
Immune checkpoint inhibitors in liver transplant: a case series
Highlight box
Key findings
• Immune checkpoint inhibitors carry the risk of graft rejection even four years after liver transplant.
• There are no obvious benefits of checkpoint inhibitors in patients with liver transplants with recurrent hepatocellular carcinoma.
What is known and what is new?
• Rejection of grafts may be related to the length of time between transplantation and checkpoint inhibition.
• Graft versus host disease may be a possible risk of neoadjuvant immune checkpoint inhibition.
What is the implication, and what should change now?
• Checkpoint inhibition may have more utility in the pre-transplant setting than in the post-transplant setting.
• Further prospective studies are needed to evaluate the safety and efficacy of immune checkpoint inhibitors in the peri-transplant setting.
• Insight into the mechanisms of graft tolerance and checkpoint mediated graft rejection may provide treatment options when immunotherapy is utilized in patients with transplants.
Introduction
Cancers originating in the liver—hepatocellular carcinoma (HCC) and cholangiocarcinoma—can be cured with resection or liver transplant (LT). A large proportion of patients with early disease need an LT, given concomitant liver disease due to parenchymal pathology (often the precursor for HCC) or biliary pathology (a precursor in some cases of cholangiocarcinoma). Surgical anatomy imperatives (such as vascular involvement or hilar tumors) can also drive transplant decisions (1,2). LT alone, however, leads to suboptimal cures. Until recently, systemic therapy options to treat these cancers in the neoadjuvant/adjuvant setting were limited. The advent of immune checkpoint inhibitors (ICIs) has offered an opportunity to effect better cures. Success in HCC and cholangiocarcinoma has been demonstrated with combination atezolizumab/bevacizumab (3), tremelimumab/durvalumab (4) and gemcitabine/cisplatin with durvalumab/tremelimumab (5).
However, a significant barrier to ICI in the peri-transplant setting is the potentially disastrous consequence of organ rejection. Studies in this setting are limited to case reports, case series and reviews. A recent analysis of 28 published patients with LT treated with ICI for various malignancies identified a graft rejection rate of 32%. Those treated for HCC specifically had an overall response rate of 11% (6). Since its publication, there have been three additional reported cases of patients with LT treated for HCC with the newer treatment option, atezolizumab/bevacizumab. All three of patients had disease progression without liver rejection (7,8).
In this retrospective chart review, we add to the growing collection of patients with LT who have received ICI in the neoadjuvant setting. We describe an additional case of fatal ICI-related rejection. We also discuss two patients treated with atezolizumab/bevacizumab post-LT without liver rejection. Finally, we detail a case of graft versus host disease (GVHD) in a patient with an LT who had recently received nivolumab. We present the following article in accordance with the AME Case Series reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-922/rc).
Methods
A retrospective institution wide search of the electronic medical record was done to find patients with LT treated with ICIs at the University of Cincinnati. Patients received immunotherapy between 2019 and 2021 and status was followed until study submission. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Publication of this case series was waived from patient consent according to the institutional review board of the University of Cincinnati.
Case presentation
Case 1
A 56-year-old male with a history of an MSH2 gene mutation (Lynch syndrome) was diagnosed with stage 1B cholangiocarcinoma with pathology positive for programmed cell death protein 1 (PD-1) (Table 1). He initially received 3 months of gemcitabine & cisplatin, but had an increase in size of liver lesions. He was deemed unresectable. Therapy was changed to pembrolizumab. He completed 20 doses of pembrolizumab over 16 months with a decrease in tumor burden. His last cycle of pembrolizumab was 13 days prior to LT. Post-transplant immunosuppression included prednisone, tacrolimus and mycophenolate. Due to a post-transplant rise in liver enzymes, he underwent graft biopsy, which showed signs of mild acute cellular rejection in addition to findings of viral hepatitis, consistent with a history of both hepatitis B (HBV) and hepatitis C virus (HCV) in the donor. He was treated with entecavir for HBV and immunosuppression was continued and liver function enzymes soon normalized. He remains disease free 31 months since transplant.
