
Suspicious findings observed retrospectively on CT imaging performed before the diagnosis of pancreatic cancer
Highlight box
Key findings
• Pancreatic cancer can be incidentally detected in CT performed for other purposes, but suspicious findings of pancreatic cancer may also be overlooked.
What is known and what is new?
• In this study, 51.9% of the patients who underwent CT before pancreatic cancer diagnosis had findings suggestive of pancreatic cancer in a retrospective review.
• Suspicious findings of pancreatic cancer included a hypoattenuating mass, focal pancreatic duct dilatation, and distal parenchymal atrophy of the pancreas.
What is the implication, and what should change now?
• To diagnose pancreatic cancer in the early stage, clinicians need to always pay attention to the presence of these features.
Introduction
Pancreatic cancer is a fatal disease in which most patients are diagnosed at an advanced stage. Although early diagnosis of pancreatic cancer is the most important way to improve prognosis, most of early pancreatic cancer are asymptomatic and an effective screening tool for its early diagnosis in high-risk groups has not been established (1,2).
Risk factors for pancreatic cancer include a family history of pancreatic cancer, diabetes, obesity, chronic pancreatitis, hereditary pancreatitis, smoking, and alcohol intake (3,4). To obtain an early diagnosis of pancreatic cancer, computed tomography (CT), endoscopic ultrasonography (EUS), magnetic resonance imaging (MRI), and endoscopic retrograde cholangiopancreatography have been used in patients who have a high risk of pancreatic cancer. However, clear evidence of the clinical benefits of these tests has not been established (5-7).
CT is the most widely used imaging modality for the diagnosis of pancreatic cancer, and it is helpful for determining the status of local and remote diseases and vascular invasion (8). The sensitivity for diagnosing small pancreatic cancers <10 mm in size by CT is 33–44% (5). A characteristic CT finding of early pancreatic cancer is hypoattenuation with contrast enhancement. In addition, main pancreatic duct dilatation and distal parenchymal atrophy are characteristic findings of early pancreatic cancer (9-12).
CT is commonly performed for many reasons, such as abdominal pain, fever of unknown origin, and regular follow-up examinations to detect the recurrence of cancers. Pancreatic cancer or suspicious lesions of pancreatic cancer can be incidentally observed on CT performed for reasons unrelated to the pancreas. Earlier findings of pancreatic cancer may be observed on imaging for the examination of an unrelated disease. In addition, pancreatic cancer can be incidentally detected. With the widespread use of imaging studies in medical practice, the occurrence of abnormal pancreatic lesions found incidentally in asymptomatic subjects is increasing. Pancreatic cancer incidentally detected without symptoms has a higher rate of early-stage diagnosis, a higher resection potential, and a higher 5-year survival rate than that with symptoms (13-15). A suspected small lesion of the pancreas on CT conducted for other purposes may be easily overlooked unless a distinct finding is detected in the pancreas. Suspected lesions of pancreatic cancer have been found in 40–85% of cases where CT was conducted before a pancreatic cancer diagnosis (11,16-18).
However, few studies have focused on CT findings before a pancreatic cancer diagnosis, and the nature of the lesions of suspected pancreatic cancer on CT is unknown. In this study, to diagnose pancreatic cancer early with CT conducted for other purposes, we aimed to determine the CT findings before a pancreatic cancer diagnosis in patients who had undergone CT within 1 year of a pancreatic cancer diagnosis (prediagnostic CT). We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-863/rc).
Methods
Patients
Between January 2008 and December 2019, patients who were diagnosed with pancreatic cancer and hospitalized at the National Health Insurance Service Ilsan Hospital (Goyang, Korea) were selected. We reviewed all the CT scans of the selected patients. Among them, those who underwent contrast-enhanced abdominal CT or chest CT including the images of the pancreas within 1 year of a pancreatic cancer diagnosis, were enrolled in this study. Patients who were not initially diagnosed with pancreatic cancer but had reported suspected lesions for pancreatic cancer based on CT imaging and were later diagnosed with pancreatic cancer after follow-up were excluded. Only patients with no report of suspected lesions of pancreatic cancer in the prediagnostic CT were included. The criteria for the diagnosis of pancreatic cancer were as follows: the cancer was histologically confirmed, or if no histological confirmation existed, then imaging findings (e.g., CT, MRI, and positron emission tomography) were reasonable for pancreatic cancer, and the clinical course of the patient was appropriate for pancreatic cancer.
