
Immune-related adverse events as independent prognostic factors for camrelizumab in patients with esophageal squamous cell carcinoma: a retrospective cohort study
Highlight box
Key findings
• irAEs are the prognostic indicators of outcome for camrelizumab (anti-PD-1 antibody) treatment in patients with ESCC.
What is known and what is new?
• The effectiveness of immunotherapy in advanced ESCC is heterogeneous, and there is currently a lack of reliable and practical biomarkers for identifying and evaluating treatment outcomes. There is controversy surrounding the predictive value of biomarkers such as PD-L1 expression and TMB, and they are inconvenient and costly to obtain. Recent studies have demonstrated a positive correlation between the efficacy of ICIs and the occurrence of irAEs in patients with other types of solid tumors.
• IrAEs may serve as a promising and ideal marker for assessing the efficacy of immunotherapy in patients with advanced ESCC.
What is the implication, and what should change now?
• There is a strong correlation between the prevalence of irAEs and better clinical outcomes in patients with ESCC treated with camrelizumab, indicating that recognizing and monitoring irAEs throughout anti-PD-1 therapy is vitally important. When irAEs occur, effective intervention should be given immediately to prevent severe adverse reactions and improve outcomes.
Introduction
In 2020, esophageal carcinoma (EC) ranked as the sixth most fatal malignancy and the seventh most frequently diagnosed cancer (1). The most frequent histologic subtype of EC is esophageal squamous cell carcinoma (ESCC), which accounts for more than 90% of EC cases (2). Despite improvements in surgical techniques and treatment strategies, such as the use of platinum-based doublet systemic chemotherapy as the standard of care (3,4), the mortality associated with advanced ESCC remains severe, with a 5-year overall survival (OS) rate of less than 20% (5).
Immune checkpoint inhibitors (ICIs) that target programmed cell death protein 1 (PD-1) and programmed death ligand 1 (PD-L1) have completely changed the landscape in which patients with ESCC have been treated over the past decade. Trials studying the PD-1/PD-L1 pathway have demonstrated prolonged survival and safety benefits with anti-PD-1 antibodies compared with chemotherapy in patients with advanced ESCC. In the KEYNOTE-181 study, pembrolizumab increased the OS in the PD-L1 combined positive score (CPS) ≥10 subgroups as compared to chemotherapy (6). In the ATTRACTION-3 trial, regardless of PD-L1 expression, nivolumab demonstrated a statistically significant and clinically meaningful improvement in OS compared to chemotherapy in patients with advanced ESCC (7). In the ESCORT study, camrelizumab significantly increased OS in patients with advanced or metastatic ESCC compared to chemotherapy (8). Overall, PD-1 monotherapy has become the standard of care for second-line advanced ESCC.
However, only a small percentage of people can benefit from ICIs, and the effectiveness of anti-PD-1 treatment differs among individuals (9). The rate of response to PD-1 inhibitors in patients previously treated for ESCC is relatively low compared to other cancer types (10). Despite the emerging evidence indicating that anti-PD-1 therapies may be beneficial for patients with positive PD-L1 expression, high tumor mutation burden (TMB), and high microsatellite instability (MSI-H) (11-13), the optimum prognostic biomarkers for ESCC are lacking. PD-L1 is well-known for predicting the efficacy of immunotherapy, it directly reflects the immune status of tumors and has predictive value in many types of cancer, with simple detection methods. However, PD-L1 expression is influenced by multiple factors, such as detection methods, tissue source, sampling time and method, and the determination of PD-L1 expression threshold is controversial. In addition, some PD-L1-negative patients may also benefit from immunotherapy, so PD-L1 cannot be used as a single predictive indicator. Tumors with high TMB usually have more neoantigens, which can induce stronger immune responses. However, TMB detection methods vary and different methods may yield different results. The assessment of TMB is expensive and time-consuming, making it difficult to meet clinical needs. MSI-H is rare in patients with ESCC, so routine testing for MSI alone is not realistic, despite the potential value to those rare patients. Establishing easily accessible, cost-effective predictive factors to recognize patients with ESCC who might benefit from PD-1 inhibition is therefore urgently needed.