Table 1
| Case ID | Age at first diagnosis | M/F | Indication for LT | Treatments prior to LT | ICI [# of doses] | Days between last ICI and LT | Peri-transplant pRBC | Complications | Post-LT IS |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 56 | M | Cholangiocarcinoma | Gemcitabine + cisplatin | Pembrolizumab [20] | 13 | 0 | Mild ACR | Prednisone, tacrolimus, mycophenolate |
| 2 | 47 | F | HCC | Resection, bland embolization, SIRT, microwave ablation, lenvatinib | Nivolumab [7] | 55 | 5 | GVHD | Prednisone, tacrolimus, mycophenolate |
M/F, male/female; LT, liver transplant; ICI, immune checkpoint inhibitor; pRBC, packed red blood cells; IS, immunosuppression; HCC, hepatocellular carcinoma; SIRT, selective internal radiation therapy; ACR, acute cellular rejection; GVHD, graft versus host disease.
Case 2
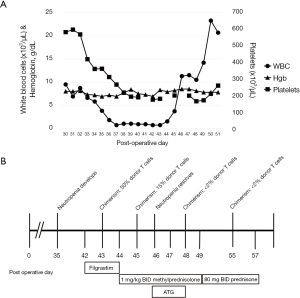
A 47-year-old female was diagnosed with stage 1b fibrolamellar HCC and underwent successful hepatectomy. Five years after diagnosis, she had recurrence of multifocal HCC, without a clear fibrolamellar component. She underwent bland embolization, selective internal radiation therapy (SIRT) and microwave ablation, followed by 7 doses of nivolumab over 7 months (Table 1). She was switched to lenvatinib due to progression of disease. Then, 55 days after the last dose of nivolumab, she underwent LT. Immediate post-transplant immunosuppression included prednisone, tacrolimus and mycophenolate. Post-transplant course was complicated by GVHD, manifested by fevers and neutropenia, which developed by postoperative day (POD) 35 (Figure 1A,1B). Infectious workup, including parvovirus, human herpesvirus-8 (HHV-8), herpes simplex virus (HSV), Epstein-Barr virus (EBV) and cytomegalovirus (CMV), was negative. Ferritin was 5,775 ng/mL. Bone marrow biopsy was normal. Skin biopsy of a developing rash was suggestive of morbilliform drug eruption but not GVHD. She initially received filgrastim on POD 42–44 but this was stopped when chimerisms resulted. Initial peripheral blood chimerism studies on POD 43 revealed 50% donor T cells. On POD 44 she was started on 1 mg/kg twice daily (BID) of methylprednisolone. On POD 45, repeat chimerism studies showed improvement with 15% donor T cells. On POD 46–48, anti-thymocyte globulin (ATG) was given. Chimerisms improved to less than 2% donor T cells by POD 48 and stabilized. She was continued on tacrolimus throughout. She continues to remain HCC-free 48 months after LT with no signs of GVHD. She remains on mycophenolate and tacrolimus.
Case 3
A 48-year-old male with a history of non-alcoholic steatohepatitis (NASH) cirrhosis was diagnosed with stage II HCC. Programmed death-ligand 1 (PD-L1) was negative by immunohistochemistry (IHC). He underwent SIRT and trans-arterial chemoembolization (TACE), followed by LT (Table 2). Post-operative immunosuppression included tacrolimus, mycophenolate and prednisone. However, 24 months after transplant, he was found to have disease progression and started on atezolizumab and bevacizumab for 7 doses over 4 months without rejection. Unfortunately, he had progression of disease and enrolled in hospice. He died 7 months after initiation of ICI (31.5 months after LT).
Table 2
| Case ID | Age at first dx | M/F | Indication for LT | Malignancy | Tx prior to LT | Rejection | Recurrence [months post-LT] | ICI [# of doses] | Time between LT & ICI (months) | ICI response? | Other tx post-LT | Death (months post-LT) | Death (months post-ICI initiation) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 48 | M | HCC | HCC | TACE, SIRT | No | Yes [24] | Atezolizumab + bevacizumab [7] | 24 | None | None | Yes; 31.5 | 7 |
| 4 | 67 | F | HCC | HCC | SIRT | No | Yes [6] | Atezolizumab + bevacizumab [4] | 9.5 | None | Lenvatinib, cabozantinib | Yes; 19 | 9 |
| 5 | 42 | M | PSC | Small bowel adenocarcinoma | None | Yes | N/A | Nivolumab [1] | 50.5 | None | FOLFOX | Yes; 53.5 | 3 |
| 6 | 59 | F | PSC/cirrhosis | Rectal SCC | None | No | N/A | Nivolumab [1] | 122 | None | Carboplatin + paclitaxel; 5-FU + cisplatin | Yes; 126 | 3.5 |
Dx, diagnosis; M/F, male/female; LT, liver transplant Tx, treatment; ICI, immune checkpoint inhibitor; HCC, hepatocellular carcinoma; PSC, primary sclerosing cholangitis; SCC, squamous cell carcinoma; TACE, trans-arterial chemoembolization; SIRT, selective internal radiation therapy; N/A, not applicable; FOLFOX, folinic acid, fluorouracil & oxaliplatin; 5-FU, fluorouracil.
