
Overall survival and toxicity of hepatocellular carcinoma Barcelona Clinic Liver Cancer B patients receiving Y90 radioembolization: analysis of the radiation-emitting SIR-spheres in non-resectable liver tumor (RESiN) registry
Highlight box
Key findings
• Bolondi subgroup classification predicts overall (OS) and progression-free survival (PFS) for Barcelona Clinic Liver Cancer (BCLC) Class B disease.
• Median OS for patients in subgroups 1 wasn’t reached at a mean of 28.8 months. Median OS for subgroup 2 was 24.9 months.
• Hepatic function toxicities were most common in subgroup 4.
What is known and what is new?
• Chemoembolization is the standard of care for initial treatment of BCLC B disease.
• Previous research had demonstrated similar survival stratification with best supportive care and chemoembolization.
• Bolondi subgroup class can predict survival and toxicity following radioembolization.
What is the implication, and what should change now?
• Radioembolization is a reasonable treatment option for patients with intermediate-stage (BCLC B) hepatocellular carcinoma.
• Radioembolization should only be used for patients in subgroup 4 in patients that can be treated with segmental infusion.
Introduction
Primary liver cancer is the sixth most common type of cancer and the third leading cause of cancer death worldwide, with hepatocellular carcinoma (HCC) comprising 75–80% of these tumors (1). The most widely accepted classification scheme is the Barcelona Clinic Liver Cancer (BCLC) system (2-5). BCLC has been validated as a predictor of overall survival (OS) and is endorsed both by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver (6-12). BCLC intermediate-stage (BCLC B) patients have a diverse range of presentation regarding tumor burden and liver function (11-13).
Transarterial chemoembolization (TACE) is the first-line treatment recommendation for HCC in this group with a reported OS ranging from 14–45 months (7,11). Due to limited evidence-based and standardized treatment recommendations across the spectrum of BCLC B patients, Bolondi et al. proposed a subclassification system for intermediate stage HCC focused on tumor burden and liver function (14). Given the common overlap of cirrhosis and HCC, both these factors are critical in treatment planning. Previous papers have demonstrated that stratifying patients by the Bolondi subclassification stratified OS with best supportive care, chemoembolization and other treatments (15-18).
Transarterial radioembolization (TARE) using Yttrium-90 (Y-90) is performed in BCLC B patients (19). The OS of BCLC B patients treated with TARE compares favorably with those treated with TACE (18). Additionally, patients maintain better health-related quality of life with TARE compared to TACE (20). The Radiation-Emitting Sir-Spheres in Non-resectable tumor (RESiN) registry (NCT 02685631) is a prospectively gathered observational study on patients treated with Y-90 embedded microspheres (Sirtex Medical, Woburn, MA, USA). Previously, outcomes of 448 HCC patients in the RESiN registry were reported (21). The purpose of the current analysis is to evaluate OS and toxicities of BCLC B patients broken down by the Bolondi sub-classification method. We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-972/rc).
Methods
Registry/patients
The RESiN registry is an observational study collecting data on patients over 18 years of age with primary or secondary liver cancer scheduled to receive Y-90 microsphere therapy as part of their treatment. Patients were enrolled from 2015–2020. The decision to treat with Y-90 was made at an institutional level based on collaborative decision making with the treating IR and referring physician(s). Exclusion criteria included (I) prior treatment with arterial Y-90 therapy, even if new areas were being targeted, and (II) the need for consent from a surrogate in cases where patients were unable to consent on their own. This registry was an online collaboration between 43 hospitals across The United States utilizing a Research Electronic Data Capture online database. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study protocol was approved and reviewed by the Vanderbilt Ingram Cancer Center and Vanderbilt University Institutional Review Board (IRB) as GI 1523 (IRB No. 150407) and subsequently approved at the other sites. All patients signed informed consent to participate.
In this review, all patients had HCC diagnosed by radiologic appearance and/or biopsy. All tumors qualified as BCLC B. The subgroups were determined by liver function and whether tumor burden was within or beyond the “Up to 7” Criteria: the sum of the number of tumors plus the diameter of the largest tumor. The subgroups were determined as outlined by Bolondi in Table 1. All patients followed local imaging and laboratory follow-up guidelines given the observational nature of the registry. Follow-up imaging was interpreted at each institution by abdominal imagers. Baseline demographic values included age, sex, race, ethnicity, cause of cirrhosis, hepatic functions including ascites and encephalopathy, and previous arterial and surgical treatments. Patients were treated by trained interventional radiologists. The treated portion of the liver (whole liver, lobar, segmental), the delivered activity and dosimetry method were tracked.
