
Predictive value of apparent diffusion coefficient for neoadjuvant chemotherapy in locally advanced colorectal cancer patients
Highlight box
Key findings
• The ADC could be used as a predictor of the efficacy of neoadjuvant chemotherapy in locally advanced CRC patients.
What is known and what is new?
• The ADC reflects the density of tumor cells. In other malignant tumors, it has been shown to be related to the efficacy of neoadjuvant chemotherapy, but in patients with CRC, there is still a lack of relevant research.
• This study confirmed that the ADC is related to the efficacy of neoadjuvant chemotherapy in patients with CRC.
What is the implication, and what should change now?
• The ADC could be used as a predictor for the efficacy of neoadjuvant chemotherapy in locally advanced CRC patients. Combined with the ADC, clinicians can better evaluate the efficacy of neoadjuvant chemotherapy, providing a theoretical basis for clinicians to choose appropriate treatment plans.
Introduction
The incidence rate and mortality of colorectal cancer (CRC) rank third among malignant tumors, and the number of its patients accounts for about 8% of malignant tumor cases (1). Since early CRC can have no obvious symptoms, more than 20% of patients with CRC are at a locally advanced stage at the first diagnosis. Neoadjuvant chemotherapy is an important method for the treatment of locally advanced CRC. Its purpose is to reduce the stage of CRC before surgery, at the same time promote the sensitivity of tumor cells to chemotherapy drugs, and finally achieve the goal of improving the prognosis of patients (2-4). Neoadjuvant chemotherapy can significantly improve the prognosis of CRC patients (5), but 30–50% of patients are not sensitive to neoadjuvant chemotherapy. Such patients are prone to progress and even lose the opportunity to receive surgical treatment (6,7). Therefore, the key to further improve the prognosis of CRC is to accurately identify patients who are not sensitive to neoadjuvant chemotherapy at an early stage. Previous studies have confirmed that many clinical indicators of patients with malignant tumors can be affected by chemotherapeutic drugs (8,9), and these indicators can also be used to predict the efficacy of neoadjuvant chemotherapy. Carcinoembryonic antigen (CEA) is a common biological indicator for CRC patients (10,11), but a study showed that CEA level at baseline was not significantly related to the progression-free survival (PFS) rate of CRC patients with liver metastasis who received neoadjuvant treatment (12). Therefore, it is of great significance to find new biological indicators to predict the efficacy of neoadjuvant chemotherapy in CRC patients. Apparent diffusion coefficient (ADC) reflects the density of tumor cells. The lower its level, the greater the density of tumor cells, and the higher the degree of malignancy will be, so the tumors become more likely to metastasize (13). Studies in patients with breast cancer and osteosarcoma have confirmed that the ADC was related to the clinicopathological characteristics of patients, and had a good value in predicting the efficacy of neoadjuvant chemotherapy (14-16). In addition, exploring prognostic indicators of malignant tumors is also one of the purposes of clinical research (17). A study in patients with liver metastasis of CRC showed that ADC had good value in predicting the prognosis of patients with CRC (18). Therefore, we speculated that the ADC was related to the efficacy of neoadjuvant chemotherapy in locally advanced CRC patients, but there is no relevant research at present. The purpose of this study was to explore the predictive value of ADC for neoadjuvant chemotherapy in locally advanced CRC patients. We present the following article in accordance with the STARD reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-124/rc).
Methods
General information
A total of 128 patients with CRC treated with neoadjuvant chemotherapy in The First Affiliated Hospital of Xiamen University from January 2016 to January 2017 were retrospectively collected. According to the response after neoadjuvant chemotherapy, the patients were divided into an objective response group (n=80) and a control group (n=48). The inclusion criteria were as follows: (I) patients with locally advanced CRC (pathological diagnosis obtained through colonoscopy before operation); (II) age ≥18 years old; (III) receiving neoadjuvant chemotherapy; (IV) the presence of measurable lesions. The exclusion criteria were as follows: (I) recurrent CRC; (II) combination with other malignant tumors; (III) liver and kidney insufficiency (any liver or kidney disease); (IV) immune insufficiency; (V) lost to follow-up; (VI) neuroendocrine tumors, stromal tumors, and other special types of malignant tumors. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of The First Affiliated Hospital of Xiamen University (No. c202200174) and individual informed consent was waived in this retrospective study. The patient inclusion flow chart is shown in Figure 1.
