
Hepatic arterial infusion chemotherapy and immune checkpoint inhibitors, alone or in combination, in advanced hepatocellular carcinoma with macrovascular invasion: a single-centre experience in Taiwan
Highlight box
Key findings
• Hepatic arterial infusion chemotherapy combined with immune checkpoint inhibitors had a superior response on portal vein tumour thrombus compared with hepatic arterial infusion chemotherapy alone in patients with advanced hepatocellular carcinoma and macrovascular invasion.
What is known and what is new?
• Hepatic arterial infusion chemotherapy combined with immune checkpoint inhibitors is known to have a superior overall tumour response compared with hepatic arterial infusion chemotherapy alone in patients with advanced hepatocellular carcinoma;
• This study suggests that the combination therapy may have a beneficial response in portal vein tumour thrombus.
What is the implication, and what should change now?
• The combination of immunotherapy and hepatic arterial infusion chemotherapy may serve as a viable alternative treatment option for patients with hepatocellular carcinoma and tumour thrombi.
Introduction
Hepatocellular carcinoma (HCC) is the most common type of liver cancer, accounting for 75–85% of primary liver cancer worldwide (1). In Taiwan, HCC is the third most commonly diagnosed malignancy and the second leading cause of cancer-related deaths (2). Vascular invasion is present in approximately 10–40% of patients with HCC at diagnosis, and the presence of vascular invasion is associated with poor survival after curative and noncurative treatment for HCC (3-5).
Systemic therapies including tyrosine kinase inhibitors (TKIs), immune checkpoint inhibitors (ICIs), vascular endothelial growth factor (VEGF) inhibitors, and a combination of an ICI and VEGF inhibitor (atezolizumab and bevacizumab) are recommended for the treatment of advanced HCC (6-9). Sorafenib, a first generation TKI, is frequently used as the first-line treatment for advanced HCC. However, the efficacy of sorafenib in patients with advanced HCC and vascular invasion was suboptimal with a median overall survival (OS) of 3.1–8.1 months and a median progression-free survival (PFS) of around 2 months (10,11). In Asia, hepatic arterial infusion chemotherapy (HAIC) has been recommended for treatment of advanced HCC with vascular invasion when systemic therapies cannot be used or have failed (12), and has shown to improve OS and time to progression compared with sorafenib (13-16). In addition, HAIC allows repeated delivery of high concentrations of intrahepatic drugs without simultaneous embolization of the hepatic vasculature and leads to acceptable levels of toxicity (17,18).
Building on the positive data of ICIs in advanced HCC (19,20), a recent, retrospective study in China, including 229 patients with advanced HCC who received either HAIC alone or in combination with ICIs, was conducted. It showed that the addition of ICIs improved disease control rate (DCR) in overall response and intrahepatic response, and prolonged OS and PFS when compared with HAIC alone (21). Here, we compared the real-world effectiveness of HAIC and ICIs, alone or in combination, in patients with advanced HCC and macrovascular invasion (MVI); furthermore, we also investigated the predictors of survival outcomes in the whole cohort. We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-858/rc).
Methods
Study design and patients
This study was a retrospective, single-centre review of medical records of adult patients with unresectable HCC treated with HAIC and ICIs alone or in combination at the National Cheng Kung University Hospital, Tainan between November 1, 2016 and December 31, 2020. This study was approved by the Institutional Review Board of the National Cheng Kung University Hospital (No. B-ER-110-300) and was performed according to the ethical principles for medical research of the World Medical Association’s Declaration of Helsinki (as revised in 2013). Informed consent was waived because of the retrospective nature of the study and the analysis used anonymous clinical data.
Eligible patients were diagnosed with unresectable HCC as primary tumour and had MVI. Diagnosis of HCC was based on either tissue histology or typical radiographic findings (22). The presence and extent of vascular invasion were determined by characteristic findings using multiphase dynamic computed tomography (CT) or magnetic resonance imaging (MRI) (3,23,24). Typical malignant tumour thrombus was defined as thrombus enhancement after the administration of contrast media compared to pre-contrast images (≥20 Hounsfield units on CT and ≥15% on MRI), thrombus expansion within the involved vessel, and continuity of thrombus within the tumour (25). Patients must have received at least one dose of HAIC, ICIs or HAIC plus ICIs. Patients who failed to complete the screening radiographic assessment, died before the first radiographic assessment, and had atypical image pattern of tumour thrombus, hepato-cholangiocarcinoma, Vp1 or Vp2 invasion [the presence of portal vein tumour thrombus (PVTT) distal to or in the second-order branches of the portal vein], hepatic vein tumour thrombus, renal vein thrombus and superior vena cava thrombus, were not eligible.
Treatment
One-shot HAIC with doxorubicin and cisplatin in combination was administrated once a month. Doxorubicin and cisplatin were administered intra-arterially at doses of 50 and 65 mg/m2, respectively. Doxorubicin was diluted in 100 mL normal saline, and the infusion time was 10 minutes. Cisplatin was diluted in 500 mL normal saline, and the infusion time was 3 hours. Pre-medications included dexamethasone and adequate hydration (26). ICIs included nivolumab (Opdivo®, Bristol Myers Squibb), pembrolizumab (Keytruda®, Merck), nivolumab plus ipilimumab (Yervoy®, Bristol Myers Squibb), atezolizumab (Tecentriq®, Roche) plus bevacizumab (Avastin®, Genentech Inc.) or spartalizumab (Novartis AG).
Assessment
Serial contrast-enhanced CT or MRI were used to assess tumour responses including objective response rate (ORR), DCR, complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD) based on Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1 and modified RECIST (mRECIST) (27,28). For vascular response, the largest diameters of the tumour thrombi were measured and compared with the recorded basal values and categorized into CR defined as complete disappearance of the tumour thrombus, PR defined as at least a 30% decrease in thrombus diameters, SD defined as a decrease of <30% or an increase of <20%, and PD defined as an increase of ≥20% in the sum of the diameters. Objective response rate of tumour thrombi (ORRT) was defined as the total number of patients achieving CR or PR, and DCR of tumour thrombi (DCRT) was defined as the proportion of patients achieving CR, PR, or SD (24). In the cases involving concurrent PVTT and inferior vena cava vein tumour thrombus (IVCTT), the vascular responses of the PVTT and IVCTT were assessed individually (24). Recurrence of new hepatic tumour and new distal metastasis were evaluated by CT or MRI every 8–12 weeks.