Review of medical reports
The medical records of the enrolled patients were retrospectively analyzed based on sex, age, the reason for undergoing CT before a pancreatic cancer diagnosis, the department where CT was conducted, and the type of contrast-enhanced CT. In addition, the reason for undergoing CT at the time of diagnosis of pancreatic cancer was investigated by dividing cases into “symptomatic” or “asymptomatic”. For those diagnosed with pancreatic cancer, the size of pancreatic cancer, location, histological confirmation, resectability, treatment method, stage, date of the last follow-up, recurrence, and death were investigated. All patients were followed up until the date of death or December 2020.
Image analysis of prediagnostic CT
The prediagnostic CT image analysis was independently conducted by two board-certified abdominal radiologists (C An and S Park, who had 5 and 23 years of experience in abdominal imaging, respectively). All disagreements were resolved by discussion to reach a consensus. The reviewers were informed that all patients were later confirmed as having pancreatic cancer. They reviewed whether any lesions of suspected pancreatic cancer existed by dividing their findings into pancreatic parenchyma and pancreatic duct findings. The CT findings of the pancreatic parenchyma were classified into “normal”, “mass-like lesion”, “diffuse swelling” (suggestive of acute pancreatitis), “diffuse parenchymal atrophy” (suggestive of chronic pancreatitis), and “distal parenchymal atrophy”. A mass-like lesion was analyzed for its diameter and contrast enhancement pattern. Diffuse parenchymal atrophy was defined as atrophy in the entire pancreas without focal lesions. Distal parenchymal atrophy was defined as parenchymal atrophy in the distal part of the pancreatic focal lesion or disproportional atrophy in the absence of a pancreatic focal lesion (11). The findings of the pancreatic duct were classified as “normal”, “focal dilatation”, and “diffuse dilatation”. Pancreatic duct dilatation was defined as the maximum diameter of the main pancreatic duct of ≥3 mm. The findings of both the pancreatic parenchyma and pancreatic duct were analyzed together.
Statistical analysis
Continuous variables are presented as median values, and categorical variables are presented as numbers of patients and percentages. Prediagnostic CT scans were analyzed by merging the findings of pancreatic parenchyma and pancreatic duct. The agreements between two radiologists for findings of pancreatic parenchyme and pancreatic duct were calculated using Cohen’s kappa. All statistical analyses were conducted using SPSS, version 23.0 (IBM Corp., Armonk, NY, USA).
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study design was reviewed and approved by the Institutional Review Board of National Health Insurance Service Ilsan Hospital (NHIMC 2018-09-014). The requirement for obtaining patient informed consent was waived by the board due to the retrospective design of the current study.
Results
Characteristics of the patients
Between January 2008 and December 2019, 736 patients were diagnosed with pancreatic cancer at the National Health Insurance Service Ilsan Hospital and hospitalized. Among them, 27 patients who underwent prediagnostic CT within 1 year of a pancreatic cancer diagnosis and had no reports of suspected pancreatic cancer lesion on CT were finally enrolled in this study.
The median age of the patients was 73.6 (range, 47–87) years, and the group included 19 men and 8 women. The median time interval between the prediagnostic CT and pancreatic cancer diagnosis was 6.6 (range, 1.9–12.0) months. The prediagnostic CT examinations included abdominal CT in 25 patients and chest CT in two patients. Based on the phase of contrast enhancement, single-phase CT was conducted in 22 patients, and multiphase CT was conducted in five patients (Table 1).
Table 1
| Parameters | No. of patients |
|---|---|
| Age (years), median [range] | 73.6 [47–87] |
| Sex | Male: 19; female: 8 |
| Reasons for undergoing prediagnostic CT | |
| Regular follow-up of previous diagnosed other cancers | 11 |
| Acute pancreatitis | 3 |
| Regular follow-up of chronic pancreatitis | 3 |
| Fever | 2 |
| Abdominal pain | 2 |
| Regular follow-up of liver cirrhosis | 1 |
| Lower back pain | 1 |
| Liver abscess | 1 |
| Common bile duct stone with cholangitis | 1 |
| Abdominal trauma | 1 |
| Fall injury | 1 |
| Department of undergoing prediagnostic CT | |
| Gastroenterology | 11 |
| Other than gastroenterology | 16 |
| Types of contrast-enhanced CT | |
| Single-phase | 22 |
| Multiphase | 5 |
CT, computed tomography.
