
Lesion size, elevated morphology, and non or closed-type atrophy are predictive factors for gastric adenocarcinoma of the fundic gland type rather than oxyntic gland adenoma
Highlight box
Key findings
• We revealed the differences in clinical features between oxyntic gland adenoma and gastric adenocarcinoma of the fundic gland type.
What is known and what is new?
• There is no prediction model for differentiating oxyntic gland neoplasms confined to the mucosa from tumors invading the submucosa.
• Lesion size ≥5 mm, elevated morphology, and non- or closed-type atrophy are predictive factors for gastric adenocarcinoma of the fundic gland type.
What is the implication, and what should change now?
• Our predictive model will serve as a practical guide for the management of oxyntic gland neoplasms.
Introduction
The first case of gastric adenocarcinoma of the fundic gland type (GA-FG) was reported in 2007 as a well-differentiated adenocarcinoma occurring in the cardia of the remnant stomach (1). Subsequently, several researchers collected similar cases and proposed a new concept of disease entities (2-12). Currently, oxyntic gland neoplasms confined to the mucosal layer (T1a) are classified as oxyntic gland adenomas, and oxyntic gland neoplasms with submucosal invasion (T1b) are defined as GA-FG (13). Compared with conventional gastric cancers, oxyntic gland adenoma and GA-FG have unique clinicopathological features, including frequent development in Helicobacter pylori (H. pylori) infection-negative patients and endoscopic appearance of subepithelial lesion-like morphology with superficial vascular dilatation (14). Additionally, a higher prevalence of submucosal invasion is well-known in this disease as well (13). However, to the best of our knowledge, there is no prediction model for differentiating tumors confined to the mucosa (i.e., oxyntic gland adenoma) from tumors invading the submucosa (i.e., GA-FG). Therefore, in the current study, we analyzed the differences in features between oxyntic gland adenomas and GA-FG. We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-870/rc).
Methods
This study is a subgroup analysis of our recently published article reporting the clinicopathologic features of 165 patients with 180 oxyntic gland adenomas and GA-FG lesions (15). We excluded 24 patients for whom H. pylori infection status was not available. We further excluded five patients in whom the depth of invasion was not available because the tumor was not resected. Finally, 136 patients with 150 oxyntic gland adenomas and GA-FG lesions were enrolled in this study.
Histological diagnoses were based on endoscopic biopsy, endoscopic mucosal resection, endoscopic submucosal dissection, or surgical resection (14,15). We compared the patient’s sex, age at diagnosis, H. pylori infection status, lesion size, location, morphology, and other endoscopic features between oxyntic gland adenoma and GA-FG. The morphology of the neoplastic lesions was classified according to the Japanese Classification of Gastric Carcinoma (16). In the current study, we defined an “elevated lesion” as a tumor with 0–I (polypoid-protruding tumors), 0–IIa (slightly elevated superficial tumors), or 0–IIa+IIc morphology (slightly elevated superficial tumors with slightly depressed area). H. pylori infection status was classified as active gastritis (patients with current H. pylori infection), inactive gastritis (patients with past infection), or uninfected (H. pylori-uninfected patients).
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the ethics committees of Okayama University Hospital (No. 2107-011) and other participating institutions. The requirement for written informed consent was waived because of the observational, noninterventional, and retrospective study design. All investigations were performed in accordance with relevant guidelines and regulations.
Statistical analysis
For univariate analysis, variables were analyzed by using a t-test, chi-square test or Fisher’s exact test. Factors exhibiting significant values in the univariate analysis were further analyzed by multivariate analysis using logistic regression analysis. Statistical analyses were performed by JMP Pro 14.0.0 software (SAS Institute, Cary, NC, USA), and P<0.05 was considered significant.
Results
Eight patients had two lesions: one patient had two oxyntic gland adenomas, four patients had two GA-FGs, and the remaining three patients had one oxyntic gland adenoma and one GA-FG. Additionally, two patients had four lesions: one patient had four oxyntic gland adenomas, and the other patient had three oxyntic gland adenomas and one GA-FG lesion. The current study included a total of 136 patients with 150 oxyntic gland adenomas and GA-FG lesions (Figure 1). Representative endoscopic images of GA-FG and oxyntic gland adenoma are shown in Figures 2,3.