OS was defined as the length of time from the date HAIC or ICIs was administered (index date) to death from any cause. Patients without documented death at the time of the final analysis were censored at the date of the last follow-up. PFS was defined as the length of time from the index date to progression as per RECIST version 1.1 or death from any cause, whichever came first. Patients without documented disease progression or death at the time of the final analysis were censored at the date of the last follow-up. Adverse events (AEs) were recorded based on National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE; version 5.0).
Statistical analysis
Descriptive statistics were used for patient characteristics and laboratory values, expressed in percentage, median (interquartile range) and mean ± standard deviation. Comparison between groups was performed with chi-square test or Fisher’s exact test for categorical variables. Comparison between groups was performed with Kruskal-Wallis test for continuous variables. Survival curves were estimated using the Kaplan-Meier method and compared using the log-rank test. Pairwise comparison was performed using Scheffe’s multiple comparisons test. For overall tumour response, vascular response and AEs, comparison between any two groups was performed using Post-hoc test with Bonferroni correction. The univariate and multivariate analyses were performed using the Cox proportional hazards model to identify prognostic factors for survival. Variables with P value <0.05 in the univariate analysis and variables of treatment modalities entered the multivariate analysis. All statistical assessments were considered statistically significant as P value <0.05, except for the Bonferroni correction, where P values <0.017 were considered statistically significant. All analyses were conducted using the SAS statistical package (v. 9.4 for Windows; SAS Institute, Cary, NC, USA, RRID:SCR_008567).
Results
Patient characteristics
A total of 130 patients with advanced HCC and MVI (ICIs alone: 46; HAIC alone: 70; HAIC+ICI: 14) were included in the analysis (Figure 1), among whom, 99 (76.15%) were female and 80.77% were 55 years or older (Table 1). Most patients had Eastern Cooperative Oncology Group (ECOG) ≥1 (65.38%), were classified as Child-Pugh A (74.62%) and had Cancer of the Liver Italian Program Scoring System (CLIP) scores of 2–5 (86.15%). More than half (65.4%) of patients had chronic hepatitis B, and 50% of patients were treated with nucleotide analogue therapies. Thirty percent of patients had chronic hepatitis C. Less than 10% of patients had alcoholic hepatitis (9.2%) and nonalcoholic steatohepatitis (0.8%). More than a quarter (28.46%) of patients had distal metastases and there was significant difference between the proportions of patients with distal metastasis in the three groups (P=0.025). However, no significant difference in the proportions of patients with distal metastases of lung, bone and lymph node were detected between the three groups. Regarding thrombus location, 19 (14.62%) patients had IVCTT and 12 (9.23%) patients had concurrent IVCTT and PVTT (Vp3 or Vp4). In contrast, nearly half (41.54%) of patients had main portal vein invasion (Vp4 alone or Vp3 and Vp4). Nearly one-third (34.62%) of patients had portal vein invasion at the first order branch (Vp3 or bilateral Vp3). There was a significant difference between the proportion of thrombus location in the three groups (P=0.001). Transcatheter arterial chemoembolization (TACE), radiofrequency ablation/percutaneous ethanol injection and TKIs were the most common prior treatments (Table S1). Previous systemic therapies in each group were summarized in Table S1. Category and dosage of PD-1 inhibitors in the ICI group and HAIC+ICI group were summarized in Table S2. Twenty (43.48%) patients in the ICI group received PD-1 inhibitors as first-line systemic therapy. The majority of patients also received concomitant TKI therapy. In both HAIC groups, more than 70% of patients received concomitant TKI; however, less than 50% of the ICI group received concomitant TKI.
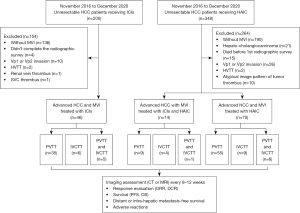
Table 1
| Characteristics | HAIC (n=70) | Systemic ICI (n=46) | HAIC and ICI (n=14) | P value |
|---|---|---|---|---|
| Sex, n (%) | 0.683 | |||
| Female | 55 (78.57) | 33 (71.74) | 11 (78.57) | |
| Male | 15 (21.43) | 13 (28.26) | 3 (21.43) | |
| Age, years, median (IQR), n (%) | 62.0 (56.0, 71.0) | 64.0 (57.0, 69.0) | 61.5 (56.0, 67.0) | 0.904 |
| <55 | 14 (20.00) | 8 (17.39) | 3 (21.43) | 0.918 |
| ≥55 | 56 (80.00) | 38 (82.61) | 11 (78.57) | |
| ECOG PS, n (%) | 0.008* | |||
| 0 | 17 (24.29) | 24 (52.17) | 4 (28.57) | |
| ≥1 | 53 (75.71) | 22 (47.83) | 10 (71.43) | |
| Alpha-fetoprotein, ng/mL, n (%) | 0.339 | |||
| <400 | 35 (50.00) | 25 (54.35) | 10 (71.43) | |
| ≥400 | 35 (50.00) | 21 (45.65) | 4 (28.57) | |