All patients underwent prediagnostic CT for reasons unrelated to pancreatic cancer. Among the 27 patients, 11 patients underwent CT as a regular follow-up examination for previously treated cancers (4 gastric cancers, 2 bladder cancers, 1 lung cancer, 1 lymphoma, 1 thymic cancer, 1 prostate cancer, and 1 liver cancer); 3 patients underwent CT for acute pancreatitis; 3 patients, for chronic pancreatitis; 2 patients for fever; and 2 patients for abdominal pain. The departments where the CT was conducted were the gastroenterology department for 11 (40.3%) patients and other departments [i.e., hematology-oncology (3 patients); infectious medicine, surgery, urology, and emergency medicine (2 patients for each department); endocrinology, rheumatology, and nephrology, pulmonology, and thoracic surgery (1 patient for each department)] for the remaining 16 (59.7%) patients.
Imaging findings of prediagnostic CT, based on the retrospective review
Two radiologists retrospectively reviewed the CT findings, based on the pancreatic parenchyma and pancreatic duct findings. Interobserver agreements between two radiologists for pancreatic parenchyma and pancreatic duct findings were good (Cohen’s kappa=0.74, P<0.001) and strong (Cohen’s kappa=0.88, P<0.001), respectively. The findings of pancreatic parenchyma were as follows: normal in 9 (33.3%) patients, mass-like lesion in 9 (33.3%) patients (Figure 1), diffuse swelling in 4 (14.8%) patients, diffuse parenchymal atrophy in 3 (11.1%) patients, and distal parenchymal atrophy in 2 (7.4%) patients (Figure 2). The findings of the pancreatic duct were as follows: normal in 15 (55.6%) patients, focal dilatation in 6 (22.2%) patients (Figure 3), and diffuse dilatation in 6 (22.2%) patients (Table 2). Collectively, 7 patients had normal pancreatic parenchyma and pancreatic duct, and 20 patients had abnormal findings in the pancreas (Table 3).
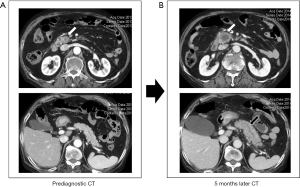
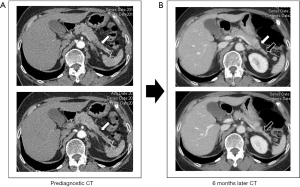
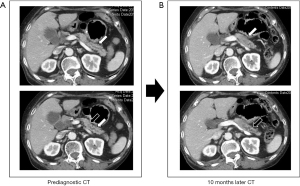
Table 2
| Findings | No. of patients (%) |
|---|---|
| Pancreas parenchyma | |
| Normal | 9 (33.3) |
| Mass-like lesion | 9 (33.3) |
| Diffuse swelling | 4 (14.8) |
| Diffuse parenchymal atrophy | 3 (11.1) |
| Distal parenchymal atrophy | 2 (7.4) |
| Pancreatic duct | |
| Normal | 15 (55.6) |
| Focal dilatation | 6 (22.2) |
| Diffuse dilatation | 6 (22.2) |
| Total | 27 (100.0) |
CT, computed tomography.
Table 3
| Findings | Pancreatic duct | Total | ||
|---|---|---|---|---|
| Normal | Focal dilatation | Diffuse dilatation | ||
| Pancreas parenchyma | ||||
| Normal | 7 | 2† | 0 | 9 |
| Mass-like lesion | 6† | 2† | 1† | 9 |
| Diffusely swelling | 1 | 1† | 2 | 4 |
| Diffuse parenchymal atrophy | 0 | 0 | 3 | 3 |
| Distal parenchymal atrophy | 1† | 1† | 0 | 2 |
| Total | 15 | 6 | 6 | 27 |
†, patients with lesions of suspected pancreatic cancer. CT, computed tomography.
The nine mass-like lesions all presented with focal hypoattenuation on contrast-enhanced CT. Among these patients, 6 patients had no pancreatic duct dilatation, and 2 patients had focal pancreatic duct dilatation. The median size of the retrospectively detected mass-like lesion was 1.2 (range, 0.8–1.9) cm. Of the 2 patients with distal parenchymal atrophy, 1 patient had focal pancreatic duct dilatation, and 1 patient had no pancreatic duct dilatation. Taken together, 3 of 6 patients with focal pancreatic duct dilatation had mass-like lesions or distal atrophy findings in the pancreatic parenchyma.