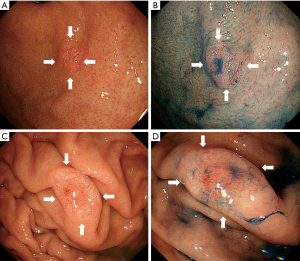
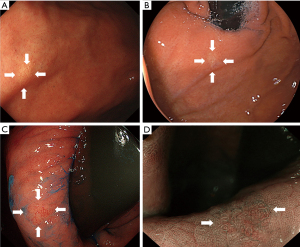
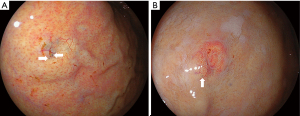
A comparison of lesion characteristics between oxyntic gland adenomas and GA-FG is summarized in Table 1. The mean size of GA-FG (7.7±5.4 mm) was significantly larger than that of oxyntic gland adenoma (5.5±3.1 mm). GA-FG showed an elevated morphology (79.1%) and a higher prevalence than that of oxyntic gland adenomas (51.8%). GA-FG more frequently had black pigmentation within the lesion (23.9%) than oxyntic gland adenomas (9.6%) (Figure 4). Open-type atrophy was less frequently observed in GA-FG (18.8%) than oxyntic gland adenomas (34.9%). No differences were observed in other features such as sex, mean age at diagnosis, lesion location, subepithelial lesion-like morphology, lesion color, vascular dilatation on the surface, and H. pylori infection status.
Table 1
| Variables | Oxyntic gland adenoma, n (%) | GA-FG, n (%) | P value |
|---|---|---|---|
| Sex | 0.508 | ||
| Male | 54 (65.1) | 47 (70.1) | |
| Female | 29 (34.9) | 20 (29.9) | |
| Age, mean ± SD, years | 66.7±9.1 | 67.1±8.3 | 0.784 |
| Size, mean ± SD, mm | 5.5±3.1 | 7.7±5.4 | 0.002 |
| Location | 0.815 | ||
| Fornix | 30 (36.1) | 22 (32.8) | |
| Cardia | 10 (12.0) | 9 (13.4) | |
| Body | 42 (50.6) | 36 (53.7) | |
| Upper third of the body | 21 | 18 | |
| Middle third of the body | 16 | 14 | |
| Lower third of the body | 5 | 4 | |
| Angle | 1 (1.2) | 0 (0) | |
| Antrum | 0 (0) | 0 (0) | |
| Pylorus | 0 (0) | 0 (0) | |
| Morphology | <0.001 | ||
| 0–I | 0 (0) | 2 (3.0) | |
| 0–IIa | 43 (51.8) | 47 (70.1) | |
| 0–IIb | 31 (37.3) | 12 (17.9) | |
| 0–IIc | 9 (10.8) | 2 (3.0) | |
| 0–IIa+IIc | 0 (0) | 4 (6.0) | |
| 0–III | 0 (0) | 0 (0) | |
| Macroscopic appearance | 0.075 | ||
| SEL-like | 40 (48.2) | 42 (62.7) | |
| Non SEL-like | 43 (51.8) | 25 (37.3) | |
| Color | 0.288 | ||
| Similar to the peripheral mucosa | 23 (27.7) | 24 (35.8) | |
| Reddish | 9 (10.8) | 11 (16.4) | |
| Whitish | 27 (32.5) | 11 (16.4) | |
| Yellowish-white | 20 (24.1) | 17 (25.4) | |
| Yellowish | 4 (4.8) | 4 (6.0) | |
| Vascular dilatation on the surface | 0.244 | ||
| Present | 52 (62.7) | 48 (71.6) | |
| Absent | 31 (37.3) | 19 (28.4) | |
| Black pigmentation | 0.018 | ||
| Present | 8 (9.6) | 16 (23.9) | |
| Absent | 75 (90.4) | 51 (76.1) | |
| H. pylori infection status | 0.541 | ||
| Uninfected | 27 (32.5) | 25 (37.3) | |
| Active gastritis | 10 (12.0) | 5 (7.5) | |
| Inactive gastritis | 46 (55.4) | 37 (55.2) | |
| Gastric atrophy | 0.028 | ||
| None or closed type | 54 (65.1) | 52 (81.3) | |
| Open type | 29 (34.9) | 12 (18.8) |
GA-FG, gastric adenocarcinoma of the fundic gland type; SD, standard deviation; SEL, subepithelial lesion.
Subsequently, we performed multivariate logistic regression analysis (Table 2), which revealed that lesion size of ≥5 mm (odds ratio, 2.96; 95% confidence interval: 1.21–7.23), elevated morphology (odds ratio, 2.40; 95% confidence interval: 1.06–5.45), and no or closed-type atrophy (odds ratio, 2.49; 95% confidence interval: 1.07–5.80) were factors that differentiated GA-FG from oxyntic gland adenoma. In contrast, the presence of black pigmentation within the lesion was not significant in the multivariate analysis, despite its significance in the univariate analysis.