| Etiology of liver disease | ||||
| No liver disease, n (%) | 6 (8.57) | 3 (6.52) | 2 (14.29) | 0.662 |
| Liver disease present, n (%) | 64 (91.43) | 43 (93.48) | 12 (85.71) | |
| Chronic hepatitis B, n (%) | 47 (73.44) | 29 (67.44) | 9 (75.00) | 0.765 |
| Undetectable HBV DNA, n (%) | 16 (34.04) | 13 (44.83) | 5 (55.56) | 0.398 |
| HBV DNA (IU/mL), mean ± SD | (1.41±4.71)×106 | (1.64±7.92)×104 | (1.04±2.85)×105 | 0.219 |
| Ongoing NUC therapy, n (%) | 38 (80.85) | 21 (72.41) | 6 (66.67) | 0.536 |
| ETV, n (%) | 32 (68.09) | 15 (51.72) | 6 (66.67) | 0.346 |
| TDF, n (%) | 3 (6.38) | 4 (13.79) | 0 (0) | 0.332 |
| TAF, n (%) | 1 (2.13) | 2 (6.90) | 0 (0) | 0.457 |
| ETV+TDF, n (%) | 1 (2.13) | 0 (0) | 0 (0) | 0.664 |
| Efavirenz/Emtricitabine/Tenofovir, n (%) | 1 (2.13) | 0 (0) | 0 (0) | 0.664 |
| Chronic hepatitis C, n (%) | 20 (31.25) | 16 (37.21) | 3 (25.00) | 0.677 |
| Alcoholic hepatitis, n (%) | 8 (12.50) | 2 (4.65) | 2 (16.67) | 0.296 |
| Nonalcoholic steatohepatitis, n (%) | 0 (0.00) | 1 (2.33) | 0 (0.00) | 0.462 |
| Child-Pugh stage, n (%) | 0.073 | |||
| A | 56 (80.00) | 29 (63.04) | 12 (85.71) | |
| B | 14 (20.00) | 17 (36.96) | 2 (14.29) | |
| CLIP, n (%) | 0.097 | |||
| 0–1 | 6 (8.57) | 8 (17.39) | 4 (28.57) | |
| 2–5 | 64 (91.43) | 38 (82.61) | 10 (71.43) | |
| Distant metastases, n (%) | ||||
| No | 57 (81.43) | 28 (60.87) | 8 (57.14) | 0.025* |
| Yes | 13 (18.57) | 18 (39.13) | 6 (42.86) | |
| Lung | 9 (69.23) | 10 (55.56) | 3 (50.00) | 0.667 |
| Bone | 2 (15.38) | 2 (11.11) | 0 (0.00) | 1.000 |
| Lymph node | 6 (46.15) | 5 (27.78) | 3 (50.00) | 0.483 |
| Other | 3 (23.08) | 5 (27.78) | 2 (33.33) | 1.000 |
| Thrombus location, n (%) | 0.001* | |||
| IVC | 9 (12.86) | 6 (13.04) | 4 (28.57) | |
| IVC + main PV | 3 (4.29) | 2 (4.35) | 1 (7.14) | |
| IVC + 1st branch PV | 3 (4.29) | 3 (6.52) | 0 (0.00) | |
| Main PV | 26 (37.14) | 10 (21.74) | 7 (50.00) | |
| Main PV + bilateral 1st branch PV | 0 (0.00) | 11 (23.91) | 0 (0.00) | |
| Bilateral 1st branch PV | 3 (4.29) | 1 (2.17) | 0 (0.00) | |
| 1st branch PV | 26 (37.14) | 13 (28.26) | 2 (14.29) | |
| Prior treatment, n (%) | 0.020* | |||
| No | 37 (52.86) | 13 (28.26) | 8 (57.14) | |
| Yes | 33 (47.14) | 33 (71.74) | 6 (42.86) | |
| Line of ICI systemic therapy, n (%) | ||||
| First-line | 0 (0.00) | 20 (43.48) | 5 (35.71) | 0.837 |
| ≥ Second-line | 0 (0.00) | 26 (56.52) | 9 (64.28) | |
| Concomitant use of TKIs, n (%) | ||||
| No | 15 (21.43) | 24 (52.17) | 4 (28.57) | 0.003* |
| Yes | 55 (78.57) | 22 (47.83) | 10 (71.43) | |
| Sorafenib | 42 (76.36) | 14 (63.64) | 5 (50.00) | 0.183 |
| Regorafenib | 1 (1.82) | 2 (9.09) | 0 (0.00) | 0.304 |
| Lenvatinib | 12 (21.82) | 6 (27.27) | 5 (50.00) | 0.177 |
*, P<0.05. BCLC, Barcelona Clinic Liver Cancer; CLIP, Cancer of the Liver Italian Program Scoring System; DNA, deoxyribonucleic acid; ECOG PS, Eastern Cooperative Oncology Group performance status; ETV, entecavir; HAIC, hepatic arterial infusion chemotherapy; HBV, hepatitis B virus; ICI, immune checkpoint inhibitor; IQR, interquartile range; IVC, inferior vena cava; NUC, nucleotide analogue; PD-1, programmed cell death protein-1; PV, portal vein; SD, standard deviation; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate; TKI, tyrosine kinase inhibitor.
Treatment response
Overall tumour responses based on RECIST and mRECIST are presented in Table 2. HAIC+ICI group had higher rates of CR and PR, ORR and DCR than HAIC group and ICI group, but no significant differences were observed between the three groups. The rates of SD and PD were also similar between three groups. Overall ORR and DCR were also similar between the HAIC group and ICI group (Table S3).
Table 2
| Response | Overall RECIST, n (%) | Overall mRECIST, n (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HAIC (n=70) | Systemic ICI (n=46) | HAIC and ICI (n=14) | P value | HAIC (n=70) | Systemic ICI (n=46) | HAIC and ICI (n=14) | P value | ||
| CR | 0 (0.00) | 0 (0.00) | 1 (7.14) | 0.108 | 0 (0.00) | 1 (2.17) | 1 (7.14) | 0.088 | |
| PR | 16 (22.86) | 12 (26.09) | 6 (42.86) | 0.299 | 17 (24.29) | 12 (26.09) | 6 (42.86) | 0.355 | |
| SD | 13 (18.57) | 6 (13.04) | 2 (14.29) | 0.717 | 12 (17.14) | 5 (10.87) | 2 (14.29) | 0.645 | |
| PD | 41 (58.57) | 28 (60.87) | 5 (35.71) | 0.230 | 41 (58.57) | 28 (60.87) | 5 (35.71) | 0.230 | |
| ORR | 16 (22.86) | 12 (26.09) | 7 (50.00) | 0.111 | 17 (24.29) | 13 (28.26) | 7 (50.00) | 0.150 | |
| DCR | 29 (41.43) | 18 (39.13) | 9 (64.29) | 0.230 | 29 (41.43) | 18 (39.13) | 9 (64.29) | 0.230 | |
CR, complete response; DCR, disease control rate; HAIC, hepatic arterial infusion chemotherapy; HCC, hepatocellular carcinoma; ICI, immune checkpoint inhibitor; MVI, macrovascular invasion; ORR, objective response rate; PD, progressive disease; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumours; mRECIST, modified Response Evaluation Criteria in Solid Tumours; SD, stable disease.