Assuming that a mass-like lesion (9/27), focal dilatation of the pancreatic duct (6/27), or distal parenchymal atrophy (2/27) could be findings suggestive of early pancreatic cancer, a total of 17 findings of suspected pancreatic cancer were found in 27 prediagnostic CT scans, and three patients had two findings at the same time. Taken together, 14 (51.9%) of 27 patients had one or more findings of suspected pancreatic cancer (Table 3). These findings were overlooked in all 14 patients.
Clinical features of pancreatic cancer among the patients
At the time of diagnosis of pancreatic cancer, 11 (40.7%) patients had no symptoms of pancreatic cancer. Among these patients, 6 patients underwent CT for the follow-up of other cancers; 1 patient for a pancreatic mass incidentally found on abdominal ultrasound; 1 patient for the follow-up of acute pancreatitis; 1 patient for ischemic colitis; 1 patient for upper gastrointestinal bleeding; and 1 patient for incidental serum amylase and lipase elevation. 16 (59.3%) symptomatic patients underwent CT because of jaundice (n=7) and abdominal pain (n=9). The median size of pancreatic cancer was 3.0 (range, 1.2–8.7) cm (Table 4). The locations were the head (18 patients), body (4 patients), and tail (5 patients). The cancer was resectable in 14 patients (51.9%), locally advanced in 7 (25.9%) patients, and had distant metastasis in 6 (22.2%) patients. Twenty-four patients had a histologically confirmed diagnosis of pancreatic cancer. Three patients without a histological diagnosis all died because of the progression of pancreatic cancer.
Table 4
| Parameters | No. of patients |
|---|---|
| Size of pancreas cancer (cm), median (range) | 3.0 (1.2–8.7) |
| Location | |
| Head | 18 |
| Body | 4 |
| Tail | 5 |
| Resectability | |
| Resectable | 14 |
| Locally advanced | 7 |
| Distant metastasis | 6 |
| Treatment | |
| Curative resection | 12 |
| PPPD | (6) |
| Distal pancreatectomy | (6) |
| Systemic chemotherapy | 8 |
| Palliative gastrojejunostomy | 2 |
| Only supportive care | 5 |
| Stage | |
| 1B | 5 |
| 2A | 1 |
| 2B | 6 |
| 3 | 9 |
| 4 | 6 |
| Outcomes | |
| Alive without recurrence after surgery | 2 |
| Alive with palliative treatment | 1 |
| Death | 24 |
PPPD, pylorus preserving pancreato-duodenectomy.
Twelve patients underwent curative surgery, and eight patients underwent palliative chemotherapy. The TNM stage of pancreatic cancer was stage 1B in 5 patients, stage 2A in 1 patient, stage 2B in 6 patients, stage 3 in 9 patients, and stage 4 in 6 patients. The median survival of all patients was 10.8 months. During follow-up, 24 patients died, and their median survival was 10.6 (range, 1.2–61.5) months.
Discussion
In this study, we analyzed the data of 27 patients who underwent contrast CT within 1 year before their pancreatic cancer diagnosis. Fourteen (51.9%) of 27 patients with pancreatic cancer had suspected pancreatic cancer lesions on the prediagnostic CT scan. All prediagnostic CT examinations were conducted for reasons unrelated to pancreatic cancer, and 59.3% of these CT scans were conducted in departments other than the gastroenterology department. We provided actual clinical results and characteristic CT findings for suspected pancreatic cancer lesions that had been overlooked in CT conducted for other purposes.
Early pancreatic cancer is mostly asymptomatic; therefore, the processes by which early pancreatic cancer is diagnosed are regular follow-up examination of high-risk patients, medical examination in a healthy general population, and the incidental detection of pancreatic lesions on imaging tests conducted for other purposes (19). Regular screening can be considered for patients in known high-risk groups for pancreatic cancer, but the selection of the patients and test method remain controversial. An international consensus recommends conducting follow-up EUS or MRI annually in individuals with a family history or genetically high risk of pancreatic cancer (20). The health examination for screening pancreatic cancer in the general population is not recommended because it is impractical and less cost-effective, and the diagnosis rate is low (21).