Recent research has shown some correlations between the effects of ICIs and immune-related adverse events (irAEs) (14). IrAEs are defined as inflammatory adverse effects of immune system activation that affect the skin, liver, lungs, and endocrine glands (15,16). The presence of irAEs was associated with positive responses and prolonged survival in patients with upper gastrointestinal cancer receiving ICIs therapies in a retrospective study (17), but the solid proof in ESCC is lacking.
We thus conducted a retrospective study to establish whether there is an association between irAEs and the treatment efficacy of camrelizumab [a humanized immunoglobin G4 (IgG4) monoclonal antibody against the PD-1 receptor] in patients with advanced ESCC in terms of antitumor response and survival. We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-75/rc).
Methods
Study design and patients
We conducted a single-center, retrospective cohort study from January 13, 2019, to February 5, 2022, at the China-Japan Union Hospital of Jilin University. The inclusion criteria were ICI-naive patients who had recurrent or metastatic ESCC and who were treated with single-agent camrelizumab for at least 1 dose; those treated previously with targeted therapy or chemotherapy were also deemed eligible. No patients were excluded. The sample size was determined by the number of cases that fulfilled the inclusion criteria over the study period. All patients were followed up until May 28, 2022. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of China-Japan Union Hospital of Jilin University (No. 20220418). Individual consent for this retrospective analysis was waived.
Data collection
The clinical data collected were as follows: sex, age, previous therapy, site of metastases, number of prior therapies, Eastern Cooperative Oncology Group (ECOG) performance status, number of prior therapies, the PD-L1 CPS [defined as the number of PD-L1-positive cells (tumor cells, macrophages, and lymphocytes) divided by the total number of tumor cells], and smoking status (current, former, or never).
End points and follow-up
Patients were treated with camrelizumab at a fixed dose of 200 mg every 21 days. The primary end point was objective response rate (ORR) as per Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 and was defined as the percentage of patients who achieved a complete response (CR) or a partial response (PR) as the best overall response assessed by investigators. The secondary end points included disease control rate (DCR), which was defined as the proportion of patients who had a CR, PR, or stable disease (SD); OS, which was defined as the duration from the initiation of anti-PD-1 treatment to death from any cause; and safety profile. Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.0. Clinical and analytical evaluations were performed every 3 weeks after the start of treatment. Every 8 to 12 weeks, or as often as clinically necessary, patients underwent enhanced computed tomography (CT) scans. Patients were contacted every 12 weeks to assess survival during follow-up. During these visits, physical examinations, routine laboratory tests, and imaging studies were performed. For patients who could not be contacted, we supplemented their information by reviewing their electronic medical records and contacting their family members. We also conducted multiple confirmations and verifications for any missing data. Throughout the entire follow-up process, we adhered to the same follow-up procedures to ensure accuracy and completeness of the data.
Statistical analysis
Continuous variables are summarized using medians and ranges (minimum and maximum). Categorical variables are summarized using frequencies and proportions and were compared using Fisher exact test or chi-squared test. Odds ratio (OR) and chi-squared test were used to identify any relationships between the occurrence of irAEs and ORR. OS was calculated using the Kaplan-Meier curves and was compared with log-rank (Mantel-Cox) test. We utilized Cox regression analysis adjusted for age and sex (in order to reduce potential bias) to investigate the correlation between the occurrence of immune-related adverse events (irAEs) and OS. Results are expressed as hazard ratios (HRs) with a 95% confidence interval (CI). Univariate and multivariate analyses were conducted to identify prognostic factors for OS. Factors with a univariate P value less than 0.1 were included in multivariate analysis. Statistical analysis was performed with R software version 4.1.1 (R Foundation for Statistical Computing). P<0.05 (two-sided) was considered statistically significant.
Results
Patient characteristics
From January 13, 2019, to February 5, 2022, 136 consecutive patients were included. As of May 28, 2022, the median follow-up was 26 months (range, 3–32 months). Table 1 shows the characteristics of all study participants. The median age was 60 years (range, 32–84 years). Males accounted for 81.6% of the sample population, 89.7% of patients received platinum-based chemotherapy as first-line therapy, and 106 patients (77.9%) had lymph node metastases at the time of diagnosis. The ECOG score was 0 in 24 patients (17.6%), 1 in 102 patients (75.0%), and 2 in 10 (7.4%). Baseline characteristics were well balanced between the 2 groups with or without irAEs.