Vascular responses based on vessel RECIST are shown in Table 3. The vascular ORRT was significantly different between three groups (P=0.023). Post-hoc analysis with Bonferroni correction also showed that ORRT was significantly higher in the HAIC+ICI group than in the HAIC group (P=0.014). ORRT was similar between the HAIC group and ICI group (P=0.572) and between the ICI group and HAIC+ICI group (P=0.064). For responses in PVTT, there were significant differences between PR rate (P=0.037), ORRT (P=0.013), DCRT (P=0.026), and PD rate (P=0.026) in the three groups. Post-hoc analysis with Bonferroni correction showed that the HAIC+ICI group had significantly higher PR rate (P=0.015), ORRT (P=0.005) and DCRT (P=0.005), and lower PD rate (P=0.005) than the HAIC group. PR rate, ORRT, DCRT, and PD rate were similar between the HAIC group and ICI group (P=0.532 for PR rate; P=0.435 for ORRT; P=0.773 for DCRT; P=0.773 for PD rate) and between the ICI group and HAIC+ICI group (P=0.077 for PR rate; P=0.031 for ORRT; P=0.020 for DCRT; P=0.020 for PD rate). There were no significant differences observed between the three groups and between the HAIC group and ICI group in IVCTT responses (Table S4).
Table 3
| Response | Vessel response, n (%) | PVTT response, n (%) | IVCTT response, n (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HAIC (n=70) | Systemic ICI (n=46) | HAIC and ICI (n=14) | P value | HAIC (n=60) | Systemic ICI (n=40) | HAIC and ICI (n=10) | P value | HAIC (n=16) | Systemic ICI (n=11) | HAIC and ICI (n=6) | P value | |||
| CR | 3 (4.29) | 2 (4.35) | 2 (14.29) | 0.246 | 2 (3.33) | 2 (5.00) | 1 (10.00) | 0.480 | 2 (12.50) | 1 (9.09) | 2 (33.33) | 0.463 | ||
| PR | 24 (34.29) | 19 (41.30) | 9 (64.29) | 0.109 | 22 (36.67) | 18 (45.00) | 8 (80.00) | 0.037‡* | 5 (31.25) | 6 (54.55) | 1 (16.67) | 0.287 | ||
| SD | 10 (14.29) | 6 (13.04) | 1 (7.14) | 0.770 | 9 (15.00) | 4 (10.00) | 1 (10.00) | 0.736 | 4 (25.00) | 2 (18.18) | 1 (16.67) | 1.000 | ||
| PD | 33 (47.14) | 19 (41.30) | 2 (14.29) | 0.075 | 27 (45.00) | 16 (40.00) | 0 (0.00) | 0.026§* | 5 (31.25) | 2 (18.18) | 2 (33.33) | 0.771 | ||
| ORRT | 27 (38.57) | 21 (45.65) | 11 (78.57) | 0.023†* | 24 (40.00) | 20 (50.00) | 9 (90.00) | 0.013¶* | 7 (43.75) | 7 (63.64) | 3 (50.00) | 0.595 | ||
| DCRT | 37 (52.86) | 27 (58.70) | 12 (85.71) | 0.075 | 33 (55.00) | 24 (60.00) | 10 (100.00) | 0.026||* | 11 (68.75) | 9 (81.82) | 4 (66.67) | 0.771 | ||
*, P<0.05. Post-hoc test with Bonferroni correction (P<0.017 was considered as statistically significant): †, vessel ORRT: HAIC and ICI vs. HAIC (P=0.014); HAIC vs. ICI (P=0.572); HAIC and ICI vs. ICI (P=0.064); ‡, PVTT PR: HAIC and ICI vs. HAIC (P=0.015); HAIC vs. ICI (P=0.532); HAIC and ICI vs. ICI (P=0.077); §, PVTT PD: HAIC vs. HAIC and ICI (P=0.005); HAIC vs. ICI (P=0.773); HAIC and ICI vs. ICI (P=0.020); ¶, PVTT ORRT: HAIC and ICI vs. HAIC (P=0.005); HAIC vs. ICI (P=0.435); HAIC and ICI vs. ICI (P=0.031); ||, PVTT DCRT: HAIC and ICI vs. HAIC (P=0.005); HAIC vs. ICI (P=0.773); HAIC and ICI vs. ICI (P=0.020). CR, complete response; DCRT, disease control rate of tumour thrombi; HAIC, hepatic arterial infusion chemotherapy; HCC, hepatocellular carcinoma; ICI, immune checkpoint inhibitor; IVCTT, inferior vena cava vein tumour thrombus; MVI, macrovascular invasion; ORRT, objective response rate of tumour thrombi; PD, progressive disease; PR, partial response; PVTT, portal vein tumour thrombus; SD, stable disease.
A total of 29 (22.31%) patients had recurrence of new hepatic tumour, and 22 (16.92%) patients had recurrent of new distal metastasis. No significant differences were observed between three groups with regard to recurrences of new primary hepatic tumour and new distal metastasis (Table 4). The treatment responses of primary hepatic tumour and distal metastasis between three groups were also not significantly different (Table 5).