Table 1
| Variables | Subgroup B1 | Subgroup B2 | Subgroup B3 | Subgroup B4 |
|---|---|---|---|---|
| Child-Pugh score | 5, 6, 7 | 5, 6 | 7 | 8–9 |
| Up to 7 criteria | Within | Outside | Outside | Within or outside |
| Portal vein thrombosis | No | No | No | No |
HCC, hepatocellular carcinoma.
Statistical analysis
Demographic differences between subgroups were assessed using Kruskal-Wallis and Pearson tests for continuous and discrete variables regarding liver function, cirrhotic etiology, tumor characteristics, and health status. OS and progression-free survival (PFS) were defined as the time from the date of treatment to death or confirmation of disease progression at any site at follow-up CT or MRI. Kaplan-Meier analysis was performed to compare OS and PFS with 95% confidence intervals reported. Fifteen patients (7 in subgroup 1, 7 in subgroup 2, and 1 in subgroup 4) had no further data entered after their baseline. OS and PFS were calculated for the remaining 129 patients using modified Response Evaluation Criteria in Solid Tumors (mRECIST). For patients that were lost to follow-up, the last date of contact was used as a censoring point. Reasons for leaving the study were tracked, including the cause of death when available with differences compared using the Pearson test. Variables concerning the nature of intra- and extrahepatic progressive disease following treatment were analyzed by way of Pearson tests. Grade 3 or greater liver function toxicities and constitutional adverse events were tracked using the Common Terminology Criteria for Adverse Events version 5. If a patient had multiple events of the same toxicity within the course of the study, then the highest grade was given and counted as a single event. A single patient could develop multiple toxicities.
Results
Demographics
Cohort demographics are included in Table 2. Subgroups B1, B2, B3 and B4 had 54, 59, 8, and 23 patients respectively. Prior to treatment, 109/144 (76%) of the patients had a diagnosis of cirrhosis, most commonly in subgroups 1 (48/54, 89%) and 4 (23/23, 100%) compared to subgroups 2 (33/59, 56%) and 3 (5/8, 62%) (P<0.001). Thirty-four patients (24%) had previous arterial therapy and 19 (14%) had previous resection. The subgroups were similar in age (P=0.30), race (P=0.65), ethnicity (P=0.45), and gender (P=0.26). Most of the patients were male (n=116, 81%) and white (n=107, 74%). The most common causes of cirrhosis were hepatitis C (n=68, 47%) and alcohol (n=35, 24%). Patients in subgroups 3 and 4 more commonly had hepatic encephalopathy (8/31, 26% compared to 3/109, 3%, P<0.001) and pre-treatment ascites (20/31, 65% compared to 4/109, 37%, P<0.001). Ten of the 11 (91%) patients with encephalopathy had Grade 1 disease at baseline. All patients responded to intervention for their encephalopathy and ascites. Patients in subgroup 4 also had a higher bilirubin (median 1.9 vs. 0.8 in the other subgroups, <0.001) and lower albumin (2.9 vs. >3 in the other subgroups, P<0.001).
Table 2
| Variables | N | Group 1 (N=54) | Group 2 (N=59) | Group 3 (N=8) | Group 4 (N=23) | Combined (N=144) | P value |
|---|---|---|---|---|---|---|---|
| Age, years | 144 | 65 [62–70] | 67 [62–73.5] | 68 [64–74.5] | 64 [60.5–68] | 62.0 [66.0–71.0] | 0.31 |
| Sex | 144 | 0.262 | |||||
| Female | 12 (22%) | 13 (22%) | 6 (75%) | 22 (96%) | 28 (19%) | ||
