
Paclitaxel combined with platinum (PTX) versus fluorouracil combined with cisplatin (PF) in the treatment of unresectable esophageal cancer: a systematic review and meta-analysis of the efficacy and toxicity of two different regimens
Highlight box
Key findings
• Compared with the PF regimen, the PTX regimen combined with radiotherapy in the CCRT of unresectable esophageal cancer exhibited benefits in the short-term therapeutic effect (CR, ORR, and DCR rates) and 2-year OS rate, and also had lower gastrointestinal toxicity.
What is known and what is new?
• CCRT is the standard therapy for patients with localized carcinoma of the esophagus who select for non-surgical treatment (category 1A). However, the locoregional failure rate after CCRT is high, and the long-term survival status is not optimistic. Chemotherapy plays an important role in definitive chemoradiotherapy strategies.
• This article aimed to systematically evaluate the efficacy and toxicity of paclitaxel/docetaxel combined with platinum (PTX) and fluorouracil combined with cisplatin (PF) in the CCRT of unresectable esophageal cancer.
What is the implication, and what should change now?
• The conclusions of this study require further confirmation through prospective randomized controlled studies with large sample sizes.
Introduction
Concurrent chemoradiotherapy (CCRT) is the standard therapy for patients with localized carcinoma of the esophagus who select for non-surgical treatment (category 1A) (1-3). However, the locoregional failure rate after CCRT is high, and the long-term survival rate is not optimistic. Chemotherapy plays an important role in definitive chemoradiotherapy strategies. The commonly used clinical concurrent chemotherapy regimens include fluorouracil and cisplatin, taxane and platinum, gemcitabine and platinum, and irinotecan. Among these, fluorouracil + cisplatin (PF) is the most classic scheme and has been used as the preferred first-line treatment based on the results of the RTOG85-01 trial (1).
Squamous cell carcinoma is the main pathological type (>90%) of esophageal cancer in Asian countries. Therefore, most scholars prefer paclitaxel combined with platinum (PTX) in clinical trial design and practical treatment (4-7). This is also a first-line recommendation in the Chinese Society of Clinical Oncology (CSCO) guidelines. Our previous study (8) found insufficient evidence to confirm the superiority or inferiority of either of these two schemes, especially for esophageal squamous cell carcinoma. Disputes on the toxicity and efficacy of the two chemotherapy regimens for esophageal squamous cell carcinoma persisted all the time. Which regimen can maximize the benefits of patients? Based on the above background, we conducted this study and the aim was to systematically evaluate the efficacy and toxicity of PTX and PF in the CCRT of unresectable esophageal cancer. We present the following article in accordance with the PRISMA-NMA reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-33/rc).
Methods
Literature search
The PubMed, China National Knowledge Infrastructure (CNKI), Google Scholar and Embase databases were searched by combining subject words and free words through December 31, 2021. The following medical subject headings were used: “Esophageal Neoplasm”, “Chemoradiotherapies”, “Paclitaxel”, and “Docetaxel”. After searching for the above MeSH terms, in order to avoid deleting important articles, two clinicians will read the title and abstract of the articles independently, manually screening, and finally determined the included and excluded articles.
Literature inclusion criteria
The inclusion criteria were as follows: (I) studies on CCRT for esophageal cancer and randomized controlled trials (RCTs) comparing the PTX and PF regimens published in Chinese or English; (II) studies that applied the three-dimensional conformal or intensity-modulated radiotherapy techniques; (III) the research observation indicators were the therapeutic effect and toxicities of the two schemes; and (IV) studies involving participants with esophageal cancer (squamous cell carcinoma/adenocarcinoma) confirmed by pathological diagnosis, without exclusion based on gender, age, or clinical stage.
Exclusion criteria
The exclusion criteria were as follows: (I) co-studies of other malignant tumors or concurrent other diseases; (II) neoadjuvant or postoperative adjuvant chemoradiotherapy studies; (III) articles that did not include the data required for this study; and (IV) studies involving combination regimens that included drugs other than PTX and PF.
Intervention measures
The experimental group (PTX group) was treated with paclitaxel/docetaxel (taxanes) combined with platinum-based chemotherapy with concurrent radiotherapy, and the control group (PF group) was treated with cisplatin combined with fluorouracil with concurrent radiotherapy.
Observation indices and reference variables
(I) Safety: Grade 3 and above toxic reactions (hematological toxicity, gastrointestinal reaction, radiation pneumonia, and radiation esophagitis) of the two chemotherapy regimens were collected from the included studies. (II) The short-term therapeutic effects included Complete Remission (CR), Partial Response (PR), Objective Response Rate (ORR), and Disease Control Rate (DCR) based on the Response Evaluation Criteria In Solid Tumors (RECIST) standards. (III) overall survival (OS) and Progression-Free Survival (PFS): this included the 1-, 2-, 3-, and 5-year OS rates and the 1-, 2-, and 3-year disease PFS rates.