Table 4
| Variables | HAIC (n=70), n (%) | Systemic ICI (n=46), n (%) | HAIC and ICI (n=14), n (%) | P value |
|---|---|---|---|---|
| Recurrent of new hepatic tumour, yes (%) | 20 (28.57) | 8 (17.39) | 1 (7.14) | 0.130 |
| Recurrent of new distal metastasis, yes (%) | 7 (10.00) | 11 (23.91) | 4 (28.57) | 0.069 |
HAIC, hepatic arterial infusion chemotherapy; ICI, immune checkpoint inhibitor.
Table 5
| Response | HAIC (n=62), n (%) | Systemic ICI (n=43), n (%) | HAIC and ICI (n=13), n (%) | P value |
|---|---|---|---|---|
| Efficacy of primary hepatic tumour† | ||||
| CR | 1 (1.61) | 1 (2.33) | 0 (0.00) | 1.000 |
| PR | 15 (24.19) | 12 (27.91) | 6 (46.15) | 0.276 |
| SD | 23 (37.10) | 13 (30.23) | 2 (15.38) | 0.295 |
| PD | 23 (37.10) | 17 (39.53) | 5 (38.46) | 0.968 |
| ORR | 16 (25.81) | 13 (30.23) | 6 (46.15) | 0.343 |
| DCR | 39 (62.90) | 26 (60.47) | 8 (61.54) | 0.968 |
| Efficacy of distal metastasis† | ||||
| CR | 1 (7.14) | 1 (5.56) | 1 (14.29) | 0.766 |
| PR | 1 (7.14) | 3 (16.67) | 2 (28.57) | 0.363 |
| SD | 4 (28.57) | 3 (16.67) | 1 (14.29) | 0.677 |
| PD | 8 (57.14) | 11 (61.11) | 3 (42.86) | 0.768 |
| ORR | 2 (14.29) | 4 (22.22) | 3 (42.86) | 0.368 |
| DCR | 6 (42.86) | 7 (38.89) | 4 (57.14) | 0.768 |
†, responses were evaluated using the RECIST. CR, complete response; DCR, disease control rate; HAIC, hepatic arterial infusion chemotherapy; ICI, immune checkpoint inhibitor; ORR, overall response rate; PD, progressive disease; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumours; SD, stable disease.
Survival
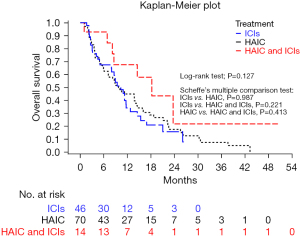
The median OS was 10.3 months [95% confidence interval (CI): 8.0–11.7], 9.7 months (95% CI: 6.0–14.1) and 18.2 months (8.2–not estimable) in the ICI group, HAIC group and HAIC+ICI group, respectively. The OS rates at 6 and 12 months were 67.4% and 31.4% in the ICI group, 63.9% and 44.9% in the HAIC group and 92.9% and 67.5% in the HAIC+ICI group, respectively. Although the median OS was longer in the HAIC+ICI group, there was no significant difference between the three groups (P=0.127) and any two groups (HAIC vs. ICI, P=0.987; ICI vs. HAIC+ICI, P=0.221; HAIC vs. HAIC+ICI, P=0.413) (Figure 2).
The median PFS was 2.5 months (95% CI: 1.8–4.9), 3.4 months (95% CI: 2.7– 5.3), and 9.8 months (95% CI: 5.5–15.4) in the ICI group, HAIC group, and HAIC+ICI group, respectively. The PFS rates at 6, and 12 months were 32.6% and 24.6% in the ICI group, 35.5% and 21.2% in the HAIC group and 78.6% and 33.2% in the HAIC+ICI group, respectively. The median PFS was numerically longer in the HAIC+ICI group than either the HAIC or the ICI monotherapy group but there was no significant difference between the three groups (P=0.091) and any two groups (HAIC vs. ICI, P=0.995; ICI vs. HAIC+ICI, P=0.225; HAIC vs. HAIC+ICI, P=0.251) (Figure 3).

Survival analysis by response showed that patients achieving CR/PR in either overall tumour or vascular responses were associated with longer PFS and OS than those achieving SD or PD (P<0.001) (Figures S1-S4).
Predictors for survival
The results of univariate and multivariate analyses are listed in Table S5 and Table S6. In multivariate analysis of death, CLIP score 2–5 was independently associated with increased risk of death [adjusted hazard ratio (HR): 2.26; 95% CI: 1.10–4.65; P=0.027]. Achievement of CR/PR in either overall tumour response (adjusted HR: 0.11; 95% CI: 0.05–0.22; P<0.001) or vascular response (adjusted HR: 0.22; 95% CI: 0.14–0.36; P<0.001) and treatment in combination with TKIs (adjusted HR: 0.51; 95% CI: 0.31–0.86; P=0.011) were independently associated with decreased risk of death. Combination use of HAIC and ICIs was also associated with reduced risk of death although this did not reach statistical significance (adjusted HR: 0.47; 95% CI: 0.21–1.04; P=0.062) (Table S5).
In multivariate analysis of PFS, Child-Pugh class B was an independent risk factor for progression or death (adjusted HR: 1.81; 95% CI: 1.15–2.84; P=0.010), while the use of HAIC plus ICIs was associated with reduced risk of progression or death compared with those who received HAIC alone (adjusted HR: 0.46; 95% CI: 0.23–0.94; P=0.032) (Table S6).