| Male | 42 (78%) | 46 (78%) | 2 (25%) | 1 (4%) | 116 (82%) | ||
| Race | 144 | 0.652 | |||||
| American Indian/Alaska | 0 (0%) | 1 (2%) | 0 (0%) | 0 (0%) | 1 (1%) | ||
| Asian | 1 (2%) | 5 (8%) | 0 (0%) | 0 (0%) | 6 (4%) | ||
| Black | 8 (15%) | 6 (10%) | 3 (38%) | 4 (17%) | 21 (15%) | ||
| White | 42 (78%) | 42 (71%) | 5 (62%) | 18 (78%) | 107 (74%) | ||
| Other | 0 (0%) | 3 (5%) | 0 (0%) | 0 (0%) | 5 (3%) | ||
| Unknown | 1 (2%) | 2 (3%) | 0 (0%) | 1 (4%) | 4 (3%) | ||
| Ethnicity | 144 | 0.452 | |||||
| Hispanic/Latino | 9 (17%) | 5 (8%) | 0 (0%) | 4 (17%) | 18 (12%) | ||
| Non-Hispanic | 43 (80%) | 48 (81%) | 7 (88%) | 17 (74%) | 115 (80%) | ||
| Other | 0 (0%) | 3 (5%) | 0 (0%) | 0 (0%) | 3 (2%) | ||
| Unknown | 2 (4%) | 3 (5%) | 1 (12%) | 2 (9%) | 8 (6%) | ||
| Cirrhosis present | 144 | <0.0012 | |||||
| Yes | 48 (89%) | 33 (56%) | 5 (62%) | 23 (100%) | 109 (76%) | ||
| No | 6 (11%) | 26 (44%) | 3 (38%) | 0 (0%) | 35 (24%) | ||
| Alcohol | 144 | 0.0032 | |||||
| Yes | 14 (26%) | 8 (14%) | 1 (12%) | 12 (52%) | 35 (24%) | ||
| No | 40 (74%) | 51 (86%) | 7 (88%) | 11 (48%) | 109 (76%) | ||
| Hepatitis B | 144 | 0.762 | |||||
| Yes | 1 (2%) | 2 (3%) | 0 (0%) | 0 (0%) | 3 (2%) | ||
| No | 53 (98%) | 59 (100%) | 8 (100%) | 22 (96%) | 141 (98%) | ||
| Hepatitis C | 144 | 0.0762 | |||||
| Yes | 32 (59%) | 21 (36%) | 3 (38%) | 12 (52%) | 68 (47%) | ||
| No | 22 (41%) | 38 (64%) | 5 (62%) | 11 (48%) | 76 (53%) | ||
| NASH | 144 | 0.962 | |||||
| Yes | 6 (11%) | 5 (8%) | 0 (0%) | 2 (9%) | 13 (9%) | ||
| No | 48 (89%) | 54 (92%) | 8 (100%) | 21 (91%) | 131 (91%) | ||
| Bilirubin | 144 | 0.80 [0.6–1.2] | 0.80 [0.5–1.1] | 0.8 [0.6–0.9] | 1.9 [1.4–2.5] | 0.9 [0.6–1.3] | <0.0011 |
| Albumin | 144 | 3.85 [3.5–4.1] | 3.7 [3.4–4.1] | 3.1 [2.6–3.3] | 2.9 [2.6–3.2] | 3.7 [3.2–4.0] | <0.0011 |
| Previous embolization | 139 | 0.112 | |||||
| Yes | 15 (28%) | 8 (14%) | 3 (38%) | 8 (36%) | 34 (24%) | ||
| No | 38 (72%) | 48 (86%) | 5 (62%) | 14 (64%) | 105 (76%) | ||
| Previous resection | 135 | 0.832 | |||||
| Yes | 10 (21%) | 9 (16%) | 0 (0%) | 0 (0%) | 19 (14%) | ||
| No | 38 (79%) | 49 (84%) | 8 (100%) | 21 (100%) | 116 (86%) | ||
| Ascites | 144 | <0.0012 | |||||
| Yes | 2 (4%) | 2 (3%) | 5 (62%) | 15 (65%) | 24 (17%) | ||
| No | 52 (96%) | 57 (97%) | 3 (38%) | 8 (35%) | 120 (83%) | ||
| Hepatic encephalopathy | 144 | <0.0012 | |||||
| Yes | 2 (4%) | 1 (2%) | 1 (12%) | 7 (30%) | 11 (8%) | ||
| No | 52 (96%) | 58 (98%) | 7 (88%) | 16 (70%) | 133 (92%) | ||
| MELD | 143 | 9 [7–11] | 8 [6–9] | 7 [6–9.5] | 13 [11.5–15] | 9 [7–11] | <0.0011 |
| Child-Pugh class | 144 | <0.0012 | |||||
| Class A | 44 (81%) | 59 (100%) | 0 (0%) | 0 (0%) | 103 (72%) | ||
| Class B/C | 10 (19%) | 0 (0%) | 8 (100%) | 23 (100%) | 41 (28%) |
Baseline imaging
Baseline imaging findings are included in Table 3. Ninety-seven patients had 1 (n=50, 35%) or 2–3 tumors (n=47, 33%). Patients in subgroup 1 had the smallest diameter index tumor (median 2.5 cm, IQR: 1.8–4.3 cm) and total tumor diameter (median 4.6 cm, IQR: 3.2–6.3 cm). Patients in subgroup 4 had the next lowest burden of disease, followed by subgroups 2 and 3, respectively. The differences in index and overall tumor burden between subgroups was significant (both P<0.001). There were no patients with portal vein thrombosis as this study addresses BCLC B patients.