Case 4
A 67-year-old female with a history of HCV cirrhosis was diagnosed with HCC. She initially underwent SIRT followed by LT (Table 2). Post-operative immunosuppression included tacrolimus, mycophenolate and prednisone. Explant was notable for 4 viable tumors. Six months after LT she was found to have recurrence of disease. She was treated with lenvatinib and was unable to tolerate side effects. She was then treated with atezolizumab/bevacizumab for four doses over two months but had progression of disease. She was switched to cabozantinib but continued to have progression. She then enrolled in hospice and died 9 months after the first dose of ICI (19 months after LT).
Case 5
A man with a history of ulcerative colitis and primary sclerosing cholangitis (PSC) underwent LT at age 39. At age 42, he was found to have small bowel obstruction secondary to adenocarcinoma of the small intestine. Next generation sequencing was notable for microsatellite instability. PD-L1 by IHC was negative. He was treated with 6 doses of FOLFOX but had disease progression (Table 2). Given microsatellite instability in the tumor, the decision was made to try ICI. He was treated with a single dose of nivolumab, which was complicated by acute liver rejection noted 23 days following nivolumab. He was then treated with high dose steroids, in addition to tacrolimus; however, liver function worsened. ATG and mycophenolate were added and tacrolimus was increased. Unfortunately, his liver function worsened and he died 3 months after receiving nivolumab (53.5 months after LT).
Case 6
A woman with a history of cirrhosis secondary to PSC underwent LT at age 50. At age 59, she was found to have stage IV squamous cell carcinoma of the rectum. She was treated with carboplatin and paclitaxel for 6 doses (Table 2). She had progression of disease and was switched to nivolumab for a single dose. Sirolimus and mycophenolate were continued for immunosuppression. She was admitted to the hospital due to elevated liver enzymes secondary to worsened liver metastases. She was treated with 2 doses of 5-fluorouracil and cisplatin, with continued progression of disease and was eventually referred to hospice and died 3.5 months after last ICI (126 months after LT).
Discussion
Due to the inherent risk of organ rejection, studies involving the utility of ICI in patients pre-LT and post-LT are limited. A recent meta-analysis of patients treated with ICI post-LT demonstrated that the risk of rejection may be related to the duration of time between LT and ICI (6). Patients who developed rejection were given ICI earlier after LT compared with patients without rejection (2.9 vs. 5.3 years, P=0.02). Our patient with rejection (Case 5), however, did not necessarily mirror this finding and received ICI for 50.5 months (>4 years) post LT. This analysis also suggested that graft PD-L1 positivity may be related to an increased risk of rejection. Unfortunately, none of the transplanted grafts in our series had PD-L1 measured to contribute to this finding, including Case 5. It may be beneficial for future studies, including studies evaluating neoadjuvant ICI prior to LT, to evaluate PD-L1 status of grafts to help clarify if graft PD-L1 status predicts rejection risk. Further studies could also evaluate the effect of checkpoint inhibition on the balance of immune regulators, such as CD8+ T cells and regulatory T cells (Tregs). Checkpoint inhibition may lead to graft rejection by interruption of mechanisms that normally facilitate graft tolerance. Utilizing a mouse model with acquired graft tolerance (utilizing CTLA4 immunoglobulin), Tanaka et al. revealed that blockade of the PD-1/PD-L1 pathway leads to rejection of cardiac allografts compared to controls. PD-L1 blockade was associated with an increase in peripheral effector CD8+ T cells, with a concomitant reduction in cardiac allograft FOXP3+ CD4+ and CD25+ Tregs (9). Thus, PD-L1 blockade may alter the balance of alloreactive CD8+ T-cells and immunosuppressive Tregs, tipping the scale away from graft tolerance and toward graft rejection. In HCC, the activity of suppressive Tregs is thought to promote tumor progression by inhibition of various immune cells (10). Therefore, balancing the role of Tregs in both tumor progression and graft tolerance will be a fine line to navigate in the post-LT setting.