Table 2
| Factor | Comparison | Odds ratio | 95% LCL | 95% UCL | P value |
|---|---|---|---|---|---|
| Lesion size | ≥5 vs. <5 mm | 2.96 | 1.21 | 7.23 | 0.017 |
| Morphology | Elevated vs. non-elevated | 2.40 | 1.06 | 5.45 | 0.036 |
| Gastric atrophy | None or closed type vs. open type | 2.49 | 1.07 | 5.80 | 0.034 |
| Black pigmentation | Present vs. absent | 1.89 | 0.68 | 5.30 | 0.224 |
GA-FG, gastric adenocarcinoma of the fundic gland type; LCL, lower confidence limit; UCL, upper confidence limit.
Based on the multivariate analysis, we considered that a lesion size of ≥5 mm (feature 1), elevated morphology (feature 2), and no or closed-type atrophy (feature 3) are characteristic of GA-FG rather than oxyntic gland adenoma. We assigned one point to each feature, and the overall score was calculated based on the number of these three features. When oxyntic gland neoplasms with no feature or at least one feature were categorized as oxyntic gland adenomas and those with two or three features were categorized as GA-FG, the sensitivity and specificity were 85.1% and 43.4% for GA-FG, respectively.
Discussion
In the present study, the number of oxyntic gland adenoma (n=83) and GA-FG (n=67) was in a ratio of approximately 4:3. Benedict et al. reported that 32 cases of pT1a, 63 cases of pT1b, and two cases of advanced-stage tumors were included in 111 published cases (17). Hence, the prevalence of oxyntic gland adenomas and GA-FG was in the ratio of 1:2. Concerning the difference in prevalence between our study and the previous reports, publication bias might have existed because more lesions with submucosal or deeper invasion (GA-FG) may have been published than lesions confined within the mucosa (oxyntic gland adenomas). Regardless of the prevalence, oxyntic gland neoplasms frequently invade the submucosa. Oxyntic gland adenomas and GA-FG are composed of highly differentiating columnar cells that mainly differentiate into chief cells and, to a lesser extent, parietal cells (12). Gastric chief cells and parietal cells are located at the base of the fundic glands (i.e., oxyntic glands), which constitute the mucosa of the fundus and body of the stomach. These cells reside at any level in the fundic glands, but the chief and parietal cells are most abundant in the deeper and middle regions of the mucosa, respectively. Therefore, the higher incidence of submucosal invasion in oxyntic gland neoplasms may be explained by the cell of origin.
We revealed that a larger lesion size, elevated tumor morphology, and no or closed-type atrophy in the background gastric mucosa were endoscopic features characteristics of GA-FG, rather than oxyntic gland adenoma. Because large lesion size reflects tumor growth in a horizontal direction and elevated morphology reflects tumor growth in a vertical direction, it is reasonable that these two features correspond to submucosal invasion. In contrast, there is no clear explanation for the association between the grade of gastric atrophy and invasion depth. A possible hypothesis is that, since oxyntic gland neoplasms are generally covered by foveolar epithelium, non or less-atrophic, thick mucosa may conceal the tumor, and small tumors are not easily identified with esophagogastroduodenoscopy.
Based on the results of the present study, we propose a discriminating algorithm to differentiate GA-FG from oxyntic gland adenoma. Our study had several limitations. First, pathologists at various institutions diagnosed the gastric lesions. Interobserver variations and differences in methodologies between the participating pathologists may have resulted in interpretation bias during the analysis of the oxyntic gland adenoma and GA-FG groups. Second, although we proposed a diagnostic algorithm for differentiating GA-FG from oxyntic gland adenoma, a validation study was not conducted. Thirdly, although the sensitivity of our algorithm was relatively high (85.1%), its specificity was low (43.4%). We consider that methods to distinguish between the two diseases are unlikely to be developed because a morphological continuum exists from oxyntic gland adenoma to GA-FG (13). Despite its low specificity, our algorithm can be used to develop appropriate disease management strategies. For instance, en bloc resection with endoscopic submucosal dissection is desirable for preoperatively suspected GA-FG lesions, to evaluate the invasion depth. At the same time, the relatively null result in the present study highlights the morphological continuum between the two categories and the necessity of a thorough pathological evaluation for diagnosis. Although statistical differences were observed in endoscopic findings, such as the size, elevated morphology, and background atrophy, these factors may not be specific for differentiating oxyntic gland adenomas from GA-FGs. Thus, to overcome these issues, validation of our scoring system in a multicenter study with a larger sample size is required.