Table 3
| Variables | N | Group 1 (N=54) | Group 2 (N=59) | Group 3 (N=8) | Group 4 (N=23) | Combined (N=144) | P value |
|---|---|---|---|---|---|---|---|
| Tumor number | 141 | 0.0022 | |||||
| 1 | 27 (50%) | 15 (25%) | 3 (38%) | 5 (25%) | 50 (35%) | ||
| 2–3 | 18 (33%) | 20 (34%) | 0 (0%) | 9 (45%) | 47 (33%) | ||
| 4–5 | 9 (17%) | 7 (12%) | 2 (25%) | 2 (10%) | 20 (14%) | ||
| >5 | 0 (0%) | 17 (29%) | 3 (38%) | 4 (20%) | 24 (17%) | ||
| Largest tumor diameter (cm) | 144 | 2.5 (1.8–4.3) | 6.7 (4.7–10.3) | 8.2 (6.9–11.9) | 4.2 (3.8–5.4) | 4.6 (2.7–6.8) | <0.0011 |
| Total tumor diameter (cm) | 114 | 4.6 (3.2–6.3) | 14.1 (9.6–20.0) | 13.9 (11.0–21.9) | 7.3 (5.7–12.8) | 7.8 (4.8–14.4) | <0.0011 |
Delivered activity
The median prescribed activity was 1.2 [interquartile range (IQR), 0.9–1.6] Gigabecquerel (GBq). Delivered activity and treatment location by subgroup is included in Table 4. The highest activities were prescribed for patients in subgroups 2 and 3. One hundred thirty-two treatments were lobar or greater. All 12 (8%) of patients that underwent segmental therapy were in the subgroups that included patients that met the Up to 7 Criteria: 10/54 patients (19%) of subgroup 1 and 2/23 patients (9%) of subgroup 4. Median activity delivered for whole liver, lobar and segmental treatments was 1.7 (IQR, 1.4–2.2), 1.2 (IQR, 0.9–1.5), and 0.7 (IQR, 0.4–1.0) GBq. This difference was significant (P<0.001).
Table 4
| Variables | N | Group 1 (N=54) | Group 2 (N=59) | Group 3 (N=8) | Group 4 (N=23) | Combined | P value |
|---|---|---|---|---|---|---|---|
| Activity (GBq) | 144 | 1.0 (0.8–1.4) | 1.4 (1.1–1.7) | 1.4 (1.2–1.8) | 1.2 (1.0–1.5) | 1.2 (0.9–1.6) | 0.022 |
| Treatment zone | 144 | 0.061 | |||||
| Whole liver | 10 (19%) | 14 (24%) | 2 (25%) | 3 (13%) | 29 (20%) | ||
| Lobar | 34 (63%) | 45 (76%) | 6 (75%) | 18 (78%) | 103 (72%) | ||
| Segmental | 10 (19%) | 0 (0%) | 0 (0%) | 2 (9%) | 12 (8%) | ||
| Dosimetry method | 98 | 0.61 | |||||
| BSA | 34 (87%) | 33 (89%) | 6 (100%) | 15 (94%) | 88 (90%) | ||
| Empiric | 0 | 1 (3%) | 0 | 1 (6%) | 2 (2%) | ||
| Partition | 5 (13%) | 3 (8%) | 0 | 0 | 8 (8%) |
Survival
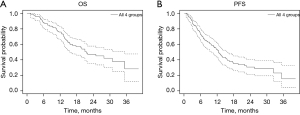
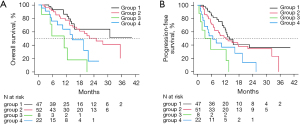
The median OS (Figure 1A) for the cohort was 21.5 months (95% CI: 15.6–35.4 months). There were no deaths within 30 days of treatment. The median PFS (Figure 1B) for the cohort was 12.4 months (95% CI: 9.8–15.4 months). Subgroup analysis for OS is demonstrated in Figure 2A. Median OS was not reached for subgroup 1 at a mean of 28.8 months, while it was 24.9 months (95% CI: 16.5–not reached) for subgroup 2 and 11.0 months (95% CI: 1.3–not reached) for subgroup 3 and 14.6 months (95% CI: 8.1–21.5 months) for subgroup 4. These differences were statistically significant (χ2=19.8, P=0.0002). Median PFS by subgroup is in Figure 2B. Subgroup 1 had the longest median PFS (13.8 months, 95% CI: 11.4–not reached). Subgroup 2 median PFS was 12.4 months (95% CI: 7.4–21.9), subgroup 3 was 4.5 months (95% CI: 0.6–not reached) and subgroup 4 was 6.6 months (95% CI: 2.9–19.5). The differences in PFS by subgroup were statistically significant as well (χ2=16.8, P=0.0008).