Quality evaluation
We conducted a systematic review of randomized controlled trials. Two English databases and one Chinese database were subjected to a systematic search from inception to December 2021. Two reviewers independently assessed eligibility, extracted data, and evaluated methodological quality using the Cochrane risk of bias (RoB 2.0) tool. RoB’s evaluation results provide an important assessment of the quality of evidence in the meta-analysis, it evaluated five aspects: randomization process, intervention measures deviating from expectations, missing result data, measurement of results, and selection of reported results. If all criteria are met, the study is low bias risk, if one or more criteria are partially met, the study is medium bias risk, if one or more criteria are not met, the study is high bias risk. Figure 1 is the result of assessment.
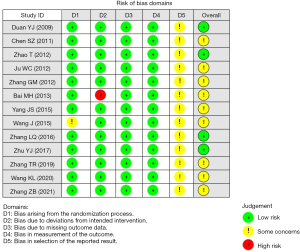
Data extraction
Two reviewers independently extracted the included research data, and after cross-checking, inconsistent results were resolved through discussion or soliciting the opinions of a third party. The extracted data included the basic information, sample size, pathology type, chemotherapy regimen, radiotherapy dose, study type, short-term therapeutic effect, OS rate, PFS rate, and grade 3 or above toxicity. For studies of which the long-term survival rates could not be obtained directly, Engauge-Digitizer software (Free Software Foundation, Inc., Boston, MA, USA) was used to extract the information from the Kaplan-Meier curve. First, set the coordinate points and the three points (0, 0), (0, 1), and (n, 0) respectively. Then collect each data point, open the data, and the time corresponds to the survival rate. You can perform a simple filter to delete duplicate and redundant data, and only take data from the main key time points, such as 12 months (1 year). The time is on the X-axis, and the survival rate is on the Y-axis.
Statistical analysis
Stata 11.1 (Stata Corp, College Station, TX) was used, the relative risk (RR) was selected as the effect index, and the 95% confidence intervals (CI) were calculated. To evaluate the rationality of the combined analysis of the test groups, the Chi-square test was used to assess the heterogeneity among the test groups. A random effects model (REM) was used if the heterogeneity was significant (P<0.05 or I2>50%); otherwise, a fixed effects model (FEM) was applied. If there was considerable heterogeneity, the causes and sources of heterogeneity were further analyzed, and if necessary, subgroup and sensitivity analyses were performed to test the stability of the results. Begg analysis of the included studies was performed using Stata 11.1. The Begger analysis and egger analysis were used to assess publication bias, and the robustness of the pooled results further assessed by the Trim and Fill analysis. P<0.05 was considered to indicate publication bias.
Results
Basic information on the literature retrieval
Our search retrieved 809 potentially relevant articles, 77 relevant articles were identified by reading the abstracts, and 13 articles were ultimately included after reading the full texts. The specific process is shown in Figure 2.
Basic characteristics of the included studies
A total of 13 papers (9-21) were included in the systematic evaluation (Table 1), all of which were RCTs from China. A total of 962 patients with esophageal cancer were included in the systematic evaluation, the pathological type in nine studies was squamous cell carcinoma and two included both squamous and adenocarcinoma, while two studies were not explicitly reported. Together, these studies comprised a total of 798 cases of squamous carcinoma and five cases of adenocarcinoma, while 159 cases were not explicitly reported. There were 480 cases (49.9%) in the PTX group and 482 cases (50.1%) in the PF group. The statistical tests of the baseline data in the PTX and PF groups were reported in all 13 studies, and all were comparable. The radiation doses and tumor stages differed among the studies, but there was no difference in the comparative results after pooling the 13 studies.