Table 1
| Characteristic | All patients (n=136) | Non-irAEs (n=55) | irAEs (n=81) | P value |
|---|---|---|---|---|
| Sex | 0.103 | |||
| Female | 25 (18.4) | 6 (10.9) | 19 (23.5) | |
| Male | 111 (81.6) | 49 (89.1) | 62 (76.5) | |
| Age (years) | 0.912 | |||
| <65 | 77 (56.6) | 31 (56.4) | 46 (56.8) | |
| ≥65 | 59 (43.4) | 24 (43.6) | 35 (43.2) | |
| Previous therapy | ||||
| Surgery | 50 (36.8) | 16 (29.1) | 34 (42.0) | 0.178 |
| Radiotherapy | 87 (64.0) | 33 (60.0) | 54 (66.7) | 0.540 |
| First-line platinum-based chemotherapy | 122 (89.7) | 53 (96.4) | 69 (85.2) | 0.069 |
| Site of metastases | ||||
| Liver | 31 (22.8) | 17 (30.9) | 14 (17.3) | 0.099 |
| Lung | 58 (42.6) | 24 (43.6) | 34 (42.0) | 0.988 |
| Bone | 18 (13.2) | 5 (9.1) | 13 (16.0) | 0.359 |
| Lymph node | 106 (77.9) | 42 (76.4) | 64 (79.0) | 0.877 |
| Other | 33 (24.3) | 13 (23.6) | 20 (24.7) | 0.937 |
| ECOG performance status | 0.829 | |||
| 0 | 24 (17.6) | 9 (16.4) | 15 (18.5) | |
| 1 | 102 (75.0) | 41 (74.5) | 61 (75.3) | |
| 2 | 10 (7.4) | 5 (9.1) | 5 (6.2) | |
| Number of prior therapies | 0.272 | |||
| 1 | 83 (61.0) | 30 (54.5) | 53 (65.4) | |
| ≥2 | 53 (39.0) | 25 (45.5) | 28 (34.6) | |
| PD-L1 combined positive score | 0.965 | |||
| <10 | 56 (41.2) | 22 (40.0) | 34 (42.0) | |
| ≥10 | 66 (48.5) | 27 (49.1) | 39 (48.1) | |
| Not evaluable | 14 (10.3) | 6 (10.9) | 8 (9.9) | |
| Smoking status | 0.982 | |||
| Former | 41 (30.1) | 17 (30.9) | 24 (29.6) | |
| Current | 77 (56.6) | 31 (56.4) | 46 (56.8) | |
| Never | 18 (13.2) | 7 (12.7) | 11 (13.6) |
Data are presented as n (%), unless otherwise specified. ECOG, Eastern Cooperative Oncology Group; PD-L1, programmed death ligand-1; irAE, immune-related adverse event.
irAEs
A total of 128 irAEs were observed in 81 out of 136 patients (59.6%). The details are shown in Table 2. The most frequent irAE was reactive cutaneous capillary endothelial proliferation (RCCEP) solely caused by camrelizumab (n=48 events; 45 cases in grade 1–2, 3 cases in grade 3), followed by aspartate aminotransferase (AST) increase (n=12; all in grade 1), diarrhea (n=10), hypothyroidism (n=10), and fatigue (n=10). Of the 136 patients, 22 (16.2%) had interrupted treatment, and 9 (6.6%) of them discontinued anti-PD-1 treatment owing to irAEs. No treatment-related deaths occurred during the follow-up.