Adverse events
The safety profiles of the treatment groups are listed in Table 6. AEs were reported for 50% or more of patients in each group. The overall incidence of AEs was similar between three groups (P=0.620). HAIC+ICI group had higher incidence of AEs Grade ≥3 than HAIC group and ICI group, but the difference did not reach statistical significance between three groups (P=0.297). Significant difference between the incidences of neutropenia was detected in the three groups (P=0.026), and HAIC+ICI group had a higher rate than the other two groups. Among the patients with neutropenia, two were Grade 3 and recovered after dose adjustment. Significant difference between the incidences of nausea, vomit, dyspepsia or anorexia was detected in the three groups (P=0.003) with the incidences being most frequent in the HAIC group. Post-hoc analysis with Bonferroni correction showed that the HAIC group had significantly higher rates of nausea, vomit, dyspepsia or anorexia compared with those of the ICI group (P=0.002). Significant difference between the incidences of skin rash was detected in the three groups (P<0.001) with the most frequent being the ICI group. Post-hoc analysis with Bonferroni correction showed that the HAIC group had significantly lower rate of skin rash than the ICI group (P<0.001). Furthermore, the levels of total bilirubin tended to elevate in the ICI group (β-coefficient =0.94, P=0.010) and HAIC group (β-coefficient =0.40, P=0.001), and there was no change in the HAIC+ICI group (β-coefficient =0.23, P=0.120) after 12 weeks of treatment (Figure S5 and Table S7). It indicated that liver functional reserve in patients who received combination therapy was not significantly changed during the 12-week treatment period.
Table 6
| Variables | HAIC (n=70) | Systemic ICI (n=46) | HAIC and ICI (n=14) | P value |
|---|---|---|---|---|
| Side effect | 0.620 | |||
| No | 29 (41.43) | 23 (50.00) | 7 (50.00) | |
| Yes | 41 (58.57) | 23 (50.00) | 7 (50.00) | |
| Side effect type | ||||
| Nausea or vomit or dyspepsia or anorexia | 13 (31.71) | 0 (0.00) | 2 (28.57) | 0.003†* |
| Alopecia | 4 (9.76) | 0 (0.00) | 1 (14.29) | 0.202 |
| Diarrhoea or colitis | 4 (9.76) | 1 (4.35) | 0 (0.00) | 0.793 |
| Neutropenia | 7 (17.07) | 0 (0.00) | 2 (28.57) | 0.026‡* |
| Fever | 7 (17.07) | 0 (0.00) | 0 (0.00) | 0.064 |
| Skin rash | 1 (2.44) | 10 (43.48) | 1 (14.29) | <0.001§* |
| Fatigue or weakness | 4 (9.76) | 4 (17.39) | 0 (0.00) | 0.526 |
| Hepatitis | 1 (2.44) | 4 (17.39) | 1 (14.29) | 0.067 |
| Pneumonitis | 0 (0.00) | 3 (13.04) | 0 (0.00) | 0.086 |
| Hypothyroidism | 0 (0.00) | 1 (4.35) | 0 (0.00) | 0.423 |
| Side effect grade | 0.185 | |||
| 1 | 25 (60.98) | 11 (47.83) | 4 (57.14) | |
| 2 | 9 (21.95) | 8 (34.78) | 0 (0.00) | |
| 3 | 6 (14.63) | 2 (8.70) | 3 (42.86) | |
| 4 | 1 (2.44) | 2 (8.70) | 0 (0.00) | |
| Side effect grade | 0.297 | |||
| <3 | 34 (82.93) | 19 (82.61) | 4 (57.14) | |
| ≥3 | 7 (17.07) | 4 (17.39) | 3 (42.86) |
*, P<0.05. Post-hoc test with Bonferroni correction (P<0.017 was considered statistically significant): †, nausea or vomit or dyspepsia or anorexia: HAIC vs. Systemic ICI (P=0.002); HAIC vs. HAIC and ICI (P=1.000); systemic ICI vs. HAIC and ICI (P=0.048); ‡, neutropenia: HAIC vs. systemic ICI (P=0.043); HAIC vs. HAIC and ICI (P=0.601); systemic ICI vs. HAIC and ICI (P=0.048); §, skin rash: HAIC vs. systemic ICI (P<0.001); HAIC vs. HAIC and ICI (P=0.273); systemic ICI vs. HAIC and ICI (P=0.215). HAIC, hepatic arterial infusion chemotherapy; ICI, immune checkpoint inhibitor.
Discussion
This real-world study in Taiwan showed that HAIC in combination with PD-1 inhibitors achieved better vascular response than HAIC or systemic ICIs alone in patients with advanced HCC and vascular invasion. Moreover, this study also indicated that the strategy of combining PD-1 inhibitors and HAIC is an independent prognostic factor for prolonged PFS. HAIC in combination with ICIs was generally well tolerated. Compared with HAIC or ICIs alone, the combination therapy may be associated with increased incidence of Grade ≥3 AEs but the overall AE incidences were similar between groups. Higher CLIP scores were associated with increased risk of death. Achievement of CR/PR in either overall tumour or vascular responses and concomitant use of TKIs were associated with reduced risk of death.
The efficacy of HAIC combined with ICIs was also reported in previous studies. A retrospective study in China that included HCC patients with Barcelona Clínic Liver Cancer B or C and Child-Pugh A reported that HAIC+ICI therapy had better DCR in overall response and intrahepatic response and longer OS and PFS than HAIC alone. HAIC+ICI therapy, compared with HAIC alone, provided the OS benefit in patients with PVTT Vp1–3, but not in those with main PVTT (Vp4), and PFS benefit was not observed in patients with PVTT (Vp1–4) (21). Our study showed that the combination of HAIC and ICIs was an independent prognostic factor for prolonged PFS, and indeed HAIC+ICI therapy had longer median PFS and OS than HAIC and ICIs alone. However, there was no significant difference in median PFS or OS between the three treatments. This may be due to the majority of patient (85.38%) with PVTT Vp3 and/or Vp4, small number of patients included in the HAIC+ICI group, and the imbalance in the patient number between HAIC+ICI group and HAIC or ICI group. Another retrospective study at 3 hospitals in China included patients who had unresectable HCC with MVI and/or extrahepatic spread. Among patients receiving HAIC plus toripalimab, the majority (77.4%) had PVTT, and the median OS and PFS were 17.13 and 9.3 months, respectively (29). A real-world study in China included patients who had advanced HCC with vascular invasion or metastases receiving HAIC + PD-1 inhibitors + TKIs. The median PFS was 10.6 months. Similarly, a majority of included patients (74.1%) had PVTT (3.7% with Vp1–2, 37.0% with Vp3 and 33.3% with Vp4) (30). The median OS and PFS for HAIC+ICI treatment were similar to the previous studies in broadly similar patient populations (21,29,30). Our findings are in agreement with previous studies which HAIC combined with PD-1 inhibitor could provide a survival benefit in advanced HCC patients. These observations support the clinically important implication that combination of HAIC and ICIs may contribute to the effective control of tumor thrombi, preserve liver function, and provide an opportunity for patients to receive further treatment. Further large cohort-based, long-term follow-up studies are required to evaluate the treatment effect of combination therapy in HCC patients.