While the standard of care for unresectable HCC is atezolizumab/bevacizumab, only three patients with prior LT treated with atezolizumab/bevacizumab have been previously reported. All of these reported patients had no graft rejection but died of disease progression (7,8). Here, we report an additional two patients with LT (Cases 3 and 4) who had progression of disease without organ rejection after receiving atezolizumab/bevacizumab. Further investigation into whether atezolizumab is a safer alternative with regards to graft rejection compared to other ICI is warranted. However, the lack of documented organ rejection may simply be due to fewer patients treated with atezolizumab as it was only recently approved for unresectable HCC. Based on the few cases from our study, in addition to prior reported cases, most patients do not have obvious response to ICI when treated post-LT and the risk of rejection seems to be higher than the overall response (6). Use should be done with extreme caution. Of note, the two reported cases who did benefit from post-LT pembrolizumab had resolution of their metastatic disease (11,12) but this response should be considered more of an exception than the rule. A risk-benefit discussion should be had prior to giving ICI in this setting, and in patients who opt for a trial of ICI, evaluation of whether one ICI has a higher risk of rejection, and if tumor characteristics, such as mutational burden or PD-L1 status, predicts response could be useful.
Given the rise of ICI, neoadjuvant immunotherapy has become a dilemma in the pre-transplant period given the risk of rejection should a liver become available for transplant. Two patients in our series were treated with ICI prior to LT (Cases 1 and 2). Case 1 was actively receiving ICI as he was last treated with pembrolizumab 13 days prior to LT and Case 2 was last treated with nivolumab 55 days prior to LT. Case 1 had mild acute cellular rejection, while Case 2 did not. The mild rejection in Case 1 did not appear to be clinically significant as liver enzymes improved 3 weeks after LT. This improvement could be explained by the initiation of entecavir for HBV as well. Regardless, the half-lives of nivolumab and pembrolizumab are 25 days (13) and 27 days (14), respectively, which implies both should have had a continued physiologic effect of the ICI. Peri-transplant blood loss and transfusion requirement has been postulated as a potential, but unproven, factor in reducing risk of rejection given the rapid loss of circulating ICI (15). Of note, Case 1 did not have any peri-transplant related transfusion. In addition, the short period of time between ICI and LT (13 days) could theoretically have made him more prone to rejection.
Reports of ICI in the neoadjuvant LT setting are limited to case reports and case series and are summarized in Table 3. Successes in pre-transplant ICI include patients who have been downstaged to fit transplant criteria and have gone on to receive a successful graft (15-17). There are, however, several examples of fatal hepatic necrosis and acute cellular rejection presumably related to checkpoint inhibition (18-20). Schnickel et al. detail 2 patients out of 5 who had nivolumab-induced LT rejection and suggest that a period of less than 3 months between ICI and LT may make one prone to rejection (17). However, a different case series reported no instances of acute rejection in a series of 9 patients treated with nivolumab between 1 and 253 days prior to LT (15). Standardization of both the timing between ICI and LT and the use of additional immunosuppressive agents, such as ATG, should continue to be evaluated as neoadjuvant ICI becomes more common.
Table 3
| Author | # of patients | Malignancy | ICI | Complications | Time between ICI and LT |
|---|---|---|---|---|---|
| Tabrizian (15) | 9 | HCC | Nivolumab | Mild ACR (attributed to sub-therapeutic tacrolimus) in a single patient | <4 weeks in 8 out of the 9 patients |
| Schwacha-Eipper (16) | 1 | HCC | Nivolumab | None | 15 weeks |
| Schnickel (17) | 5 | HCC | Nivolumab | Graft failure requiring re-transplant* Acute cellular rejection** |
5 weeks* & 10 days** |
| Nordness (18) | 1 | HCC | Nivolumab | Fatal hepatic necrosis | 8 days |
| Chen (19) | 1 | HCC | Toripalimab | Fatal hepatic necrosis | 93 days |
| Aby (20) | 1 | HCC | Nivolumab | Non-fatal ACR | 16 days |
* and ** refer to individual patients in the case series. ICI, immune checkpoint inhibitor; LT, liver transplant; HCC, hepatocellular carcinoma ACR, acute cellular rejection.