Table 2
| Immune-related adverse event | Grade 1–2 | Grade 3–5 |
|---|---|---|
| RCCEP | 45 (33.1) | 3 (2.2) |
| AST increased | 12 (8.8) | 0 (0.0) |
| Diarrhea | 10 (7.4) | 3 (2.2) |
| Hypothyroidism | 10 (7.4) | 2 (1.5) |
| Fatigue | 10 (7.4) | 3 (2.2) |
| Decreased appetite | 7 (5.1) | 2 (1.5) |
| Nausea | 7 (5.1) | 1 (0.7) |
| Vomiting | 3 (2.2) | 1 (0.7) |
| Stomatitis | 2 (1.5) | 0 (0.0) |
| Constipation | 2 (1.5) | 0 (0.0) |
| Weight decreased | 1 (0.7) | 0 (0.0) |
| WBC count decreased | 1 (0.7) | 0 (0.0) |
| Neutrophil count decreased | 1 (0.7) | 0 (0.0) |
Data are presented as n (%). Listed are immune-related adverse events that occurred during the study period or within 30 days thereafter (within 90 days for events higher than grade 3). RCCEP, reactive cutaneous capillary endothelial proliferation; AST, aspartate aminotransferase; WBC, white blood cell.
Treatment efficacy
Of the 136 patients, objective response was observed in 40 patients (29.4%): CR in 10 cases (7.4%) and PR in 30 (22.1%). SD was detected in 44 cases (32.4%), progressive disease was detected in 52 cases (38.2%), and 84 patients (61.8%) achieved disease control (Table 3). The median OS for all patients was 8.4 (95% CI: 6.1–12.9) months.
Table 3
| Variables | All patients (n=136) | Non-irAEs (n=55) | irAEs (n=81) | P value |
|---|---|---|---|---|
| Best overall response | 0.001 | |||
| Complete response | 10 (7.4) | 4 (7.3) | 6 (7.4) | |
| Partial response | 30 (22.1) | 4 (7.3) | 26 (32.1) | |
| Stable disease | 44 (32.4) | 18 (32.7) | 26 (32.1) | |
| Progressive disease | 52 (38.2) | 29 (52.7) | 23 (28.4) | |
| Objective response rate | 40 (29.4; 21.9–37.8) | 8 (14.5; 6.50–26.7) | 32 (39.5; 28.8–51.0) | 0.003 |
| Disease control | 84 (61.8; 53.0–70.0) | 26 (47.3; 33.7–61.2) | 58 (71.6; 60.5–81.1) | 0.007 |
Data are n (%; 95% CI) or n (%), unless stated otherwise. Percentages might not add up to 100% due to rounding. CI, confidence interval; irAE, immune-related adverse event; PD-1, programmed cell death-1.
Correlation of irAEs with treatment efficacy
Patients who presented irAEs showed an increase in the probability of achieving an objective response (OR =3.84; 95% CI: 1.60–9.18; P=0.003). As shown in Table 3, 32 of the 81 (39.5%) patients who experienced toxicity demonstrated an objective response, while this occurred in only 8 of the 55 (14.5%) patients who did not experience toxicity (P=0.003).
Figure 1 demonstrates that the OS of patients with and without irAEs was 13.5 (95% CI: 7.9–24.3) and 5.6 (95% CI: 3.0–8.5) months, respectively (adjusted HR =0.56, 95% CI: 0.41–0.76; P=0.0013). The 12- and 24-month survival rates for the patients with and without irAEs were 54.0% vs. 25.6% and 38.1% vs. 15.0%, respectively. In the subgroup of patients with PD-L1 CPS <10, patients who developed irAEs had a remarkably increased median OS when compared with patients without irAEs (13.5 vs. 2.3 months; adjusted HR =0.36, 95% CI: 0.19–0.68; P=0.0009), while in the subgroup of patients with PD-L1 CPS ≥10, patients who developed irAEs had a longer median OS over patients without irAEs (15.4 vs. 9.7 months), but this difference was not statistically significant (P=0.24). Details are shown in Figure 2 and Figure 3.