Table 1
| Author | Publication | Country | Pathology | Clinical stage | Cases (PTX/PF) | Chemotherapy regimen | Radiotherapy dose (Gy) | Research |
|---|---|---|---|---|---|---|---|---|
| Duan YJ (9) | 2009 | China | SCC | – | 68 (34/34) | Paclitaxel + cisplatin vs. 5-FU + cisplatin | 60–70 | RCT |
| Chen SZ (10) | 2011 | China | SCC | III–IVa | 48 (24/24) | Docetaxel + cisplatin vs. cisplatin + fluorouracil | 60 | RCT |
| Zhao T (11) | 2012 | China | SCC | II–IVa | 90 (45/45) | Docetaxel + cisplatin vs. 5-FU + cisplatin | 50.4 | RCT |
| Ju WC (12) | 2012 | China | SCC | – | 72 (36/36) | Paclitaxel + cisplatin vs. 5-FU + cisplatin | 60–66 | RCT |
| Zhang GM (13) | 2012 | China | SCC/AC | IIa–III | 68 (33/35) | Docetaxel + cisplatin vs. 5-FU + cisplatin | 56–60 | RCT |
| Bai MH (14) | 2013 | China | SCC | IIb–IVb | 74 (36/38) | Docetaxel + cisplatin vs. 5-FU + cisplatin | 60–70 | RCT |
| Yang JS (15) | 2015 | China | SCC | III–IVa | 68 (34/34) | Paclitaxel + lobaplatin vs. cisplatin + 5-FU | 60–70 | RCT |
| Wang J (16) | 2015 | China | SCC/AC | – | 53 (25/28) | Paclitaxel + cisplatin vs. 5-FU + cisplatin | 56–60 | RCT |
| Zhang LQ (17) | 2016 | China | SCC | IIa–III | 61 (31/30) | Paclitaxel + cisplatin vs. 5-FU + cisplatin | 64 | RCT |
| Zhu YJ (18) | 2017 | China | SCC | II–IVa | 86 (45/41) | Docetaxel + cisplatin vs. cisplatin + 5-FU | 60–64 | RCT |
| Zhang TR (19) | 2019 | China | SCC | IIb–IIIc | 120 (60/60) | Docetaxel + cisplatin vs. 5-FU + cisplatin | 54–66 | RCT |
| Wang KL (20) | 2020 | China | – | – | 70 (35/35) | Docetaxel + lobaplatin vs. cisplatin + 5-FU | 50–60 | RCT |
| Zhang ZB (21) | 2021 | China | – | IIb–IIIc | 84 (42/42) | Docetaxel + cisplatin vs. cisplatin + 5-FU | 54–66 | RCT |
PTX, toxicity of paclitaxel combined with platinum; PF, fluorouracil combined with cisplatin; SCC, squamous cell carcinoma; AC, adenocarcinoma; RCT, randomized controlled trial.
Eleven studies provided data on chemoradiotherapy-related hematological toxic reactions (myelosuppressive reactions in leukocytes), 10 studies provided data on chemoradiotherapy-related gastrointestinal reactions (nausea, vomiting, and diarrhea) and radiation esophagitis, and six studies provided data on radiation pneumonia. Moreover, 10 studies reported on the short-term therapeutic effects, including CR, PR, ORR, and DCR. The 1-, 2-, 3-, and 5-year OS rates were reported in 12, 10, 7, and 2 studies, respectively. Furthermore, three studies reported 1-year PFS rates or survival curves, and two studies reported 2- and 3-year PFS rates or survival curves. The basic characteristics of the included studies are shown in Table 1.
Comparison of the toxicities between the PTX and PF regimens in CCRT
A total of 11 studies reported on the hematological toxicity, 10 reported on the gastrointestinal reactions and radiation esophagitis, and six provided data on radiation pneumonia. All of these studies were analyzed according to the cut-off point of ≥ grade 3 toxicities.
In terms of hematological toxicity, there was significant heterogeneity among the 11 studies (heterogeneity test: I2=50.3%, P=0.028), and the REM was applied for analysis. After all the studies were combined, the overall test results showed that the hematological toxicities of the two regimens were similar, and there was no overall difference (RR =1.43, 95% CI: 0.98–2.10, P=0.065, Figure 3A).
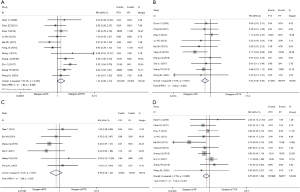
The gastrointestinal reactions were analyzed using the FEM (I2=0%, P=0.591). The results showed that the gastrointestinal reactions from the PF regimen were the most serious (RR =0.54, 95% CI: 0.36–0.80, P=0.003, Figure 3B).
Radiation esophagitis and radiation pneumonitis were analyzed using the FEM (I2=0%, P=0.326), and no overall differences were observed between the regimens (RR =0.70, 95% CI: 0.35–1.42, P=0.326, Figure 3C). Ten studies provided data on radiation esophagitis, and the FEM (I2=4.1%, P=0.402) was used to analyze the data. The overall test results indicated of the two regimens was similar, and there was no overall difference (RR =0.75, 95% CI: 0.57–1.00, P=0.052, Figure 3D).
Comparison of the short-term therapeutic effects of the PTX and PF regimens in CCRT
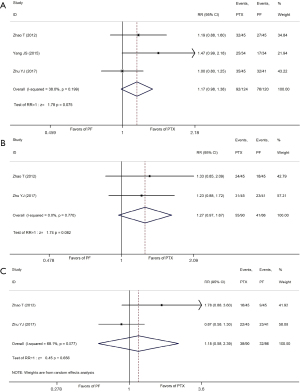
A total of 10 studies provided data on the CR, PR, ORR, and DCR of tumors, and the specific efficacy data are shown in Table 2. After combined analysis, the results showed a difference in the CR rates between the two regimens, with a range of 14.71–33.33% for the PTX regimen and 5.71–32.14% for the PF regimen, and the former exhibited higher rates than the latter (RR =1.35, 95% CI: 1.03–1.76, P=0.030). A difference was also observed in the ORR rates between the two regimens, with a range of 72.22–91.67% for the PTX regimen and 50.00–89.29% for the PF regimen, and the former exhibited higher rates than the latter (RR =1.12, 95% CI: 1.03–1.22, P=0.006). Moreover, the results also showed a difference in the DCR rates between the two regimens, with a range of 88.24–97.22% for the PTX regimen and 70.59–100.00% for the PF regimen, and the former exhibited higher rates than the latter (RR =1.05, 95% CI: 1.01–1.09, P=0.022). There was no significant difference between the two regimens in terms of the PR rate.