The proportion of pancreatic cancer incidentally detected during imaging tests for other diseases is 16% among all pancreatic cancers (14). In a Japanese study that analyzed data from 200 patients with stage 0 and stage 1 pancreatic cancer, 51.5% of the cases were incidentally detected (19). Therefore, CT conducted for other purposes may be an important modality for the early diagnosis of pancreatic cancer. Among solid lesions found incidentally in the pancreas, pancreatic cancer is the most commonly found lesion, with a rate of approximately 31–34% (22). In a review of 569 patients with pancreatic cancer, the investigators reported that patients with incidentally detected pancreatic cancer had higher tumor resectability (64% vs. 36%) and longer median survival (16 vs. 10 months) compared to patients with symptomatic lesions (14). Winter et al. (15) reported a greater number of cases of stage 1 cancer and a better 5-year survival rate in incidentally detected pancreatic cancer.
The sensitivity and specificity of CT for pancreatic cancer diagnosis is 76–92% (23-25) and 67% (23), respectively. CT that is appropriate for the diagnosis of pancreatic cancer should be taken in the arterial phase (approximately 30 s after contrast injection) and portal venous phase (approximately 60–70 s after contrast injection), which are based on the pancreatic protocol (26). If CT is conducted for purposes other than those related to pancreatic disease, diagnosing early pancreatic cancer may be more difficult because routine CT is often conducted with a single-phase protocol rather than with the pancreatic protocol (27). In this study, prediagnostic CT was conducted using the single-phase protocol in 22 of 27 patients. Therefore, for the incidental detection of early pancreatic cancer lesions, not only multiphase but also single-phase CT findings are essential in actual clinical practice.
Typical CT findings of pancreatic cancer are hypoattenuating tumor in the arterial and portal venous phases with secondary findings such as pancreatic duct dilatation, pancreatic duct break, common bile duct dilatation, and pancreatic atrophy (8,18). However, these typical CT findings are commonly found in advanced pancreatic cancer. Diagnosis is not easy if the CT shows an isoattenuating tumor, which occurs in 11–14% of all pancreatic cancers, and most of them are small pancreatic cancers (12,28). In this study, nine mass-like lesions had a median size of 1.2 cm, and all showed hypoattenuation with contrast enhancement. The isoattenuating mass might have existed, but it could not be detected. Prokesch et al. (12) reported that 11% of 53 patients with pancreatic cancer showed isoattenuating tumors and mass effect; atrophic distal parenchyma and an interrupted duct sign were important findings in the diagnosis of pancreatic cancer.
Pancreatic duct dilatation and interruption are the most important secondary findings of pancreatic cancer, and they can be a crucial key in diagnosing small pancreatic cancer (11,12,16,28). In this study, six patients had focal pancreatic duct dilatation, but it was not detected initially. Based on a study by Yoon et al. (18), 99% of pancreatic cancers with a size of 21–30 mm and 76% of pancreatic cancers with a size of ≤20 mm showed pancreatic duct dilatation and interruption on CT. In a study of 36 patients with pancreatic cancer ≤1 cm, 57% of patients had only focal pancreatic duct dilatation with no mass findings (29). In a study of the imaging findings of 10 patients with high-grade pancreatic intraepithelial neoplasia, as the premalignant lesion, localized pancreatic duct stricture was found in all patients (30). However, localized pancreatic duct stricture can also occur in benign diseases such as chronic pancreatitis. In three case series studies of localized main pancreatic duct structure without a detectable mass, 47–54% of cases were actually diagnosed with pancreatic cancer (31-33). Therefore, if focal pancreatic duct dilatation without a mass is incidentally detected, careful evaluation is necessary because of the possibility of small pancreatic cancer.
Distal parenchymal atrophy is another characteristic feature of early pancreatic cancer and may be associated with a hypoattenuating mass or focal pancreatic duct dilatation (11,32,34). In this study, distal parenchymal atrophy was found in two patients and an accompanying focal pancreatic duct dilatation was found in one patient in them. Distal atrophy of pancreatic cancer can be a secondary change because of prolonged duct obstruction caused by lesions (11). The incidence of pancreatic parenchymal atrophy in pancreatic cancer varies from 13.3% to 53.0% (34). However, distal parenchymal atrophy can be observed sometimes even in benign conditions. Distal parenchymal atrophy was present in 4% of controls without pancreatic diseases (11,35) and in 7.1–15.8% of patients with benign main pancreatic duct stricture (32,34,36).