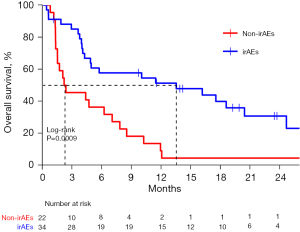
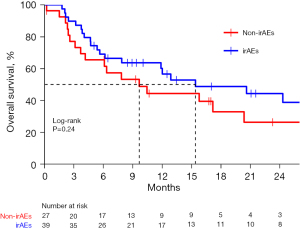
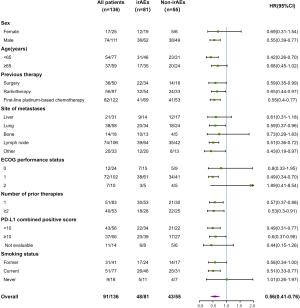
The correlation between irAEs and OS was significant (HR =0.57, 95% CI: 0.42–0.77; P=0.0002) in the multivariate analysis of OS and was unaffected by age, sex, ECOG, smoking status, prior lines of treatment, or any other demographic or clinical characteristic examined (Table 4). Moreover, the OS benefit with patients who developed irAEs was observed across almost all the subgroups (Figure 4).
Table 4
| Factors | Cases (n=136) | Overall survival | ||||
|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | |||||
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | |||
| Sex (male/female) | 111/25 | 1.22 (0.72–2.07) | 0.465 | – | – | |
| Age (≥65/<65 years) | 59/77 | 1.12 (0.74–1.71) | 0.588 | – | – | |
| Previous therapy | ||||||
| Surgery (yes/no) | 50/86 | 0.97 (0.64–1.48) | 0.883 | – | – | |
| Radiotherapy (yes/no) | 87/49 | 0.79 (0.52–1.21) | 0.283 | – | – | |
| First-line platinum-based chemotherapy (yes/no) | 122/14 | 0.99 (0.50–1.97) | 0.972 | – | – | |
| Site of metastases | ||||||
| Liver (yes/no) | 31/105 | 1.12 (0.69–1.82) | 0.652 | – | – | |
| Lung (yes/no) | 58/78 | 0.79 (0.52–1.19) | 0.258 | – | – | |
| Bone (yes/no) | 18/118 | 1.34 (0.75–2.37) | 0.322 | – | – | |
| Lymph node (yes/no) | 106/30 | 1.25 (0.74–2.12) | 0.409 | – | – | |
| Other (yes/no) | 33/103 | 0.76 (0.46–1.24) | 0.272 | – | – | |
| ECOG performance score | ||||||
| 0 | 24 | Ref | – | Ref | – | |
| 1 | 102 | 2.04 (1.1–3.76) | 0.023 | 2.01 (1.07–3.77) | 0.031 | |
| 2 | 10 | 2.44 (0.96–6.21) | 0.061 | 2.25 (0.88–5.78) | 0.091 | |
| Number of prior therapies (≥2/1) | 53/83 | 1.36 (0.90–2.06) | 0.145 | – | – | |
| PD-L1 combined positive score | ||||||
| <10 | 56 | Ref | – | Ref | – | |
| ≥10 | 66 | 1.55 (1.06–2.26) | 0.023 | 1.5 (1.02–2.22) | 0.041 | |
| Not evaluable | 14 | 1.00 (0.63–1.6) | 0.995 | 0.95 (0.59–1.52) | 0.833 | |
| Smoking status | ||||||
| Former | 41 | Ref | – | – | – | |
| Current | 77 | 0.96 (0.62–1.51) | 0.870 | – | – | |
| Never | 18 | 0.73 (0.35–1.54) | 0.413 | – | – | |
| Group (irAEs/non-irAEs) | 81/55 | 0.62 (0.46–0.83) | 0.002 | 0.57 (0.42–0.77) | 0.0002 | |
CI, confidence interval; irAE, immune-related adverse event; ECOG, Eastern Cooperative Oncology Group; PD-L1, programmed death ligand-1; Ref, reference.
Discussion
To the best of our knowledge, this is the first study to examine the presence of irAEs as a prognostic factor in patients with advanced ESCC receiving anti-PD-1 monotherapy. The development of irAEs was directly and significantly correlated with improved objective response and prolonged OS in the current study, regardless of sex and age, suggesting that irAEs may serve as prognostic indicators of immunotherapy effectiveness in patients with ESCC.