Unlike the previous study, our study did not observe a significant difference in overall response between HAIC+ICI treatment, HAIC alone and ICI alone. However, our study showed that based on vessel RECIST, DCRT and ORRT in HAIC+ICI group were improved and HAIC+ICI treatment achieved significantly better vascular response than HAIC alone, especially in PVTT. It may indicate that HAIC+ICI treatment is effective in managing PVTT. It has been reported that soluble PD-L1 levels in plasma were associated with PVTT (31), which may explain the good response to PVTT observed in patients receiving HAIC combined with PD-1 inhibitor. The efficacy of HAIC combined with ICIs for vascular thrombi is not well-established up to now. Our study was the first to demonstrate the comparison of treatment outcomes between these three treatment modalities for HCC with vascular metastases. In Asia, besides systemic target therapies, more aggressive treatments such as TACE, radiotherapy, systemic chemotherapy and local treatments are recommended for the treatment of HCC with PVTT (32,33). The novel findings in our study showed that the combination of immunotherapy and HAIC may serve as a feasible treatment option for HCC patients with tumour thrombi, and may be considered as a potential alternative therapy for clinically difficult cases.
The current study showed that vascular response of IVCTT was not significantly different between the three treatments. It has been reported that the location of tumour thrombus can affect the response rate and survival in HCC. Two retrospective studies in China included HCC patients with PVTT and/or IVCTT. One study reported that after receiving external-beam radiation therapy (EBRT), patients with PVTT had poorer survival and response rates than those with IVCTT (34). Another study showed that survival in patients with IVCTT was worse than those with PVTT in non-EBRT group. However, IVCTT became a protective factor for patients treated with EBRT (35). Further studies are required to investigate how the location of tumour thrombus affects the efficacy of HAIC and ICIs.
Our study showed that there was no significant difference in the treatment responses of distal metastasis between the three treatments, which indicated that systemic ICI might not compensate for the limitations of HAIC in controlling extra-hepatic metastases. The previous study reported the similar results which showed that OS and PFS were not significantly different between HAIC+ICI group and HAIC group in the subgroup analysis of extra-hepatic metastases (21).
The strength of our study is to focus on vascular response in HCC patients and provide evidence of HAIC+ICI in the management of PVTT. Furthermore, the findings showed that the efficacy and incidence of severe (Grade ≥3) AEs in HAIC alone and ICIs alone were comparable. In Taiwan, HAIC is covered by national health insurance, while ICIs are not. When patients cannot afford ICIs, HAIC can be an alternative. However, due to the limited patient numbers, prospective studies detailing head-to-head comparisons are needed to verify the results of the current study. Future advancements in molecular profiling techniques and a better understanding of tumor biology and biomarkers could help to identify this subset of patients (36). There are some limitations in our study. First, there might be selection bias due to retrospective design. Second, heterogeneity may exist across three groups in terms of ECOG score, liver function reserve, distant metastases, thrombus location, prior treatment, and TKIs combination therapy. In the future, more homogenous and large-scale prospective studies are needed to verify the results of the current study. Finally, small number of patients in the HAIC+ICI group and imbalance of patients among three groups may result in underestimation and overestimation.
Conclusions
The present study showed that HAIC combined with ICIs was well tolerated and had a superior vascular response of PVTT, but not IVCTT, compared with HAIC and ICIs alone. Further studies are needed to confirm the findings, and to address the survival benefit and the efficacy of combination therapy of HAIC and ICIs in HCC patients with different locations and extent of tumour thrombus.
Acknowledgments
Medical writing assistance was provided by Ruby Lu and Magdalene Chu of MIMS (Hong Kong) Ltd., which was funded by the National Cheng Kung University Hospital and complied with Good Publication Practice 3 ethical guidelines.