Progression
Seventy of the 144 patients (49%) developed progressive disease. The majority of patients with progression (66/70, 94%) had new disease in the liver, while 10/70 (14%) developed extrahepatic metastases. Six of 70 patients with new disease (8.6%) developed both intrahepatic and extrahepatic metastases. Of the 60/144 patients (42%) with isolated intrahepatic progression, 52/144 (36%) were in an area of previous treatment. By subgroup, 16/50 (32%) patients in subgroup 1, 21/52 (40%) in subgroup 2, 6/8 (75%) in subgroup 3 and 9/23 (39%) in subgroup 4 developed progression in a previous treatment zone (P=0.5). The most common extrahepatic sites were the lungs (n=5, 3.5%) and skeletal system (n=4, 2.8%). BCLC subgroup did not predict whether progression would occur in the liver or extrahepatically (P=0.28), or the specific location where extrahepatic metastases would develop (P=0.31).
Off study
Eighty-five patients left the study as demonstrated in Table 5. The most common reason was death (63/85, 74%) followed by loss to follow-up (11/85, 13%) and entry to hospice (6/85, 7%). The cause of death was known in 41/63 (65%) patients. The most common cause of death was tumor progression (23/41, 56%). There was no difference between the subgroups in reasons to leave the study or cause of death (both P=0.1).
Table 5
| Combined (n=144) [%] | Subgroup 1 (n=54) [%] | Subgroup 2 (n=59) [%] | Subgroup 3 (n=8) [%] | Subgroup 4 (n=23) [%] | P value | |
|---|---|---|---|---|---|---|
| Off study reason | 85/144 [59] | 25/54 [46] | 35/59 [59] | 6/8 [75] | 19/23 [83] | 0.1 |
| Death | 63/85 [74] | 16/54 [64] | 27/35 [77] | 6/6 [100] | 14/19 [74] | |
| Lost to follow-up | 11/85 [13] | 4/54 [16] | 7/35 [20] | 0/6 [0] | 0/19 [0] | |
| Hospice | 6/85 [7] | 3/54 [12] | 0/35 [0] | 0/6 [0] | 3/19 [16] | |
| Withdrew consent | 3/85 [4] | 2/54 [8] | 1/35 [3] | 0/6 [0] | 0/19 [0] | |
| Treatment elsewhere | 1/85 [1] | 0/54 [0] | 0/35 [0] | 0/6 [0] | 1/19 [5] | |
| Other | 1/85 [1] | 0/54 [0] | 0/35 [0] | 0/6 [0] | 1/19 [5] | |
| Cause of death | 41/63 [65] | 11/16 [69] | 18/27 [67] | 5/6 [83] | 7/14 [50] | 0.1 |
| Progressive disease | 23/41 [56] | 7/11 [56] | 13/18 [72] | 1/5 [20] | 2/7 [29] | |
| Hepatic decompensation | 5/41 [12] | 0/11 [0] | 2/18 [11] | 2/5 [40] | 1/7 [14] | |
| Other | 13 [32] | 4/11 [36] | 3/18 [17] | 2/5 [40] | 4/7 [57] |
No differences between the subgroups were identified. BCLC B, Barcelona Clinic Liver Cancer B.
Toxicity
A total of 62 Grade 3 or 4 toxicities were reported. These are outlined in Table 6. Fifty-two liver function tests and 10 constitutional events were identified. While hepatic function toxicities were negligible one month after treatment, more elevations were identified later in follow-up. Table 7 outlines toxicities at one month and at any point afterward. The most common Grade 3 or 4 liver function toxicities were elevated bilirubin (n=16, 13%) and decreased albumin (n=15, 12%). A higher proportion of significant bilirubin toxicities occurred in subgroup 4 (6/19, 32% vs. 10/101, 10%, P=0.03). Five patients in subgroup 4 (26%) developed Grade 3 albumin toxicity compared to 10/119 (10%) in subgroups 1–3 (P=0.03). Other liver function toxicities were less common and without significant difference between subgroups for aspartate aminotransferase (AST) (P=0.7), alanine aminotransferase (ALT) (P=0.5) and international normalized ratio (INR) (P=0.2). There was no identifiable difference in hepatic toxicity rates when comparing whole liver, lobar and segmental infusion (Table 8).
Table 6
| Toxicity | 30 days Grade 3 (n=35) | 30 days Grade 4 (n=35) | Total Grade 3 (n=120) | Total Grade 4 (n=120) | Total |
|---|---|---|---|---|---|
| Bilirubin | 0 | 0 | 13 | 3 | 16 |
| Albumin | 0 | 0 | 15 | 0 | 15 |
| AST | 0 | 0 | 5 | 5 | 10 |
| ALT | 0 | 0 | 7 | 3 | 10 |
| INR | 0 | 0 | 1 | 0 | 1 |
| Abdominal pain | 1 | 0 | 3 | 0 | 3 |
| Abdominal distension | 1 | 0 | 1 | 0 | 1 |
| Encephalopathy | 1 | 0 | 1 | 0 | 1 |
| Fatigue | 1 | 0 | 1 | 0 | 1 |
| Fever | 1 | 0 | 1 | 0 | 1 |
| Hyperglycemia | 0 | 1 | 0 | 1 | 1 |
| Nausea | 1 | 0 | 1 | 0 | 1 |
| Renal disorder, other | 1 | 0 | 0 | 1 | 1 |
| Total | 7 (20%) | 1 (3%) | 49 (41%) | 13 (11%) | 62 |
AST, aspartate aminotransferase; ALT, alanine aminotransferase; INR, international normalized ratio.