Only a few studies exist on CT findings before a pancreatic cancer diagnosis. Most patients underwent CT for the evaluation of diseases other than pancreatic cancer. Gangi et al. (16) reported that findings of suspected pancreatic cancer were detected in 50% of 62 CT scans of 28 patients who underwent CT within 18 months of a pancreatic cancer diagnosis. Ahn et al. (11) reported that findings indicative of pancreatic cancer were observed on 85% (17/20) of the prediagnostic CTs, and that focal hypoattenuation, pancreatic duct dilatation, and distal parenchymal atrophy were significant findings to observe to avoid missing a diagnosis of pancreatic cancer. Moreover, Yoon et al. (18) reported that detectable tumors were found in 24 (72.3%) of 33 patients with prediagnostic CT; the tumor size was <20 mm in 21 patients and 21–30 mm in three patients. The average size was 16.4 mm. Higashi et al. (17) also reported that, among 14 patients, small nodules were found in 40% of patients and pancreatic duct dilatation in 36% of patients on prediagnostic CT within 18 months before a pancreatic cancer diagnosis. In a recently published study, focal pancreatic abnormalities were found in 55 (53.4%) of 103 patients with stage 1 pancreatic cancer on CT performed at least 1 year before the diagnosis of pancreatic cancer (37).
In this study, 14 (51.9%) of 27 patients had one or more findings of suspected pancreatic cancer on the prediagnostic CT scan. As the radiologists reviewed the CT images with information on patients with pancreatic cancer, it would have affected the detection of suspicious lesions in pancreas. These imaging findings had been overlooked in all patients. A radiologist had overlooked the findings of suspected pancreatic cancer, although these lesions could have been detected if the clinician had carefully observed the pancreas on the CT scan. However, as shown in this study, many departments, other than the gastroenterology department, conduct CT and there will be practical limitations for all clinicians to always observe the pancreas with interest. Nevertheless, as part of an effort to early diagnose pancreatic cancer, radiologists and clinicians need to pay more attention to pancreatic lesions that are incidentally detected while conducting imaging examinations for other purposes. In addition, two patients in this study had suspected lesions on chest CT. Careful observation is also needed even in chest CT which includes the pancreas.
In this study, 40.7% of patients were asymptomatic at the time of pancreatic cancer diagnosis, the median size of pancreatic cancer was 3.0 cm, and curative resections were conducted in 44.4%. In general, 15–20% of patients are eligible for curative surgery (38), and the patients in this study were often diagnosed with less disease progression. The interval between prediagnostic CT and pancreatic cancer diagnosis was not long, and many patients underwent CT for regular follow-up examination of other diseases; therefore, the number of patients diagnosed at the advanced stage was relatively small.
Our study has several limitations. This study is a retrospective analysis with a small number of patients recruited from a single center, and the results may not reflect the practice of other institutions. In addition, only patients who underwent CT before their pancreatic cancer diagnosis were eligible. A more significant number of patients in our institution may have incidentally found suspected pancreatic cancer lesions on CT conducted for other purposes, and they were diagnosed as having pancreatic cancer without delay. However, their data could not be collected. Therefore, the results of this study cannot represent all pancreatic cancers detected incidentally. It was difficult to select an appropriate control group for patients with suspicious lesions of pancreatic cancer; therefore, statistical analysis could not be performed. Finally, the time interval between the time of CT examination and the pancreatic cancer diagnosis was not consistent, ranging from 1.9 to 12 months, which indicated no refined findings of the prediagnostic CT.
Conclusions
In conclusion, pancreatic cancer is often incidentally detected on CT conducted for other purposes. Therefore, to diagnose pancreatic cancer in the early stage, clinicians need to always pay attention to the presence of a hypoattenuating mass, focal pancreatic duct dilatation, or distal parenchymal atrophy of the pancreas in contrast-enhanced CT conducted for other purposes, which may be clues for an early diagnosis of pancreatic cancer.
