the MAP-kinase pathway. IET Syst Biol 2008;2:8-15. [Crossref] [PubMed]
- Cheng SY, Sun G, Schlaepfer DD, et al. Grb2 promotes integrin-induced focal adhesion kinase (FAK) autophosphorylation and directs the phosphorylation of protein tyrosine phosphatase alpha by the Src-FAK kinase complex. Mol Cell Biol 2014;34:348-61. [Crossref] [PubMed]
- Sondergaard BC, Schultz N, Madsen SH, et al. MAPKs are essential upstream signaling pathways in proteolytic cartilage degradation--divergence in pathways leading to aggrecanase and MMP-mediated articular cartilage degradation. Osteoarthritis Cartilage 2010;18:279-88. [Crossref] [PubMed]
- Jiang W, Xu Z, Yu L, et al. MicroRNA-144-3p suppressed TGF-beta1-induced lung cancer cell invasion and adhesion by regulating the Src-Akt-Erk pathway. Cell Biol Int 2020;44:51-61. [Crossref] [PubMed]
- Sandilands E, Serrels B, McEwan DG, et al. Autophagic targeting of Src promotes cancer cell survival following reduced FAK signalling. Nat Cell Biol 2011;14:51-60. [Crossref] [PubMed]