Treatment strategy
After admission, all patients completed the baseline assessment and were treated with 3 cycles of XELOX (capecitanine and oxaliplatin) regimen neoadjuvant chemotherapy after excluding the contraindications of chemotherapy. A total of 21 days constituted 1 cycle. After neoadjuvant chemotherapy, radical resection of CRC was performed within a definite time (6–8 weeks after the last neoadjuvant chemotherapy).
Efficacy evaluation
According to the Response Evaluation Criteria in Solid Tumors 1.1 (RECIST 1.1), the magnetic resonance imaging (MRI) examination result and pathological findings after neoadjuvant chemotherapy, the patients’ response to neoadjuvant chemotherapy was evaluated. Complete response (CR) meant complete disappearance of lesions for a duration of more than 4 weeks; partial response (PR) meant that the sum of the maximum diameters of the target lesions had decreased by more than 30% compared with the baseline level, for a duration of more than 4 weeks, and no new lesions had appeared; stable disease (SD) was between progressive disease (PD) and PR; PD meant that the sum of the maximum diameters of the target lesions had increased by more than 20% or new had lesions appeared. Objective response rate (ORR) = CR rate + PR rate.
Study variables
Age at diagnosis, gender, body mass index (BMI), diabetes, tumor size, lymph node metastasis, number of lymph node dissection, CEA level, ADC level, leukocyte number, neutrophil number, lymphocyte number, albumin level, primary site, degree of differentiation, pathological type, vascular tumor thrombus, invasion of adjacent tissue, and 5-year mortality.
Detection methods
Examination instrument: Siemens 3.0T MRI (Siemens, Germany), contrast agent: gadolinium gluconate, dose: 0.2 mmol/kg, forearm intravenous injection, flow rate: 2.5 mL/s. The images were collected for 6 consecutive times before and after enhancement to evaluate the ADC (total tumor volume analysis, b values were set to 50, 400, and 800 s/mm2).
Statistical analysis
The software SPSS 26.0 (IBM Corp., Armonk, NY, USA) was used to complete the data analysis of this study. A two-tailed P<0.05 indicated that the difference was statistically significant. The measurement data of the two groups of patients were expressed by mean ± standard deviation, and the differences between the two groups were analyzed by independent sample t-test; the counting data of the two groups were expressed by n (%), and the difference between the two groups was analyzed by chi-square test; the predictive value of different biological indicators on the efficacy of neoadjuvant chemotherapy and 5-year survival rate after neoadjuvant chemotherapy was analyzed using the receiver operating characteristic (ROC) curve, and the value of the point closest to the upper left of the ROC curve was the best diagnostic threshold; multivariate logistic regression analysis was used to explore the influencing factors of objective response after neoadjuvant chemotherapy.
Results
Comparison of clinical characteristics of the two groups
Compared with the control group, in the objective response group the tumor size was significantly lower (3.32±1.60 vs. 5.07±2.19 cm, P=0.000); the ADC was significantly higher (1.23±0.18 vs. 0.98±0.18 ×10−3 mm2/s, P=0.000); albumin was significantly higher (39.32±4.14 vs. 37.46±4.18 g/L, P=0.016); the proportion of patients with poorly differentiated or undifferentiated tumor cells was significantly lower (51.25% vs. 72.92%, P=0.016); and the 5-year mortality was significantly lower (40.00% vs. 58.33%, P=0.044) (Table 1).