Table 2
| Index | Effect model | Test value | Short-term therapeutic effect (%)# | RR | 95% CI | P | |
|---|---|---|---|---|---|---|---|
| PTX | PF | ||||||
| Complete remission | Fixed effects model | I2=0%, P=0.676 | 14.71–33.33 | 5.71–32.14 | 1.35 | 1.03–1.76 | 0.030 |
| Partial remission | Fixed effects model | I2=0%, P=0.747 | 40.00–69.44 | 28.89–77.14 | 1.04 | 0.91–1.91 | 0.563 |
| Objective response rate | Fixed effects model | I2=22.6%, P=0.235 | 72.22–91.67 | 50.00–89.29 | 1.12 | 1.03–1.22 | 0.006 |
| Disease control rate | Fixed effects model | I2=28.3%, P=0.184 | 88.24–97.22 | 70.59–100.00 | 1.05 | 1.01–1.09 | 0.022 |
#, we obtained data from the original text, and the short-term therapeutic effect (%) was the number of people reaching complete remission/partial remission/objective response rate/disease control rate divided by the total number of samples, so as to obtain the short-term therapeutic effect range of the different studies of the two regimens. PTX, toxicity of paclitaxel combined with platinum; PF, fluorouracil combined with cisplatin; CCRT, concurrent chemoradiotherapy; RR, relative risk; CI, confidence interval.
Comparison of the OS and PFS rates between the PTX and PF regimens in CCRT
The reported survival benefits included 1-, 2-, 3-, and 5-year OS rates and the 1-, 2-, and 3-year PFS rates. Among these, if the annual OS/FPS data were not specifically reported, then the data were extracted from the Kaplan-Meier curve using the Engauge-Digitizer software. The specific OS and PFS meta-analysis results from the 13 studies are shown in Table 3, Figure 4A-4D, and Figure 5A-5C. The meta-analysis results showed that the 2-year overall survival rates of the PTX regimen were higher than those of the PF regimen. As for the overall survival data, 12 studies (9-14,16-21) reported the 1-year OS rate, 11 studies (9-14,16-21) reported the 2-year OS rate, and two studies (14,17) reported the 5-year OS rate. There was no significant difference between the two regimens in terms of the 1-, 3- and 5-year OS (RR =1.06, 95% CI: 1.00–1.13, P=0.064; RR =1.15, 95% CI: 0.95–1.38, P=0.144; RR =1.35, 95% CI: 0.73–2.52 P=0.341, respectively). The combined meta-analysis results also showed that there was no significant statistical difference in 1-, 3-, and 5-year survival rates between the two CCRT regimens. In terms of disease PFS, the combined meta-analysis results showed that the 1-, 2-, and 3-year PFS of two the regimens were not significantly different (RR =1.17, 95% CI: 0.98–1.38, P=0.075; RR =1.27, 95% CI: 0.97–1.67, P=0.082; RR =1.18, 95% CI: 0.58–2.39, P=0.656, respectively), as shown in Figure 5A-5C.
Table 3
| Index | Effect model | Test value | Survival rate range (%) | RR | 95% CI | P | |
|---|---|---|---|---|---|---|---|
| PTX | PF | ||||||
| 1-year survival rate | Fixed effects model | I2=0.0%, P=0.644 | 76.00–92.90 | 65.10–93.60 | 1.06 | 0.997–1.13 | 0.064 |
| 2-year survival rate | Fixed effects model | I2=29.3%, P=0.166 | 48.50–76.00 | 37.10–86.20 | 1.18 | 1.05–1.32 | 0.005 |
| 3-year survival rate | Fixed effects model | I2=0.0%, P=0.635 | 34.00–60.10 | 24.20–62.60 | 1.15 | 0.95–1.38 | 0.144 |
| 5-year survival rate | Fixed effects model | I2=0.0%, P=0.387 | 17.40–34.29 | 8.20–27.78 | 1.35 | 0.73–2.52 | 0.341 |
| 1-year PFS rate | Fixed effects model | I2=38.0%, P=0.199 | 70.30–78.80 | 48.60–77.40 | 1.17 | 0.98–1.38 | 0.075 |
| 2-year PFS rate | Fixed effects model | I2=0.0%, P=0.770 | 52.30–69.40 | 41.00–55.00 | 1.27 | 0.97–1.67 | 0.082 |
| 3-year PFS rate | Random effects model | I2=68.1%, P=0.077 | 36.30–49.30 | 20.00–54.90 | 1.18 | 0.58–2.39 | 0.656 |
PTX, toxicity of paclitaxel combined with platinum; PF, fluorouracil combined with cisplatin; CCRT, concurrent chemoradiotherapy; PFS, progression-free survival; RR, relative risk; CI, confidence interval.