Table 7
| Subgroup 1 (n=47) [%] | Subgroup 2 (n=48) [%] | Subgroup 3 (n=6) [%] | Subgroup 4 (n=19) [%] | Total (n=120) [%] | P value | |
|---|---|---|---|---|---|---|
| Bilirubin | 0.03 | |||||
| Grade 3 | 4 [8] | 5 [10] | 0 [0] | 4 [21] | 13 [11] | |
| Grade 4 | 0 [0] | 1 [2] | 0 [0] | 2 [10] | 3 [2] | |
| Albumin | 0.03 | |||||
| Grade 3 | 7 [15] | 3 [6] | 0 [0] | 5 [26] | 15 [12] | |
| AST | 0.7 | |||||
| Grade 3 | 2 [4] | 2 [4] | 0 [0] | 1 [5] | 5 [4] | |
| Grade 4 | 2 [4] | 1 [2] | 0 [0] | 2 [10] | 5 [4] | |
| ALT | 0.5 | |||||
| Grade 3 | 2 [4] | 3 [6] | 0 [0] | 2 [10] | 7 [6] | |
| Grade 4 | 2 [4] | 0 [0] | 0 [0] | 1 [5] | 3 [3] | |
| INR | 0.2 | |||||
| Grade 3 | 1 [2] | 0 [0] | 0 [0] | 0 [0] | 1 [0.8] |
Significant differences were identified for bilirubin and albumin. AST, aspartate aminotransferase; ALT, alanine aminotransferase; INR, international normalized ratio.
Table 8
| Whole liver (n=26) [%] | Lobar (n=86) [%] | Segmental (n=8) [%] | Total (n=120) [%] | P value | |
|---|---|---|---|---|---|
| Bilirubin | 0.1 | ||||
| Grade 3 | 3 [12] | 9 [10] | 1 [12] | 13 [11] | |
| Grade 4 | 1 [4] | 2 [2] | 0 [0] | 3 [3] | |
| Albumin | 0.1 | ||||
| Grade 3 | 1 [4] | 14 [16] | 0 [0] | 15 [12] | |
| AST | 0.5 | ||||
| Grade 3 | 2 [8] | 3 [3] | 0 [0] | 5 [4] | |
| Grade 4 | 2 [8] | 2 [2] | 1 [12] | 5 [4] | |
| ALT | 0.5 | ||||
| Grade 3 | 2 [8] | 4 [5] | 1 [12] | 7 [6] | |
| Grade 4 | 1 [4] | 1 [1] | 0 [0] | 3 [3] | |
| INR | 0.1 | ||||
| Grade 3 | 0 [0] | 1 [1] | 0 [0] | 1 [0.8] |
AST, aspartate aminotransferase; ALT, alanine aminotransferase; INR, international normalized ratio.
Discussion
The current work reports a statistically significant correlation between individual patient BCLC B Bolondi subgroup and OS and PFS following TARE. Tumor diameter is not incorporated in assessment scores such as albumin-bilirubin (ALBI) grade or Child-Pugh and the BCLC score doesn’t account for liver function. The Bolondi subgroups provide value by combining tumor size as well as liver function to predict outcome in this heterogeneous patient group with tumor involvement extending from just outside Milan criteria to multifocal diffuse disease (7). Median OS in subgroups B1 and B2 were longer than subgroups B3 and B4. The median OS time was not reached for subgroup B1 at a mean follow-up time of 28.8 months while median OS of subgroup 2 was 24.9 months compared to 11.0 and 14.6 months for subgroups 3 and 4. There were no deaths within 30 days of treatment. Additionally, the classification scheme demonstrated significant differences between subgroups by tumor size and liver function measure. Bilirubin and albumin toxicities occurred at a significantly higher rate in patients from subgroup 4. The majority of the hepatic function toxicities developed more than a month after treatment, making separation of treatment effect from progression of cirrhosis challenging. Subgroup 3 had the largest tumor burden and shortest OS. This study completed enrollment prior to the publication of the DosiSphere study which demonstrated OS benefit with personalized dosimetry using higher tumor doses (19). The outcomes in subgroup 3 would likely have improved with personalized dosimetry.