Table 1
| Variables | Objective response group (n=80) | Control group (n=48) | t/χ2 value | P value |
|---|---|---|---|---|
| Age at diagnosis (years) | 57.86±11.34 | 60.31±12.33 | 1.145 | 0.254 |
| BMI (kg/m2) | 24.69±3.64 | 23.60±3.81 | 1.624 | 0.107 |
| Tumor size (cm) | 3.32±1.60 | 5.07±2.19 | 5.230 | 0.000 |
| Lymph node metastasis | 0.333 | 0.563 | ||
| Yes | 67 (83.75) | 42 (87.50) | ||
| No | 13 (16.25) | 6 (12.50) | ||
| Lymph node dissection number | 13.56±5.27 | 15.33±6.42 | 1.694 | 0.093 |
| CEA (U/mL) | 255.79±147.70 | 210.75±121.68 | 1.780 | 0.077 |
| ADC (×10−3 mm2/s) | 1.23±0.18 | 0.98±0.18 | 7.810 | 0.000 |
| Leukocyte number (×109/L) | 5.36±2.34 | 6.14±1.83 | 1.969 | 0.051 |
| Neutrophil number (%) | 0.64±0.11 | 0.63±0.12 | 0.490 | 0.625 |
| Lymphocyte number (%) | 0.23±0.10 | 0.26±0.10 | 1.465 | 0.145 |
| Ratio of neutrophil number to lymphocyte number | 3.79±2.69 | 3.33±2.61 | 0.933 | 0.353 |
| Albumin level (g/L) | 39.32±4.14 | 37.46±4.18 | 2.453 | 0.016 |
| Gender | 0.460 | 0.498 | ||
| Male | 61 (76.25) | 34 (70.83) | ||
| Female | 19 (23.75) | 14 (29.17) | ||
| Diabetes | 2.743 | 0.098 | ||
| Yes | 7 (8.75) | 9 (18.75) | ||
| No | 73 (91.25) | 39 (81.25) | ||
| Primary site | 0.212 | 0.645 | ||
| Colon | 23 (28.75) | 12 (25.00) | ||
| Rectum | 57 (71.25) | 36 (75.00) | ||
| Degree of differentiation | 5.839 | 0.016 | ||
| Low differentiated and undifferentiated | 41 (51.25) | 35 (72.92) | ||
| Moderately and well differentiated | 39 (48.75) | 13 (27.08) | ||
| Pathological type | 0.723 | 0.395 | ||
| Adenocarcinoma | 75 (93.75) | 43 (89.58) | ||
| Non-adenocarcinoma | 5 (6.25) | 5 (10.42) | ||
| Vascular tumor thrombus | 0.035 | 0.853 | ||
| Yes | 32 (40.00) | 20 (41.67) | ||
| No | 48 (60.00) | 28 (58.33) | ||
| Invasion of adjacent tissue | 0.332 | 0.564 | ||
| Yes | 26 (32.50) | 18 (37.50) | ||
| No | 54 (67.50) | 30 (62.50) | ||
| 5-year mortality | 4.049 | 0.044 | ||
| Yes | 32 (40.00) | 28 (58.33) | ||
| No | 48 (60.00) | 20 (41.67) |
Data are presented as mean ± standard deviation or n (%). BMI, body mass index; CEA, carcinoembryonic antigen; ADC, apparent diffusion coefficient.
The predictive value of different biological indicators on the efficacy of neoadjuvant chemotherapy in patients with locally advanced CRC
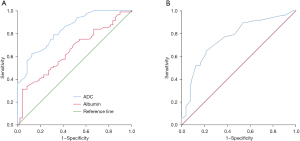
ADC had the highest predictive value of objective response for locally advanced CRC patients after neoadjuvant chemotherapy, with an area under the curve (AUC) of 0.834 [95% confidence interval (CI): 0.765–0.903, P=0.000]; the best diagnostic threshold was 1.055×10−3 mm2/s, and the specificity and sensitivity were 0.687 and 0.788, respectively (Table 2, Figure 2).
Table 2
| Variables | Areas under the curve | Standard error | P value | 95% CI | Diagnostic threshold | Sensitivity | Specificity |
|---|---|---|---|---|---|---|---|
| ADC | 0.834 | 0.035 | 0.000 | 0.765–0.903 | 1.055×10−3 mm2/s | 0.788 | 0.687 |
| Albumin | 0.648 | 0.049 | 0.005 | 0.552–0.744 | 37.9 g/L | 0.663 | 0.542 |
| Tumor size | 0.761 | 0.045 | 0.000 | 0.674–0.848 | 4.1 cm | 0.646 | 0.787 |
ADC, apparent diffusion coefficient; CRC, colorectal cancer; CI, confidence interval.