Sensitivity and bias analyses
Sensitivity analysis was performed due to the high heterogeneity in terms of hematological toxicity, ORR rate, DCR rate, and 2-year overall survival rate, and the more divergent articles were deleted.
There were 11 studies involving the hematological toxicity, which we analyzed using leave one out method. The results of 8 studies were not statistically significant (95% CI including 1), consistent with the original combined results (RR =1.43, 95% CI: 0.98–2.10), while the results of the remaining 3 studies were statistically significant (95% CI does not include 1), respectively: Chen SZ (2011), Bai MH (2013), Yang JS (2015) (see Table 4), the significant impact of these 3 studies indicates that the results are not robust.
Table 4
| Items | Deleted studies | Heterogeneity analysis I2 | Heterogeneity P value | RR | 95% CI | P |
|---|---|---|---|---|---|---|
| Hematological toxicity | Chen SZ (2011) | 52.1% | 0.027 | 1.502 | 1.003–2.251 | 0.048 |
| Hematological toxicity | Bai MH (2013) | 41.7% | 0.080 | 1.097 | 2.267–1.578 | 0.014 |
| Hematological toxicity | Yang JS (2015) | 41.4% | 0.081 | 1.093 | 2.302–1.586 | 0.015 |
RR, relative risk; CI, confidence interval.
ORR sensitivity analysis elimination method
The study outcome included 10 studies and none of the remaining studies were statistically significant (95% CI including 1), which was consistent with the original combined results (RR =1.05, 95% CI: 1.01–1.09), indicating stable results.
For ORR, the results showed that there was publication bias (Egger’s test, t=0.049, P=0.013<0.05). Therefore, the trim and filling method was used to evaluate the stability of the combined results. The heterogeneity test was Q=9.622, P=0.382, so we used a fixed effect model, with logOR =0.068, 95% CI: −0.008 to 0.143. Shows the number of missing studies after 3 iterations using the Linear method, resulting in 2. Finally, after including the data from two virtual studies, Meta-analysis was conducted for all studies, and the results showed heterogeneity test: Q=15.918, P=0.144, fixed effect model was used: logOR =1.040, 95% CI: 0.971–1.115. After adding 2 studies, the results were not statistically significant and reversed, so the pooled results were not robust. There was a publication bias present.
DCR individually removed method sensitivity analysis
The study outcome included 10 studies and none of the remaining studies were statistically significant (95%CI including 1), which was consistent with the original combined results (RR =1.12, 95% CI: 1.03–1.22), indicating that the results were stable. For DCR, the results showed that there was publication bias (Egger’s test, t=7.26, P=0.000<0.05), the trim and filling method was used to evaluate the stability of the combined results. Heterogeneity test, Q=8.232, P=0.511, using a fixed effect model, combined with log OR =0.013, 95% CI: −0.020 to 0.046). Shows the number of missing studies after 4 iterations using the Linear method, resulting in 4. Finally, after including the data from four virtual studies, the Meta-analysis of all the studies showed heterogeneity test: Q=17.787, P=0.166, fixed effect model: logOR =0.994, 95% CI: 0.965–1.025. After adding four studies, the results were reversed, and therefore the pooled results were not robust.
Two-year OS sensitivity analysis method
The study outcome included 10 studies and none of the remaining studies were statistically significant (95% CI including 1), which is consistent with the original combined results (RR =1.18, 95% CI: 1.05–1.32), indicating that the results were stable.
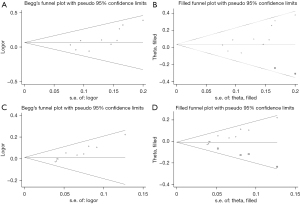
Publication bias was evaluated based on the Begg’s test results of the included studies. Funnel plots were drawn with Standard Error logarithm Risk Ratio (SElogRR) and logRR as the horizontal and vertical coordinates, respectively. The results showed that only the ORR rate indicator was biased, and the funnel plot of Begg’s analysis was not symmetrically distributed.
We also applied the trim-and-fill analysis method to evaluate the impact of publication bias on the results. If the impact is not large, the results are more robust, and if the impact is large, the publication bias must be discussed in the results. In this study, the ORR and DCR exhibited publication bias. According to the combination of the RR values of the FEM (shown in Table 5), there was a statistical difference between the PTX and PF groups (P<0.05) (Figure 6A,6B). However, no significant differences between the two groups were observed following the application of the cut-and-fill method (P>0.05, Figure 6C,6D). Therefore, the conclusions based on ORR and DCR are not robust enough and require further research.
Table 5
| Items | Test for heterogeneity | Method | Pooled Est | 95% CI | Asymptotic | |||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | Z value | P value | |||||
| ORR | Q=15.918 (P=0.144) | Fixed | 1.040 | 0.971 | 1.115 | 1.122 | 0.262 | |
| DCR | Q=17.787 (P=0.166) | Fixed | 0.994 | 0.965 | 1.025 | -0.380 | 0.704 | |
CI, confidence interval; ORR, objective response rate; DCR, disease control rate.