Acknowledgments
Funding: This work was supported by the National Health Insurance Service Ilsan Hospital grant (No. NHIMC2018CR077).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-863/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-863/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-863/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-863/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study design was reviewed and approved by the Institutional Review Board of National Health Insurance Service Ilsan Hospital (NHIMC 2018-09-014). The requirement for obtaining patient informed consent was waived by the board due to the retrospective design of the current study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lindquist CM, Miller FH, Hammond NA, et al. Pancreatic cancer screening. Abdom Radiol (NY) 2018;43:264-72. [Crossref] [PubMed]
- Moutinho-Ribeiro P, Coelho R, Giovannini M, et al. Pancreatic cancer screening: Still a delusion? Pancreatology 2017;17:754-65. [Crossref] [PubMed]
- Maisonneuve P, Lowenfels AB. Risk factors for pancreatic cancer: a summary review of meta-analytical studies. Int J Epidemiol 2015;44:186-98. [Crossref] [PubMed]
- Midha S, Chawla S, Garg PK. Modifiable and non-modifiable risk factors for pancreatic cancer: A review. Cancer Lett 2016;381:269-77. [Crossref] [PubMed]
- Hanada K, Okazaki A, Hirano N, et al. Diagnostic strategies for early pancreatic cancer. J Gastroenterol 2015;50:147-54. [Crossref] [PubMed]
- Lennon AM, Wolfgang CL, Canto MI, et al. The early detection of pancreatic cancer: what will it take to diagnose and treat curable pancreatic neoplasia? Cancer Res 2014;74:3381-9. [Crossref] [PubMed]
- Matsubayashi H, Ishiwatari H, Sasaki K, et al. Detecting Early Pancreatic Cancer: Current Problems and Future Prospects. Gut Liver 2020;14:30-6. [Crossref] [PubMed]
- Lee ES, Lee JM. Imaging diagnosis of pancreatic cancer: a state-of-the-art review. World J Gastroenterol 2014;20:7864-77. [Crossref] [PubMed]
- Takeshita K, Kutomi K, Haruyama T, et al. Imaging of early pancreatic cancer on multidetector row helical computed tomography. Br J Radiol 2010;83:823-30. [Crossref] [PubMed]
- Hijioka S, Ikari T, Kamei A, et al. CT and MRI findings with contrast enhancement of small pancreatic adenocarcinoma in the late phase. Hepatogastroenterology 2007;54:389-92. [PubMed]
- Ahn SS, Kim MJ, Choi JY, et al. Indicative findings of pancreatic cancer in prediagnostic CT. Eur Radiol 2009;19:2448-55. [Crossref] [PubMed]
- Prokesch RW, Chow LC, Beaulieu CF, et al. Isoattenuating pancreatic adenocarcinoma at multi-detector row CT: secondary signs. Radiology 2002;224:764-8. [Crossref] [PubMed]
- Lahat G, Ben Haim M, Nachmany I, et al. Pancreatic incidentalomas: high rate of potentially malignant tumors. J Am Coll Surg 2009;209:313-9. [Crossref] [PubMed]
- Takeda Y, Saiura A, Takahashi Y, et al. Asymptomatic Pancreatic Cancer: Does Incidental Detection Impact Long-Term Outcomes? J Gastrointest Surg 2017;21:1287-95. [Crossref] [PubMed]
- Winter JM, Cameron JL, Lillemoe KD, et al. Periampullary and pancreatic incidentaloma: a single institution's experience with an increasingly common diagnosis. Ann Surg 2006;243:673-80; discussion 680-3. [Crossref] [PubMed]
- Gangi S, Fletcher JG, Nathan MA, et al. Time interval between abnormalities seen on CT and the clinical diagnosis of pancreatic cancer: retrospective review of CT scans obtained before diagnosis. AJR Am J Roentgenol 2004;182:897-903. [Crossref] [PubMed]
- Higashi M, Tanabe M, Onoda H, et al. Incidentally detected pancreatic adenocarcinomas on computed tomography obtained during the follow-up for other diseases. Abdom Radiol (NY) 2020;45:774-81. [Crossref] [PubMed]
- Yoon SH, Lee JM, Cho JY, et al. Small (≤ 20 mm) pancreatic adenocarcinomas: analysis of enhancement patterns and secondary signs with multiphasic multidetector CT. Radiology 2011;259:442-52. [Crossref] [PubMed]
- Kanno A, Masamune A, Hanada K, et al. Multicenter study of early pancreatic cancer in Japan. Pancreatology 2018;18:61-7. [Crossref] [PubMed]
- Goggins M, Overbeek KA, Brand R, et al. Management of patients with increased risk for familial pancreatic cancer: updated recommendations from the International Cancer of the Pancreas Screening (CAPS) Consortium. Gut 2020;69:7-17. [Crossref] [PubMed]