In addition to being effective against the tumor cells, PD-1 blockade may also cause autoimmunity, which might lead to mild or severe adverse reactions irAEs (18,19). Although the exact pathophysiology underlying the start of irAE is yet to be known, potential explanations include the stimulation of autoantibodies, overactivation of T cells, and an increase in cytokine levels (15,20). It has been discovered that irAEs that occur with particular malignancies are associated with better clinical outcomes. For instance, it has been demonstrated that vitiligo is strongly associated with successful outcomes in patients with melanoma (21-23), but other real-world research has fallen short of establishing clear relationships between irAEs and the efficacy of ICIs combined with chemotherapy or targeted therapy (24,25). We restricted our analysis to patients with advanced ESCC receiving single-agent camrelizumab in order to avoid this heterogeneity and enhance the study comparability.
Our study revealed a strong correlation between the incidence of irAEs and better clinical outcomes, including ORR, DCR, and OS, in patients with ESCC treated with camrelizumab. The odds of obtaining a CR or PR were nearly 4 times higher for those who experienced irAEs. Patients who experienced irAEs of any kind were 44% less likely to die than those who did not. In particular, in the subgroup of PD-L1 CPS <10, the risk of death was reduced by 51%. For those with PD-L1 CPS ≥10, a tendency for a longer median OS was observed but not statistically significant, perhaps due to the small sample size. In most subgroups, the risk of death was lower in patients who developed adverse reactions than in those who did not, especially in patients who had received prior platinum-based chemotherapy, were younger than 65 years of age, had lymph node metastases, or were former or current smokers. These findings suggest that irAEs are crucial in predicting the effectiveness of PD-1 treatment in patients with ESCC.
Interestingly, we found that patients with RCCEP had a remarkably better prognosis. The possible reason for this is the high incidence of RCCEP in patients treated with camrelizumab and the better-than-average prognosis in this group of patients. The appearance of RCCEP has been reported to be associated with good outcome in hepatocellular carcinoma (26). More studies are warranted to determine whether this particular irAE is associated with a better treatment effect.
Our study found that 59.6% of the patients experienced at least 1 irAE, which is comparable to findings from other trials using anti-PD-1 antibodies as the sole treatment for advanced ESCC (6-8). Furthermore, with timely and effective interventions, the majority of patients’ symptoms were mild and manageable. Only 22 (16.2%) of 136 patients experienced interrupted treatment, and 9 (6.6%) of these discontinued anti-PD-1 treatment due to irAEs. Neither unanticipated severe adverse events nor deaths associated with the treatment occurred. Our study featured a longer follow-up time and a higher percentage of patients with an ECOG performance score of 2, which more closely approximates actual clinical practice compared to other randomized controlled trials.
Our study highlights the need for recognizing and monitoring irAEs throughout anti-PD-1 therapies by showing the correlations between irAEs and improved immunological responses to anti-PD-1 antibodies. Patients with ESCC who develop moderate irAEs may fare better than those who do not. Severe irAEs, such as myocarditis and pneumonia, can occasionally be fatal (15,17,27,28), and patients who experience them may have to discontinue anti-PD-1 therapy. As a result, careful observation and early identification of irAEs might lead to less severe side effects (29,30), as would classifying patients with efficient immune response to PD-1 inhibitors (30) and preventing the development of irAEs into more serious adverse events (30). When irAEs are found in a patient, effective intervention should be given immediately to prevent adverse reactions from becoming more severe and to enhance patient outcomes (31). Our study provides insight into on the nuanced but significant role that irAEs play in the utilization of anti-PD-1 therapy in patients with ESCC, which may help to modernize ESCC treatment. Furthermore, with appropriate monitoring and the application of standardized treatment to recognize and address toxic effects, ICI rechallenge would be safe (32). A rechallenge of ICI therapy may be an option for those patients with ESCC who experience ≥ grade 2 irAEs (33).
The study has several limitations. First, it was a retrospective study. In order to reduce the heterogeneity, we conducted Cox regression adjusted by sex and age. Second, the sample size was limited. The results of subgroup analysis should be cautiously interpreted. Third, our analysis demonstrates correlations rather than causal results. More research is warranted to clarify the underlying mechanisms through which irAEs can predict the results of ICIs and to determine whether other biomarkers are related to the occurrence of irAEs.
Conclusions
This study found a statistically significant and clinically meaningful association between the presence of irAEs and a favorable prognosis for patients with advanced ESCC treated with anti-PD-1 antibodies, suggesting that irAEs might serve as clinical prognostic indicators for the therapeutic efficacy of anti-PD-1 antibodies in patients with ESCC.