Discussion
For locally advanced unresectable esophageal cancer, CCRT is the first-line recommended treatment mode in Europe, America, and Asian countries, including China (1A evidence) (1-3). Chemotherapy with fluorouracil and cisplatin and concurrent radiotherapy of 50.4 Gy is considered the standard scheme (1,3). Esophageal cancer cases are predominantly adenocarcinoma (>70%) in Europe and the USA, and fluorouracil + cisplatin are the preferred therapeutic agents. Therefore, the National Comprehensive Cancer Network (NCCN) recommends fluorouracil + cisplatin chemotherapy for both locally advanced esophageal cancer and metastatic esophageal cancer. However, squamous cell carcinoma is the main pathological type (>90%) of esophageal cancer in Asian countries. In clinical trial design and practical treatment, most scholars are more inclined to choose taxane combined with platinum drugs (4-7), which is also a first-line recommendation in the CSCO guidelines. In the current study, especially for esophageal squamous cell carcinoma, there remained insufficient evidence to confirm the superiority or inferiority of either of the two regimens. Therefore, we conducted this meta-analysis based on the background information to evaluate the overall difference in survival benefit and toxicity between the PTX and PF regimens combined with concurrent radiotherapy in the context of precision radiotherapy, so to provide a basis for the selection of clinical treatment.
In terms of toxicity and side effects, 5-fluorouracil (5-FU) and its derivatives are anti-metabolic anti-tumor drugs that are recommended in the treatment of multiple system tumors owing to their wide anti-tumor spectrum and can be used alone or in combination with other anti-tumor drugs. The main adverse reactions include serious gastrointestinal reactions and bone marrow suppression, as well as others such as alopecia, hepatotoxicity, allergic skin reactions, hand-foot syndrome, and oral ulcers/stomatitis. Taxanes are cytotoxic anti-tumor drugs that act on the microtubule/tubulin system, which can block the G2 and M phases of the cell cycle and inhibit the mitosis and proliferation of cancer cells. Further, in vitro experiments have shown that paclitaxel exerts a significant radiosensitization effect.
In addition to bone marrow suppression, gastrointestinal reactions, and cardiovascular and liver toxicity, the common adverse drug reactions also include allergic reactions (paclitaxel), body fluid retention (docetaxel), and neurotoxicity. When chemotherapy is applied concurrently with radiotherapy, radiation esophagitis and radiation pneumonitis may be aggravated. In this meta-analysis, the treatment-related toxicities that could be obtained and statistically compared included hematological toxicity (myelosuppressive reaction of white blood cells, platelets, and hemoglobin), gastrointestinal reactions (nausea, vomiting, diarrhea), acute radiation pneumonitis, and radiation esophagitis. These were analyzed with ≥ grade 3 toxic reactions as the cut-off point. The results showed no significant difference between the two regimens in terms of the common chemotherapy-related adverse events (hematological toxicity), radiation esophagitis, and radiation pneumonitis synergized by radiotherapy and chemotherapy. In terms of gastrointestinal reactions, 10 studies provided comparable data, and nine of the studies (9-11,12,14,17-20) did not show significant differences (right studies favored the PF regimen for more severe gastrointestinal reactions), while one study (15) showed a higher incidence of ≥ grade 3 gastrointestinal reactions with the PF regimen. An overall test of the combined results of the 10 studies suggested more severe gastrointestinal reactions with the PF regimen. This may be related to the damaging effects of fluorouracil on the proliferating mucosal cells of the digestive tract (stomatitis, oral ulcers, glossitis, esophagitis). In a study of Liu et al. (8), patients with unresectable esophageal squamous cell carcinoma have comparable survival benefit between TP and FP regimens during concurrent chemoradiation, and the regimen of PTX presents with less adverse severe acute radioesophagitis and upper gastrointestinal adverse reactions. Therefore, when the PF regimen is combined with radiotherapy to treat esophageal cancer concurrently, the occurrence of gastrointestinal reactions should be an aspect of clinical treatment and nursing care.
To sum up, in terms of drug selection for patients, we should fully weigh the patient’s physical conditions, drug efficacy, and related side effects to make a choice. For example, in the drug selection of cisplatin and carboplatin, as well as the selection of paclitaxel and docetaxel, we should understand that cisplatin is the most effective and has the most sufficient evidence. Carboplatin did not show any more advantage in the treatment of oesophageal cancer as compared to cisplatin. However, its nephrotoxicity, gastrointestinal reaction, ototoxicity were lower than cisplatin, therefore, carboplatin can be selected as an alternative treatment for clinical cisplatin intolerance. Docetaxel is a new type of anti-microtubuler drug following paclitaxel, with a stronger pharmacological effect and an anti-tumor activity 1.3–12 times that of paclitaxel. Its cardiovascular toxicity is lower than that of paclitaxel. However, its application evidence in esophageal cancer is not as sufficient as paclitaxel and is generally recommended as a second-line therapy.