Our survival breakdown between subgroups is similar to previous studies evaluating best supportive care and chemoembolization for BCLC B patients (15-17). Giannini et al. described survival with best supportive care in 269 patients (15). They reported OS by subgroup of 25, 16, 9, and 5 months. Like our study, Giannini’s B3 subgroup had the smallest proportion of patients (8.2% of the 269 in the study). This finding may reflect the requirement of a single Child-Pugh score, B7, in the setting of a tumor burden beyond the Up to 7 Criteria.
Kim et al. reported chemoembolization outcomes in 821 BCLC B patients broken down by the Bolondi criteria (16). Only 5% of their patients were Bolondi subgroup 4, compared to 16% in the current study. Their survivals decreased by patient subgroup from a median of 51 months for subgroup 1 to 14.8 months for subgroup 3. Kim et al. also reported longer OS in subgroup 4 versus subgroup 3 (25 vs. 14.8 months, respectively); this finding was similar to the current study: 14.6 months for subgroup 4 vs. 11.0 months for subgroup 3. One potential reason for longer OS in subgroup 4 may be that although this subgroup is primarily defined by poor liver function (Child-Pugh score 8–9) it includes any tumor burden. Patients with lower tumor burden may be treated more selectively, with less liver toxicity. Segmental treatment in our cohort was performed only in subgroups 1 and 4. In our cohort, subgroup 4 had smaller median index tumor (4.2 vs. 8.2 cm) and total tumor diameter (7.2 vs. 13.9 cm) than subgroup 3. One difference between the current study and Kim’s report is that 2% of our patients had hepatitis B virus compared to 75.9%. Hepatitis B positive patients with HCC and viral control can have prolonged survival (22). Given the higher rate of hepatic function toxicities in the current study, we would caution against treating patients in subgroup 4 unless segmental treatment could be performed.
Nouso et al. compared BCLC B patient outcomes with radiofrequency ablation versus chemoembolization using the Bolondi subgroups (23). The majority of patients with B1 disease were treated with ablation, compared to 19.7% of the chemoembolization group. While this study documented that BCLC B patients could successfully be treated with ablation, tumor size greater than 3.0 cm adversely affected survival. Kariyama et al. later reviewed outcomes of different therapies in BCLC B patients, including resection, ablation, and chemoembolization (24). Resection patients were almost exclusively (159/165, 96%) from the B1 and 2 subgroups, with 155 (94%) Child-Pugh A and significantly fewer tumors: 136/165 patients (82%) had 3 or fewer HCC. Resection patients had the longest median OS of any group in the study at 5.6 years compared to 4.2 years for ablation and 2.5 years for chemoembolization. Resection remains a valuable option for these highly selected BCLC B patients.
The current study contains limitations. Sites entered data at self-monitored time points, resulting in less than 100% entry. Tables 2-4 include a column outlining the available and missing data for each measure. This study was performed prior to the era of personalized dosimetry which involved use of glass, rather than resin microspheres. The subgroups are not evenly matched. However, the purpose of the study was to demonstrate differences in outcomes based by stratification into the subgroups that were used. The results of this multicenter study should be validated by a larger cohort. It is encouraging that the OS breakdown by subgroup closely tracked with best previous research on supportive care and chemoembolization in BCLC B patients.
Conclusions
In summary, the current study found that the Bolondi BCLC B subclassification predicts OS and PFS. A high percent of the Grade 3 or greater adverse events were clustered in subgroup 4. Patients in this subgroup should be carefully selected prior to treatment with Y-90. Radioembolization of BCLC B subgroup 1–3 patients can be done with acceptable safety and efficacy.