- Cooperman AM, Iskandar ME, Wayne MG, et al. Prevention and Early Detection of Pancreatic Cancer. Surg Clin North Am 2018;98:1-12. [Crossref] [PubMed]
- Santo E, Bar-Yishay I. Pancreatic solid incidentalomas. Endosc Ultrasound 2017;6:S99-S103. [Crossref] [PubMed]
- Kauhanen SP, Komar G, Seppänen MP, et al. A prospective diagnostic accuracy study of 18F-fluorodeoxyglucose positron emission tomography/computed tomography, multidetector row computed tomography, and magnetic resonance imaging in primary diagnosis and staging of pancreatic cancer. Ann Surg 2009;250:957-63. [Crossref] [PubMed]
- Palazzo L, Roseau G, Gayet B, et al. Endoscopic ultrasonography in the diagnosis and staging of pancreatic adenocarcinoma. Results of a prospective study with comparison to ultrasonography and CT scan. Endoscopy 1993;25:143-50. [Crossref] [PubMed]
- Sheridan MB, Ward J, Guthrie JA, et al. Dynamic contrast-enhanced MR imaging and dual-phase helical CT in the preoperative assessment of suspected pancreatic cancer: a comparative study with receiver operating characteristic analysis. AJR Am J Roentgenol 1999;173:583-90. [Crossref] [PubMed]
- Singhi AD, Koay EJ, Chari ST, et al. Early Detection of Pancreatic Cancer: Opportunities and Challenges. Gastroenterology 2019;156:2024-40. [Crossref] [PubMed]
- Chan MG, Cassidy FH, Andre MP, et al. Delayed imaging in routine CT examinations of the abdomen and pelvis: is it worth the additional cost of radiation and time? AJR Am J Roentgenol 2014;202:329-35. [Crossref] [PubMed]
- Ishigami K, Yoshimitsu K, Irie H, et al. Diagnostic value of the delayed phase image for iso-attenuating pancreatic carcinomas in the pancreatic parenchymal phase on multidetector computed tomography. Eur J Radiol 2009;69:139-46. [Crossref] [PubMed]
- Ishikawa O, Ohigashi H, Imaoka S, et al. Minute carcinoma of the pancreas measuring 1 cm or less in diameter--collective review of Japanese case reports. Hepatogastroenterology 1999;46:8-15. [PubMed]
- Yokode M, Akita M, Fujikura K, et al. High-grade PanIN presenting with localised stricture of the main pancreatic duct: A clinicopathological and molecular study of 10 cases suggests a clue for the early detection of pancreatic cancer. Histopathology 2018;73:247-58. [Crossref] [PubMed]
- Kanno Y, Koshita S, Ogawa T, et al. Predictive Value of Localized Stenosis of the Main Pancreatic Duct for Early Detection of Pancreatic Cancer. Clin Endosc 2019;52:588-97. [Crossref] [PubMed]
- Miura S, Kume K, Kikuta K, et al. Focal Parenchymal Atrophy and Fat Replacement Are Clues for Early Diagnosis of Pancreatic Cancer with Abnormalities of the Main Pancreatic Duct. Tohoku J Exp Med 2020;252:63-71. [Crossref] [PubMed]
- Kurita A, Mori Y, Someya Y, et al. High signal intensity on diffusion-weighted magnetic resonance images is a useful finding for detecting early-stage pancreatic cancer. Abdom Radiol (NY) 2021;46:4817-27. [Crossref] [PubMed]
- Yamao K, Takenaka M, Ishikawa R, et al. Partial Pancreatic Parenchymal Atrophy Is a New Specific Finding to Diagnose Small Pancreatic Cancer (≤10 mm) Including Carcinoma in Situ: Comparison with Localized Benign Main Pancreatic Duct Stenosis Patients. Diagnostics (Basel) 2020;10:445. [Crossref] [PubMed]
- Nakahodo J, Kikuyama M, Fukumura Y, et al. Focal pancreatic parenchyma atrophy is a harbinger of pancreatic cancer and a clue to the intraductal spreading subtype. Pancreatology 2022;22:1148-58. [Crossref] [PubMed]
- Nakahodo J, Kikuyama M, Nojiri S, et al. Focal parenchymal atrophy of pancreas: An important sign of underlying high-grade pancreatic intraepithelial neoplasia without invasive carcinoma, i.e., carcinoma in situ. Pancreatology 2020;20:1689-97. [Crossref] [PubMed]
- Toshima F, Watanabe R, Inoue D, et al. CT Abnormalities of the Pancreas Associated With the Subsequent Diagnosis of Clinical Stage I Pancreatic Ductal Adenocarcinoma More Than 1 Year Later: A Case-Control Study. AJR Am J Roentgenol 2021;217:1353-64. [Crossref] [PubMed]
- Mizrahi JD, Surana R, Valle JW, et al. Pancreatic cancer. Lancet 2020;395:2008-20. [Crossref] [PubMed]