In terms of treatment benefits, the results of this meta-analysis showed that the PTX regimen had higher CR, ORR, DCR, and 2-year OS rates than the PF regimen when combined with concurrent radiotherapy in the treatment of unresectable esophageal cancer. Unfortunately, in the comparison of the long-term survival indicators, there was no significant difference in the 5-year survival rate between the two regimens. Of the 13 studies included in the meta-analysis, 2 (14,17) reported 5-year overall survival rates ranging from 17.40–34.29% for the PTX regimen and 8.20–27.78% for the PF regimen. Among them, 3 (11,15,18) favored the long-term survival benefit of the PTX regimen. Although the combined analysis result was negative, the data suggested that the PTX regimen exhibited a trend toward a long-term survival benefit (RR =1.17, 95% CI: 0.98–1.38, P=0.075; RR =1.27, 95% CI: 0.97–1.67, P=0.082; RR =1.18, 95% CI: 0.58–2.39, P=0.656). Combined with the comparison results of other therapeutic effects and survival indicators, this study suggested that the PTX regimen is the optimal regimen for the CCRT of esophageal squamous cell carcinoma.
In addition, a retrospective study (22) of PD-1 inhibitor combined with paclitaxel and platinum regimen was single regimen chemotherapy. In another meta-analysis study (23), the disease types were all nasopharyngeal carcinoma and the chemotherapy regimen was mostly paclitaxel, platinum and fluorouracil combined regimen, which could not compare the differences between fluorouracil and paclitaxel, so they did not meet the search criteria.
It is worth mentioning that immune checkpoint inhibitors have been widely used in the treatment of esophageal cancer, based on the results of KEYNOTE-590 et al., chemotherapy combined with immunotherapy has become a standard recommendation for advanced patients. However, for locally advanced esophageal cancer, the optimal mode and efficacy data of the combination of chemoradiotherapy and immunotherapy have not been published yet, and definitive chemoradiotherapy is still the standard treatment, several ongoing Phase III RCTs include KEYNOTE-975, RATIONALE 311 and ESCORT-CRT. The drug regimen chosen by KEYNOTE-975 was PF regimen combined with PD-1 inhibitors, and the other two trials were the study of paclitaxel + cisplatin combined with PD-1 inhibitors. Which regimen has higher synergistic efficacy and lower toxic response when combined with immunotherapy is worthy of our further attention.
Limitations of this study included: (I) the radiation dose, clinical stage, and specific drugs used in the studies included in the meta-analysis (eight studies in the PTX protocol group used docetaxel + platinum, five studies used paclitaxel + platinum), are all in consistent. (II) The cumulative number of cases was small, and the sum of the number of cases enrolled in both protocols in the 13 RCT studies was less than 120 (48–120 cases). (III) Most studies did not describe the specific allocation method and allocation concealment in detail, and all studies did not mention whether a blinding method was applied and did not perform an intention analysis on the results. This may cause bias in the data analysis results. In addition, all studies were from China. Therefore, the meta-analysis may be more representative of the results of Asian esophageal squamous cell carcinoma patients.
In summary, compared with the PF regimen, the PTX regimen combined with concurrent radiotherapy for unresectable esophageal cancer may have therapeutic benefits in terms of the clinical CR, ORR, DCR, and 2-year OS rates. There were no differences in hematological toxicity, esophageal toxicity, or acute radiation pneumonia between the two regimens; the PTX regimen induced fewer gastrointestinal reactions and may be the preferred CCRT regimen for esophageal squamous cell carcinoma. However, this conclusion requires further confirmation through prospective RCTs involving large sample sizes.
Conclusions
Compared with the PF regimen, the PTX regimen combined with radiotherapy in the CCRT of unresectable esophageal cancer exhibited benefits in terms of short-term therapeutic effects (CR, ORR, and DCR rates) and 2-year OS rate, and the PTX regimen involved less gastrointestinal toxicity. Therefore, the PTX regimen might be the preferred concurrent chemotherapy regimen for esophageal squamous cell carcinoma.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA-NMA reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-33/rc
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-33/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-33/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA 1999;281:1623-7. [Crossref] [PubMed]
- Kumar S, Dimri K, Khurana R, et al. A randomised trial of radiotherapy compared with cisplatin chemo-radiotherapy in patients with unresectable squamous cell cancer of the esophagus. Radiother Oncol 2007;83:139-47. [Crossref] [PubMed]
- Minsky BD, Pajak TF, Ginsberg RJ, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol 2002;20:1167-74. [Crossref] [PubMed]
- Ilson DH, Forastiere A, Arquette M, et al. A phase II trial of paclitaxel and cisplatin in patients with advanced carcinoma of the esophagus. Cancer J 2000;6:316-23. [PubMed]
- Petrasch S, Welt A, Reinacher A, et al. Chemotherapy with cisplatin and paclitaxel in patients with locally advanced, recurrent or metastatic oesophageal cancer. Br J Cancer 1998;78:511-4. [Crossref] [PubMed]
- Ajani JA, Fodor MB, Tjulandin SA, et al. Phase II multi-institutional randomized trial of docetaxel plus cisplatin with or without fluorouracil in patients with untreated, advanced gastric, or gastroesophageal adenocarcinoma. J Clin Oncol 2005;23:5660-7. [Crossref] [PubMed]
- Kim JY, Do YR, Park KU, et al. A multi-center phase II study of docetaxel plus cisplatin as first-line therapy in patients with metastatic squamous cell esophageal cancer. Cancer Chemother Pharmacol 2010;66:31-6. [Crossref] [PubMed]
- Liu LH, Jin XQ, Wang L, et al. Efficacy analysis of TP and FP regimens for concurrent chemoradiotherapy for esophageal squamous cell carcinoma. Chinese Journal of Cancer Prevention and Treatment 2022;29:940-6.