Acknowledgments
Funding: This work was supported by Sirtex Medical.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-972/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-972/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-972/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-972/coif). All authors report that the study was supported by Sirtex Medical. RTG is a consultant and speaker for Sirtex Medical and serves as a proctor for Sirtex Medical. Sirtex Medical has funded travel related to these roles. ZSC has received an institutional research grant from Sirtex Medical and serves as a speaker and consultant for Sirtex Medical. JSB is a consultant for Sirtex Medical. DYS has received institutional research grants from Sirtex Medical. He is a consultant and has received support for travel/hotel/meals for meetings with Sirtex Medical. ASK has received institutional support from Sirtex Medical, Bard Medical and ABK Biomedical. JG is a consultant for Sirtex Medical and Boston Scientific. He has also received institutional grant support from Sirtex Medical. EAW is a proctor for Sirtex Medical. DBB has received institutional research support from Sirtex Medical and Guerbet. He has served as a speaker for Cook Medical and a Data Safety Monitor for Bard Medical. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved and reviewed by the Vanderbilt Ingram Cancer Center and Vanderbilt University Institutional Review Board (IRB) as GI 1523 (IRB No. 150407) and subsequently approved at the other sites. All patients signed informed consent to participate.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology 1998;28:751-5. [Crossref] [PubMed]
- Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet 2012;379:1245-55. [Crossref] [PubMed]
- Leung TW, Tang AM, Zee B, et al. Construction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the Cancer of the Liver Italian Program staging system: a study based on 926 patients. Cancer 2002;94:1760-9. [Crossref] [PubMed]
- Nanashima A, Sumida Y, Morino S, et al. The Japanese integrated staging score using liver damage grade for hepatocellular carcinoma in patients after hepatectomy. Eur J Surg Oncol 2004;30:765-70. [Crossref] [PubMed]
- Chen LT, Martinelli E, Cheng AL, et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with intermediate and advanced/relapsed hepatocellular carcinoma: a TOS-ESMO initiative endorsed by CSCO, ISMPO, JSMO, KSMO, MOS and SSO. Ann Oncol 2020;31:334-51. [Crossref] [PubMed]
- Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol 2022;76:681-93. [Crossref] [PubMed]
- Cillo U, Vitale A, Grigoletto F, et al. Prospective validation of the Barcelona Clinic Liver Cancer staging system. J Hepatol 2006;44:723-31. [Crossref] [PubMed]
- Guglielmi A, Ruzzenente A, Pachera S, et al. Comparison of seven staging systems in cirrhotic patients with hepatocellular carcinoma in a cohort of patients who underwent radiofrequency ablation with complete response. Am J Gastroenterol 2008;103:597-604. [Crossref] [PubMed]
- Marrero JA, Fontana RJ, Barrat A, et al. Prognosis of hepatocellular carcinoma: comparison of 7 staging systems in an American cohort. Hepatology 2005;41:707-16. [Crossref] [PubMed]
- European Association for The Study of The Liver. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908-43. [Crossref] [PubMed]
- Bruix J, Sherman MAmerican Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020-2. [Crossref] [PubMed]
- de Lope CR, Tremosini S, Forner A, et al. Management of HCC. J Hepatol 2012;56:S75-87. [Crossref] [PubMed]
- Bolondi L, Burroughs A, Dufour JF, et al. Heterogeneity of patients with intermediate (BCLC B) Hepatocellular Carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis 2012;32:348-59. [PubMed]
- Giannini EG, Moscatelli A, Pellegatta G, et al. Application of the Intermediate-Stage Subclassification to Patients With Untreated Hepatocellular Carcinoma. Am J Gastroenterol 2016;111:70-7. [Crossref] [PubMed]
- Kim JH, Shim JH, Lee HC, et al. New intermediate-stage subclassification for patients with hepatocellular carcinoma treated with transarterial chemoembolization. Liver Int 2017;37:1861-8. [Crossref] [PubMed]
- Arizumi T, Ueshima K, Iwanishi M, et al. Validation of Kinki Criteria, a Modified Substaging System, in Patients with Intermediate Stage Hepatocellular Carcinoma. Dig Dis 2016;34:671-8. [Crossref] [PubMed]
- Salem R, Gordon AC, Mouli S, et al. Y90 Radioembolization Significantly Prolongs Time to Progression Compared With Chemoembolization in Patients With Hepatocellular Carcinoma. Gastroenterology 2016;151:1155-1163.e2. [Crossref] [PubMed]
- Garin E, Tselikas L, Guiu B, et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): a randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol Hepatol 2021;6:17-29. [Crossref] [PubMed]
- Das A, Gabr A, O'Brian DP, et al. Contemporary Systematic Review of Health-Related Quality of Life Outcomes in Locoregional Therapies for Hepatocellular Carcinoma. J Vasc Interv Radiol 2019;30:1924-1933.e2. [Crossref] [PubMed]
- Frantz S, Matsuoka L, Vaheesan K, et al. Multicenter Evaluation of Survival and Toxicities of Hepatocellular Carcinoma following Radioembolization: Analysis of the RESiN Registry. J Vasc Interv Radiol 2021;32:845-52. [Crossref] [PubMed]
- Hann HW, Coben R, Brown D, et al. A long-term study of the effects of antiviral therapy on survival of patients with HBV-associated hepatocellular carcinoma (HCC) following local tumor ablation. Cancer Med 2014;3:390-6. [Crossref] [PubMed]
- Nouso K, Kariyama K, Nakamura S, et al. Application of radiofrequency ablation for the treatment of intermediate-stage hepatocellular carcinoma. J Gastroenterol Hepatol 2017;32:695-700. [Crossref] [PubMed]
- Kariyama K, Nouso K, Wakuta A, et al. Treatment of Intermediate-Stage Hepatocellular Carcinoma in Japan: Position of Curative Therapies. Liver Cancer 2020;9:41-9. [Crossref] [PubMed]