Factors influencing the objective response of locally advanced CRC patients after neoadjuvant chemotherapy
ADC >1.055×10−3 mm2/s, tumor size <4.1 cm, and moderately or well differentiated tumors were favorable factors for patients with locally advanced CRC to obtain objective response after neoadjuvant chemotherapy (P<0.05) (Table 3).
Table 3
| Category | B | Standard error | Wald | P value | Relative risk (95% CI) |
|---|---|---|---|---|---|
| ADC >1.055×10−3 mm2/s | 2.203 | 0.500 | 19.402 | 0.000 | 9.050 (3.396–24.115) |
| Tumor size <4.1 cm | 1.561 | 0.473 | 10.875 | 0.001 | 4.762 (1.884–12.042) |
| Moderately or well differentiated tumor | 1.521 | 0.542 | 7.871 | 0.005 | 4.576 (1.581–13.241) |
| Albumin >37.9 g/L | 0.562 | 0.485 | 1.341 | 0.247 | 1.754 (0.678–4.540) |
| Constant | −9.213 | 1.756 | 27.539 | 0.000 | 0.000 |
CRC, colorectal cancer; CI, confidence interval; ADC, apparent diffusion coefficient.
The predictive value of ADC for 5-year survival of locally advanced CRC patients
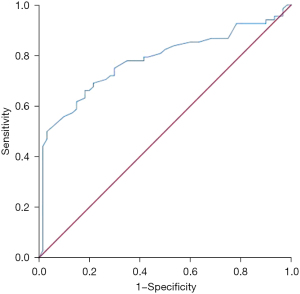
ADC had certain predictive value for the 5-year survival of locally advanced CRC patients, with an AUC of 0.778 (95% CI: 0.696–0.861, P=0.000). The best diagnostic threshold was 1.165×10−3 mm2/s, and the sensitivity and specificity were 0.691 and 0.783, respectively (Figure 3).
Discussion
The incidence rate and mortality of CRC is high, and it is one of the major diseases threatening the lives of middle-aged and elderly people. This study explored the predictive value of ADC on the efficacy of neoadjuvant chemotherapy in locally advanced CRC patients. The results showed that ADC had good predictive value on the efficacy of neoadjuvant chemotherapy in locally advanced CRC patients. ADC >1.055×10−3 mm2/s was a favorable factor for locally advanced CRC patients to achieve objective response after neoadjuvant chemotherapy (P<0.05). Moreover, the ADC had certain predictive value for the 5-year survival rate.
The ADC is currently widely used in the diagnosis and treatment of malignant tumors. Its theoretical basis is that the ADC can reflect the diffusion speed of water molecules in the tissue. When the cell density is low, the water molecules move fast, so the ADC is relatively high. When the cell density is high, the diffusion speed of water molecules decreases, so the ADC level decreases. In addition, the ADC also reflects the integrity of cell matrix and cell membrane in tissues. Due to the high cell density in solid malignant tumor tissue, the ADC in malignant tumor tissue is often in a reduced state. At present, the ADC is used to predict the prognosis of patients with malignant tumor (19-21), and has also been reported in patients with CRC (18). In the field of neoadjuvant chemotherapy, studies have also confirmed that the ADC is significantly related to the efficacy of neoadjuvant chemotherapy in patients with breast cancer and osteosarcoma (16,22,23). However, in patients with CRC, research is relatively insufficient. A study showed that the ADC had better recognition ability for patients with CRC complicated with distant metastasis after chemotherapy (24), and also showed that the ADC had certain predictive value for the curative effect after chemotherapy in patients with CRC with liver metastasis (13). These studies supported this study and suggested that the ADC could be used as a predictor of the efficacy of neoadjuvant chemotherapy for locally advanced CRC patients. However, the cases in this study were locally advanced CRC patients receiving neoadjuvant chemotherapy, which is different from the above studies. In addition, this study also showed that the ADC was significantly correlated with the long-term prognosis of patients, and the patients with higher ADC had better prognosis. A high ADC indicated that the tumor density was low, whereas a low ADC indicated that the tumor cell density was relatively high. Therefore, patients with low ADC are relatively prone to metastasis, leading to poor prognosis of patients. This had been confirmed in patients with CRC and other malignant tumors (18,25-27), supporting this study.