- Duan YJ, Tang WC, Han XL, et al. Clinical study on esophageal carcinoma treated by combined TP or PF with synchronous radiotherapy. Chinese Journal of Modern Medicine 2009;19:111-3.
- Chen SZ, Chen XM, Ding Y, et al. Therapeutic effect of combined cisplatin and docetaxel vs fluorouracil regimen with concurrent radiotherapy on advanced esophageal carcinoma. Journal of Southern Medical University 2011;31:727-9. [PubMed]
- Zhao T, Chen H, Zhang T. Docetaxel and cisplatin concurrent with radiotherapy versus 5-fluorouracil and cisplatin concurrent with radiotherapy in treatment for locally advanced oesophageal squamous cell carcinoma: a randomized clinical study. Med Oncol 2012;29:3017-23. [Crossref] [PubMed]
- Ju WC, Wang MM, Huang J, et al. Comparison on Therapeutic Efficacy of Combined TP or PF with Concurrent Radiotherapy to Advanced Esophageal Carcinoma. Cancer Research on Prevention and Treatment 2012;39:1005-7.
- Zhang GM, Han LF, Zhang SY, et al. Analysis of the efficacy of docetaxel combined with cisplatin and concurrent chemoradiotherapy in the treatment of locally advanced esophageal cancer. Chinese Medical Sciences 2012;2:14-5 + 27.
- Bai M, Wang B, Wang X, et al. Randomized trial of weekly docetaxel and cisplatin combined with concurrent 3DCRT in patients with locally advanced esophageal cancer. The Chinese-German Journal of Clinical Oncology 2013;361-4. [Crossref]
- Yang JS, Wang T, Qiu MQ, et al. Comparison of efficacy and toxicity profiles between paclitaxel/lobapoatin- and cisplatin/5-fluorouracil-based concurrent chemoradiotherapy of advanced inoperable oesophageal cancer. Intern Med J 2015;45:757-61. [Crossref] [PubMed]
- Wang J, Yu Y, Zhan BH, et al. Comparison of paclitaxel with cisplatin and 5-Fu with cisplatin concurrent radiotherapy in esophageal cancer. World Chinese Digestion Journal 2015;23:3904-8. [Crossref]
- Zhang LQ. Effects of IMRT plus different chemotherapies in the treatment of middle-advanced stage esophageal: a comparative study. Health Research 2016;36:272-5.
- Zhu Y, Zhang W, Li Q, et al. A Phase II Randomized Controlled Trial: Definitive Concurrent Chemoradiotherapy with Docetaxel Plus Cisplatin versus 5-Fluorouracil plus Cisplatin in Patients with Oesophageal Squamous Cell Carcinoma. J Cancer 2017;8:3657-66. [Crossref] [PubMed]
- Zhang TR, Lu XD. Clinical efficacy of weekly dose docetaxel combined with cisplatin concurrent radiotherapy in inoperable advanced esophageal cancer. Jiangsu Pharma 2019;45:45-47+50.
- Wang KL, Yuan L. Clinical observation of docetaxel combined with loplatinum synchronous IMRT treatment for advanced esophageal cancer. Medical Forum Magazine 2020;41:16-9.
- Zhang ZB, Liu P, Xie YY, et al. Observation of the efficacy and adv,erse effects of weekly dose docetaxel plus cisplatin chemotherapy combined with concurrent radiotherapy in advanced esophageal cancer. Guizhou Medicine 2021;45:1876-7.
- Lin W, Huang Y, Zhu L, et al. Pembrolizumab combined with paclitaxel and platinum as induction therapy for locally advanced esophageal squamous cell carcinoma: a retrospective, single-center, three-arm study. J Gastrointest Oncol 2022;13:2758-68. [Crossref] [PubMed]
- Liu Y, Yang L, Zhang S, et al. The efficacy and safety of concurrent chemoradiotherapy with induction chemotherapy vs. concurrent chemoradiotherapy alone for locally advanced nasopharyngeal carcinoma: a systematic-review and meta-analysis. Transl Cancer Res 2022;11:1207-18. [Crossref] [PubMed]
(English Language Editor: A. Kassem)