Funding: This work was supported by the National Cheng Kung University Hospital (grant Nos. NCKUH-11206002, NCKUH-11203041, and NCKUH-11203009).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-858/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-858/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-858/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-858/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board of the National Cheng Kung University Hospital (No. B-ER-110-300). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Wu J. The changing epidemiology of hepatocellular carcinoma in Asia versus United States and Europe. Adv Mod Oncol Res 2017;3:51-8. [Crossref]
- Costentin CE, Ferrone CR, Arellano RS, et al. Hepatocellular Carcinoma with Macrovascular Invasion: Defining the Optimal Treatment Strategy. Liver Cancer 2017;6:360-74. [Crossref] [PubMed]
- Lee YH, Hsu CY, Huang YH, et al. Vascular invasion in hepatocellular carcinoma: prevalence, determinants and prognostic impact. J Clin Gastroenterol 2014;48:734-41. [Crossref] [PubMed]
- Thuluvath PJ. Vascular invasion is the most important predictor of survival in HCC, but how do we find it? J Clin Gastroenterol 2009;43:101-2. [Crossref] [PubMed]
- Vogel A, Martinelli EESMO Guidelines Committee. Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO Clinical Practice Guidelines. Ann Oncol 2021;32:801-5. [Crossref] [PubMed]
- Su GL, Altayar O, O'Shea R, et al. AGA Clinical Practice Guideline on Systemic Therapy for Hepatocellular Carcinoma. Gastroenterology 2022;162:920-34. [Crossref] [PubMed]
- Benson AB, D'Angelica MI, Abbott DE, et al. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2021;19:541-65. [Crossref] [PubMed]
- Torimura T, Iwamoto H. Optimizing the management of intermediate-stage hepatocellular carcinoma: Current trends and prospects. Clin Mol Hepatol 2021;27:236-45. [Crossref] [PubMed]
- Jeong SW, Jang JY, Shim KY, et al. Practical effect of sorafenib monotherapy on advanced hepatocellular carcinoma and portal vein tumor thrombosis. Gut Liver 2013;7:696-703. [Crossref] [PubMed]
- Kuo YH, Wu IP, Wang JH, et al. The outcome of sorafenib monotherapy on hepatocellular carcinoma with portal vein tumor thrombosis. Invest New Drugs 2018;36:307-14. [Crossref] [PubMed]
- 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. Clin Mol Hepatol 2022;28:583-705. [Crossref] [PubMed]
- Lu J, Zhang XP, Zhong BY, et al. Management of patients with hepatocellular carcinoma and portal vein tumour thrombosis: comparing east and west. Lancet Gastroenterol Hepatol 2019;4:721-30. [Crossref] [PubMed]
- Song DS, Song MJ, Bae SH, et al. A comparative study between sorafenib and hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis. J Gastroenterol 2015;50:445-54. [Crossref] [PubMed]
- Choi JH, Chung WJ, Bae SH, et al. Randomized, prospective, comparative study on the effects and safety of sorafenib vs. hepatic arterial infusion chemotherapy in patients with advanced hepatocellular carcinoma with portal vein tumor thrombosis. Cancer Chemother Pharmacol 2018;82:469-78. [Crossref] [PubMed]
- Ueshima K, Ogasawara S, Ikeda M, et al. Hepatic Arterial Infusion Chemotherapy versus Sorafenib in Patients with Advanced Hepatocellular Carcinoma. Liver Cancer 2020;9:583-95. [Crossref] [PubMed]
- Moriya K, Namisaki T, Sato S, et al. Efficacy of bi-monthly hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma. J Gastrointest Oncol 2018;9:741-9. [Crossref] [PubMed]
- Moriya K, Namisaki T, Sato S, et al. Bi-monthly hepatic arterial infusion chemotherapy as a novel strategy for advanced hepatocellular carcinoma in decompensated cirrhotic patients. Clin Mol Hepatol 2019;25:381-9. [Crossref] [PubMed]
- Yau T, Hsu C, Kim TY, et al. Nivolumab in advanced hepatocellular carcinoma: Sorafenib-experienced Asian cohort analysis. J Hepatol 2019;71:543-52. [Crossref] [PubMed]
- Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J Clin Oncol 2020;38:193-202. [Crossref] [PubMed]
- Mei J, Li SH, Li QJ, et al. Anti-PD-1 Immunotherapy Improves the Efficacy of Hepatic Artery Infusion Chemotherapy in Advanced Hepatocellular Carcinoma. J Hepatocell Carcinoma 2021;8:167-76. [Crossref] [PubMed]
- Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68:723-50. [Crossref] [PubMed]
- Sherman CB, Behr S, Dodge JL, et al. Distinguishing Tumor From Bland Portal Vein Thrombus in Liver Transplant Candidates With Hepatocellular Carcinoma: the A-VENA Criteria. Liver Transpl 2019;25:207-16. [Crossref] [PubMed]
- Tsai HM, Han MZ, Lin YJ, et al. Real-world outcome of immune checkpoint inhibitors for advanced hepatocellular carcinoma with macrovascular tumor thrombosis. Cancer Immunol Immunother 2021;70:1929-37. [Crossref] [PubMed]
- Bae JS, Lee JM, Yoon JH, et al. How to Best Detect Portal Vein Tumor Thrombosis in Patients with Hepatocellular Carcinoma Meeting the Milan Criteria: Gadoxetic Acid-Enhanced MRI versus Contrast-Enhanced CT. Liver Cancer 2020;9:293-307. [Crossref] [PubMed]
- Ma MC, Chen YY, Li SH, et al. Intra-arterial chemotherapy with doxorubicin and cisplatin is effective for advanced hepatocellular cell carcinoma. ScientificWorldJournal 2014;2014:160138. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010;30:52-60. [Crossref] [PubMed]
- Xu YJ, Lai ZC, He MK, et al. Toripalimab Combined With Hepatic Arterial Infusion Chemotherapy Versus Lenvatinib for Advanced Hepatocellular Carcinoma. Technol Cancer Res Treat 2021;20:15330338211063848. [Crossref] [PubMed]
- Liu BJ, Gao S, Zhu X, et al. Real-world study of hepatic artery infusion chemotherapy combined with anti-PD-1 immunotherapy and tyrosine kinase inhibitors for advanced hepatocellular carcinoma. Immunotherapy 2021;13:1395-405. [Crossref] [PubMed]
- Kim HJ, Park S, Kim KJ, et al. Clinical significance of soluble programmed cell death ligand-1 (sPD-L1) in hepatocellular carcinoma patients treated with radiotherapy. Radiother Oncol 2018;129:130-5. [Crossref] [PubMed]
- Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int 2017;11:317-70. [Crossref] [PubMed]
- Cheng S, Yang J, Shen F, et al. Multidisciplinary management of hepatocellular carcinoma with portal vein tumor thrombus - Eastern Hepatobiliary Surgical Hospital consensus statement. Oncotarget 2016;7:40816-29. [Crossref] [PubMed]
- Hou JZ, Zeng ZC, Zhang JY, et al. Influence of tumor thrombus location on the outcome of external-beam radiation therapy in advanced hepatocellular carcinoma with macrovascular invasion. Int J Radiat Oncol Biol Phys 2012;84:362-8. [Crossref] [PubMed]
- Zeng ZC, Fan J, Tang ZY, et al. A comparison of treatment combinations with and without radiotherapy for hepatocellular carcinoma with portal vein and/or inferior vena cava tumor thrombus. Int J Radiat Oncol Biol Phys 2005;61:432-43. [Crossref] [PubMed]
- Lee SH, Jang HJ. Deep learning-based prediction of molecular cancer biomarkers from tissue slides: A new tool for precision oncology. Clin Mol Hepatol 2022;28:754-72. [Crossref] [PubMed]