Here we also report a case of a patient who developed GVHD after LT who was treated with nivolumab 55 days prior to LT (Case 2). It is possible, although difficult to prove, that this episode of GVHD is related to immune checkpoint blockade through increased T cell activity. GVHD in LT recipients occurs in ~0.1–2% of LT and carries a high mortality, often as high as 75% (21). Diagnosis is based on symptoms, often including fever, diarrhea and rash, and is supported with evidence of donor lymphocyte chimerism (21). To date, ICI related GVHD has been reported in patients with hematological malignancies with prior stem cell transplants (22). In our series, Case 2 had peripheral blood chimerisms of 50% donor T cells, which fell to 15% after high dose steroids. While it is known that donor chimerism can be detected post LT, the findings in this patient are far above the <2% average donor T cells often detected 4 weeks post LT (23). This patient’s white blood cell (WBC) count improved, which was likely related to improvement in GVHD in addition to prior administration of filgrastim. This case is somewhat atypical, as the rash was non-diagnostic of GVHD and she did not have diarrhea. This patient also had rapid improvement of chimerisms with steroids prior to starting ATG. It is possible she would have improved without intervention. While GVHD in patients with LT does occur independent of PD-L1 inhibitors, there is an increased theoretical risk of GVHD in patients with LT treated with ICI. Therefore, as the use of neoadjuvant ICI continues, close scrutiny will be needed in order to determine if ICI increases the risk of GVHD in LT recipients.
Conclusions
In summary, the field of immunotherapy in the setting of LT is new, and data seem to suggest that pre-LT ICI at least 3 months prior to LT may be safer than post-LT use. Furthermore, the benefit of ICI might be greater as well, in the early setting, rather than the recurrent disease setting, as seen in other gastrointestinal malignancies (24,25). Prospective studies are needed; we are undertaking one such trial (NCT05027425) to systematically evaluate the use of ICI in the pre-LT setting to answer these questions.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the AME case series reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-922/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-922/coif). DS reports participation in Speaker’s Bureau for Genentech and Incyte and was involved with consulting/honoraria for AstraZeneca, Transthera Sciences and Totus Medicines. DS reports Institutional research funding from AstraZeneca, Bristol-Myers Squibb, Genentech, Roche and Merck. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Publication of this case series was waived from patient consent according to the institutional review board of the University of Cincinnati.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Massarweh NN, El-Serag HB. Epidemiology of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Cancer Control 2017;24:1073274817729245. [Crossref] [PubMed]
- Katariya NN, Lizaola-Mayo BC, Chascsa DM, et al. Immune Checkpoint Inhibitors as Therapy to Down-Stage Hepatocellular Carcinoma Prior to Liver Transplantation. Cancers (Basel) 2022;14:2056. [Crossref] [PubMed]
- Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med 2020;382:1894-905. [Crossref] [PubMed]
- Kelley RK, Sangro B, Harris W, et al. Safety, Efficacy, and Pharmacodynamics of Tremelimumab Plus Durvalumab for Patients With Unresectable Hepatocellular Carcinoma: Randomized Expansion of a Phase I/II Study. J Clin Oncol 2021;39:2991-3001. [Crossref] [PubMed]
- Oh DY, Lee KH, Lee DW, et al. Gemcitabine and cisplatin plus durvalumab with or without tremelimumab in chemotherapy-naive patients with advanced biliary tract cancer: an open-label, single-centre, phase 2 study. Lancet Gastroenterol Hepatol 2022;7:522-32. [Crossref] [PubMed]
- Au KP, Chok KSH. Immunotherapy after liver transplantation: Where are we now? World J Gastrointest Surg 2021;13:1267-78. [Crossref] [PubMed]
- Ben Khaled N, Roessler D, Reiter FP, et al. Extending the Use of Atezolizumab and Bevacizumab to a Liver Transplant Recipient: Need for a Posttransplant Registry. Liver Transpl 2021;27:928-9. [Crossref] [PubMed]
- Yang Z, Sun J, Zhuang L, et al. Preliminary Evaluation of Atezolizumab Plus Bevacizumab as Salvage Treatment for Recurrent Hepatocellular Carcinoma After Liver Transplantation. Liver Transpl 2022;28:895-6. [Crossref] [PubMed]
- Tanaka K, Albin MJ, Yuan X, et al. PDL1 is required for peripheral transplantation tolerance and protection from chronic allograft rejection. J Immunol 2007;179:5204-10. [Crossref] [PubMed]
- Granito A, Muratori L, Lalanne C, et al. Hepatocellular carcinoma in viral and autoimmune liver diseases: Role of CD4+ CD25+ Foxp3+ regulatory T cells in the immune microenvironment. World J Gastroenterol 2021;27:2994-3009. [Crossref] [PubMed]
- Rammohan A, Reddy MS, Farouk M, et al. Pembrolizumab for metastatic hepatocellular carcinoma following live donor liver transplantation: The silver bullet? Hepatology 2018;67:1166-8. [Crossref] [PubMed]
- Nasr F, Al Ghoche A, Diab S, et al. Pembrolizumab monotherapy in relapsed hepatocellular carcinoma post living donor liver transplantation and sorafenib. Int J Oncol Res 2018;1:009.