Limitations
This study was a retrospective clinical study with limited sample size. This study failed to explore the dynamic change of ADC during neoadjuvant chemotherapy.
Conclusions
The ADC could be used as a predictive index of the efficacy of neoadjuvant chemotherapy in locally advanced CRC patients. Combined with the ADC, clinicians can better evaluate the efficacy of neoadjuvant chemotherapy, providing a theoretical basis for clinicians to choose appropriate treatment plans.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-124/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-124/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-124/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-124/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of The First Affiliated Hospital of Xiamen University (No. c202200174) and individual informed consent was waived in this retrospective study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]
- Sugimachi K, Sakimura S, Kuramitsu S, et al. Serial mutational tracking in surgically resected locally advanced colorectal cancer with neoadjuvant chemotherapy. Br J Cancer 2018;119:419-23. [Crossref] [PubMed]
- Lambdin J, Ryan CE, Alejandro RH, et al. Neoadjuvant Chemotherapy Plus Living Donor Transplantation (LDLT) for Non-Resectable Liver Metastases from Colorectal Cancer (CRC). Ann Surg Oncol 2023;30:18-20. [Crossref] [PubMed]
- Huang L, Xu X, Shao J, et al. Efficacy of Laparoscopic Radical Resection Combined with Neoadjuvant Chemotherapy and Its Impact on Long-Term Prognosis of Patients with Colorectal Cancer. Evid Based Complement Alternat Med 2022;2022:4774531. [Crossref] [PubMed]
- Zhang Y, Ge L, Weng J, et al. Neoadjuvant chemotherapy for patients with resectable colorectal cancer liver metastases: A systematic review and meta-analysis. World J Clin Cases 2021;9:6357-79. [Crossref] [PubMed]
- Zhang C, Wang X, Han J, et al. Histological tumor response to neoadjuvant chemotherapy correlates to Immunoscore in colorectal cancer liver metastases patients. J Surg Oncol 2021;124:1431-41. [Crossref] [PubMed]
- Yang K, Zhang F, Han P, et al. Metabolomics approach for predicting response to neoadjuvant chemotherapy for colorectal cancer. Metabolomics 2018;14:110. [Crossref] [PubMed]
- Qi A, Li Y, Yan S, et al. Effect of postoperative chemotherapy on blood glucose and lipid metabolism in patients with invasive breast cancer. Gland Surg 2021;10:1470-7. [Crossref] [PubMed]
- Qi A, Li Y, Yan S, et al. Effect of anthracycline-based postoperative chemotherapy on blood glucose and lipid profiles in patients with invasive breast cancer. Ann Palliat Med 2021;10:5502-8. [Crossref] [PubMed]
- Egbert L, Norain A, Stucky CC, et al. Cancer embryonic antigen (CEA) levels in patients with appendiceal adenocarcinoma predict response to neo-adjuvant chemotherapy and overall survival. J Surg Oncol 2023;127:688-98. [Crossref] [PubMed]
- Shan J, Gu B, Shi L, et al. Prognostic value of CEA and CA19-9 in patients with local advanced rectal cancer receiving neoadjuvant chemoradiotherapy, radical surgery and postoperative chemotherapy. Transl Cancer Res 2021;10:88-98. [Crossref] [PubMed]
- Stremitzer S, Stift J, Graf A, et al. CEA change after neoadjuvant chemotherapy including bevacizumab and clinical outcome in patients undergoing liver resection for colorectal liver metastases. Ann Surg Oncol 2015;22:1315-23. [Crossref] [PubMed]