Conclusions
In conclusion, we investigated the differences between oxyntic gland adenomas and GA-FG. We identified three possible distinctive features of GA-FG compared to oxyntic gland adenoma: lesion size ≥5 mm, elevated morphology, and no or closed-type atrophy. Oxyntic gland neoplasms with two or three features were judged as GA-FG, with 85.1% sensitivity and 43.4% specificity for GA-FG diagnosis. Future studies with larger sample sizes are required to determine whether GA-FGs have distinct endoscopic features that differentiate it from oxyntic gland adenomas.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-870/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-870/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-870/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the ethics committees of Okayama University Hospital (No. 2107-011) and other participating institutions. The requirement for written informed consent was waived because of the observational, noninterventional, and retrospective study design.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tsukamoto T, Yokoi T, Maruta S, et al. Gastric adenocarcinoma with chief cell differentiation. Pathol Int 2007;57:517-22. [Crossref] [PubMed]
- Ueyama H, Yao T, Nakashima Y, et al. Gastric adenocarcinoma of fundic gland type (chief cell predominant type): proposal for a new entity of gastric adenocarcinoma. Am J Surg Pathol 2010;34:609-19. [Crossref] [PubMed]
- Singhi AD, Lazenby AJ, Montgomery EA. Gastric adenocarcinoma with chief cell differentiation: a proposal for reclassification as oxyntic gland polyp/adenoma. Am J Surg Pathol 2012;36:1030-5. [Crossref] [PubMed]
- Hidaka Y, Mitomi H, Saito T, et al. Alteration in the Wnt/β-catenin signaling pathway in gastric neoplasias of fundic gland (chief cell predominant) type. Hum Pathol 2013;44:2438-48. [Crossref] [PubMed]
- Ueyama H, Matsumoto K, Nagahara A, et al. Gastric adenocarcinoma of the fundic gland type (chief cell predominant type). Endoscopy 2014;46:153-7. [PubMed]
- Ueo T, Yonemasu H, Ishida T. Gastric adenocarcinoma of fundic gland type with unusual behavior. Dig Endosc 2014;26:293-4. [Crossref] [PubMed]
- Nomura R, Saito T, Mitomi H, et al. GNAS mutation as an alternative mechanism of activation of the Wnt/β-catenin signaling pathway in gastric adenocarcinoma of the fundic gland type. Hum Pathol 2014;45:2488-96. [Crossref] [PubMed]
- Chiba T, Kato K, Masuda T, et al. Clinicopathological features of gastric adenocarcinoma of the fundic gland (chief cell predominant type) by retrospective and prospective analyses of endoscopic findings. Dig Endosc 2016;28:722-30. [Crossref] [PubMed]
- Chan K, Brown IS, Kyle T, et al. Chief cell-predominant gastric polyps: a series of 12 cases with literature review. Histopathology 2016;68:825-33. [Crossref] [PubMed]
- Miyazawa M, Matsuda M, Yano M, et al. Gastric adenocarcinoma of the fundic gland (chief cell-predominant type): A review of endoscopic and clinicopathological features. World J Gastroenterol 2016;22:10523-31. [Crossref] [PubMed]
- Murakami T, Mitomi H, Yao T, et al. Epigenetic regulation of Wnt/β-catenin signal-associated genes in gastric neoplasia of the fundic gland (chief cell-predominant) type. Pathol Int 2017;67:147-55. [Crossref] [PubMed]
- Ushiku T, Kunita A, Kuroda R, et al. Oxyntic gland neoplasm of the stomach: expanding the spectrum and proposal of terminology. Mod Pathol 2020;33:206-16. [Crossref] [PubMed]
- Yao T, Vieth M. Oxyntic gland adenoma. Digestive system tumours. In World Health Organization Classification of Tumours 5th edn, WHO Classification of Tumours Editorial Board. International Agency for Research on Cancer, World Health Organization; 2019:83-4.
- Iwamuro M, Kusumoto C, Nakagawa M, et al. Endoscopic resection is a suitable initial treatment strategy for oxyntic gland adenoma or gastric adenocarcinoma of the fundic gland type. Sci Rep 2021;11:7375. [Crossref] [PubMed]
- Iwamuro M, Kusumoto C, Nakagawa M, et al. Endoscopic features of oxyntic gland adenoma and gastric adenocarcinoma of the fundic gland type differ between patients with and without Helicobacter pylori infection: a retrospective observational study. BMC Gastroenterol 2022;22:294. [Crossref] [PubMed]
- Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011;14:101-12. [Crossref] [PubMed]
- Benedict MA, Lauwers GY, Jain D. Gastric Adenocarcinoma of the Fundic Gland Type: Update and Literature Review. Am J Clin Pathol 2018;149:461-73. [Crossref] [PubMed]