- Hurkmans DP, Basak EA, van Dijk T, et al. A prospective cohort study on the pharmacokinetics of nivolumab in metastatic non-small cell lung cancer, melanoma, and renal cell cancer patients. J Immunother Cancer 2019;7:192. [Crossref] [PubMed]
- Longoria TC, Tewari KS. Evaluation of the pharmacokinetics and metabolism of pembrolizumab in the treatment of melanoma. Expert Opin Drug Metab Toxicol 2016;12:1247-53. [Crossref] [PubMed]
- Tabrizian P, Florman SS, Schwartz ME. PD-1 inhibitor as bridge therapy to liver transplantation? Am J Transplant 2021;21:1979-80. [Crossref] [PubMed]
- Schwacha-Eipper B, Minciuna I, Banz V, et al. Immunotherapy as a Downstaging Therapy for Liver Transplantation. Hepatology 2020;72:1488-90. [Crossref] [PubMed]
- Schnickel GT, Fabbri K, Hosseini M, et al. Liver transplantation for hepatocellular carcinoma following checkpoint inhibitor therapy with nivolumab. Am J Transplant 2022;22:1699-704. [Crossref] [PubMed]
- Nordness MF, Hamel S, Godfrey CM, et al. Fatal hepatic necrosis after nivolumab as a bridge to liver transplant for HCC: Are checkpoint inhibitors safe for the pretransplant patient? Am J Transplant 2020;20:879-83. [Crossref] [PubMed]
- Chen GH, Wang GB, Huang F, et al. Pretransplant use of toripalimab for hepatocellular carcinoma resulting in fatal acute hepatic necrosis in the immediate postoperative period. Transpl Immunol 2021;66:101386. [Crossref] [PubMed]
- Aby ES, Lake JR. Immune Checkpoint Inhibitor Therapy Before Liver Transplantation-Case and Literature Review. Transplant Direct 2022;8:e1304. [Crossref] [PubMed]
- Akbulut S, Yilmaz M, Yilmaz S. Graft-versus-host disease after liver transplantation: a comprehensive literature review. World J Gastroenterol 2012;18:5240-8. [PubMed]
- Singh AK, Porrata LF, Aljitawi O, et al. Fatal GvHD induced by PD-1 inhibitor pembrolizumab in a patient with Hodgkin's lymphoma. Bone Marrow Transplant 2016;51:1268-70. [Crossref] [PubMed]
- Domiati-Saad R, Klintmalm GB, Netto G, et al. Acute graft versus host disease after liver transplantation: patterns of lymphocyte chimerism. Am J Transplant 2005;5:2968-73. [Crossref] [PubMed]
- Kelly RJ, Ajani JA, Kuzdzal J, et al. Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. N Engl J Med 2021;384:1191-203. [Crossref] [PubMed]
- Cercek A, Lumish M, Sinopoli J, et al. PD-1 Blockade in Mismatch Repair-Deficient, Locally Advanced Rectal Cancer. N Engl J Med 2022;386:2363-76. [Crossref] [PubMed]