- Drewes R, Pech M, Powerski M, et al. Apparent Diffusion Coefficient Can Predict Response to Chemotherapy of Liver Metastases in Colorectal Cancer. Acad Radiol 2021;28:S73-80. [Crossref] [PubMed]
- Chen Y, Wang J, Zhang X, et al. Correlation between apparent diffusion coefficient and pathological characteristics of patients with invasive breast cancer. Ann Transl Med 2021;9:143. [Crossref] [PubMed]
- Geng X, Zhang D, Suo S, et al. Using the apparent diffusion coefficient histogram analysis to predict response to neoadjuvant chemotherapy in patients with breast cancer: comparison among three region of interest selection methods. Ann Transl Med 2022;10:323. [Crossref] [PubMed]
- Wang J, Sun M, Liu D, et al. Correlation between apparent diffusion coefficient and histopathology subtypes of osteosarcoma after neoadjuvant chemotherapy. Acta Radiol 2017;58:971-6. [Crossref] [PubMed]
- Qiu Y, Chen H, Dai Y, et al. Nontherapeutic Risk Factors of Different Grouped Stage IIIC Breast Cancer Patients' Mortality: A Study of the US Surveillance, Epidemiology, and End Results Database. Breast J 2022;2022:6705052. [Crossref] [PubMed]
- Zheng X, Lu J, Zhang H, et al. Apparent diffusion coefficient is a good marker in predicting the prognosis in colorectal cancer liver metastases: a diagnostic study. J Gastrointest Oncol 2022;13:2375-81. [Crossref] [PubMed]
- Oh HC, Hong CK, Yoo J, et al. The role of apparent diffusion coefficient as a predictive factor for tumor recurrence in patients with cerebellopontine angle epidermoid tumor. Neurosurg Rev 2022;45:1383-92. [Crossref] [PubMed]
- Xiao M, Ma X, Ma F, et al. Whole-tumor histogram analysis of apparent diffusion coefficient for differentiating adenosquamous carcinoma and adenocarcinoma from squamous cell carcinoma in patients with cervical cancer. Acta Radiol 2022;63:1415-24. [Crossref] [PubMed]
- Haopeng P, Xuefei D, Zengai C, et al. High-Resolution Diffusion-Weighted Imaging of C6 Glioma on a 7T BioSpec MRI Scanner: Correlation of Tumor Cellularity and Nuclear-to-Cytoplasmic Ratio with Apparent Diffusion Coefficient. Acad Radiol 2022;29:S80-7. [Crossref] [PubMed]
- Surov A, Wienke A, Meyer HJ. Pretreatment apparent diffusion coefficient does not predict therapy response to neoadjuvant chemotherapy in breast cancer. Breast 2020;53:59-67. [Crossref] [PubMed]
- Graña-López L, Herranz M, Maciñeira FA, et al. Apparent diffusion coefficient: Potential biomarker for complete response after neo-adjuvant chemotherapy in breast cancer. Breast J 2020;26:306-8. [Crossref] [PubMed]
- Lavdas I, Rockall AG, Daulton E, et al. Histogram analysis of apparent diffusion coefficient from whole-body diffusion-weighted MRI to predict early response to chemotherapy in patients with metastatic colorectal cancer: preliminary results. Clin Radiol 2018;73:832.e9-16. [Crossref] [PubMed]
- Shaish H, Casals R, Ahmed F, et al. Impact of mandated prospectively reported apparent diffusion coefficient values on the rates of positivity for clinically significant prostate cancer by PI-RADS score. Acta Radiol 2021;62:139-44. [Crossref] [PubMed]
- Soydan L, Demir AA, Torun M, et al. Use of Diffusion-Weighted Magnetic Resonance Imaging and Apparent Diffusion Coefficient in Gastric Cancer Staging. Curr Med Imaging 2020;16:1278-89. [Crossref] [PubMed]
- Ao W, Bao X, Mao G, et al. Value of Apparent Diffusion Coefficient for Assessing Preoperative T Staging of Low Rectal Cancer and Whether This Is Correlated With Ki-67 Expression. Can Assoc Radiol J 2020;71:5-11. [Crossref] [PubMed]
(English Language Editor: J. Jones)