Acknowledgments
The authors express their gratitude to the patients and their families for participating in this study, and would like to thank Xin Su for support with statistical analysis.
Funding: This work was supported by grants from the Science and Technology Development Project of Jilin Province (Nos. 20180101124JC and 20210401174YY), the Special Project for Health Research of Jilin Province (Nos. 2018SCZ031 and 2019SCZ055), the Health Technology Innovation Project of Jilin Province (No. 3D517ED43430), and the National Key R&D Program of China (No. 2018YFC0116901).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-75/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-75/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-75/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-75/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of China-Japan Union Hospital of Jilin University (No. 20220418). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Wang QL, Xie SH, Wahlin K, et al. Global time trends in the incidence of esophageal squamous cell carcinoma. Clin Epidemiol 2018;10:717-28. [Crossref] [PubMed]
- Wang X, Hobbs B, Gandhi SJ, et al. Current status and application of proton therapy for esophageal cancer. Radiother Oncol 2021;164:27-36. [Crossref] [PubMed]
- Oshikiri T, Yasuda T, Harada H, et al. A new method (the "Bascule method") for lymphadenectomy along the left recurrent laryngeal nerve during prone esophagectomy for esophageal cancer. Surg Endosc 2015;29:2442-50. [Crossref] [PubMed]
- Thrift AP. Global burden and epidemiology of Barrett oesophagus and oesophageal cancer. Nat Rev Gastroenterol Hepatol 2021;18:432-43. [Crossref] [PubMed]
- Kojima T, Shah MA, Muro K, et al. Randomized Phase III KEYNOTE-181 Study of Pembrolizumab Versus Chemotherapy in Advanced Esophageal Cancer. J Clin Oncol 2020;38:4138-48. [Crossref] [PubMed]
- Kato K, Cho BC, Takahashi M, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2019;20:1506-17. [Crossref] [PubMed]
- Huang J, Xu J, Chen Y, et al. Camrelizumab versus investigator's choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol 2020;21:832-42. [Crossref] [PubMed]
- Humphries MP, McQuaid S, Craig SG, et al. Critical Appraisal of Programmed Death Ligand 1 Reflex Diagnostic Testing: Current Standards and Future Opportunities. J Thorac Oncol 2019;14:45-53. [Crossref] [PubMed]
- Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun 2020;11:3801. [Crossref] [PubMed]
- Dudley JC, Lin MT, Le DT, et al. Microsatellite Instability as a Biomarker for PD-1 Blockade. Clin Cancer Res 2016;22:813-20. [Crossref] [PubMed]
- Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N Engl J Med 2018;378:2093-104. [Crossref] [PubMed]
- Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019;393:1819-30. [Crossref] [PubMed]
- Xu Y, Fu Y, Zhu B, et al. Predictive Biomarkers of Immune Checkpoint Inhibitors-Related Toxicities. Front Immunol 2020;11:2023. [Crossref] [PubMed]
- Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med 2018;378:158-68. [Crossref] [PubMed]
- Bagchi S, Yuan R, Engleman EG. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu Rev Pathol 2021;16:223-49. [Crossref] [PubMed]
- Hara Y, Baba Y, Toihata T, et al. Immune-related adverse events and prognosis in patients with upper gastrointestinal cancer treated with nivolumab. J Gastrointest Oncol 2022;13:2779-88. [Crossref] [PubMed]
- Weber JS, Yang JC, Atkins MB, et al. Toxicities of Immunotherapy for the Practitioner. J Clin Oncol 2015;33:2092-9. [Crossref] [PubMed]
- Chen KB, Wu ZW, Huang Y, et al. Successful outcome of neoadjuvant PD-1 blockade, VEGFR-2 inhibitor plus chemotherapy for potentially unresectable esophagogastric junctional squamous cell carcinoma: a case report. Transl Cancer Res 2022;11:3329-36. [Crossref] [PubMed]
- Baxi S, Yang A, Gennarelli RL, et al. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ 2018;360:k793. [Crossref] [PubMed]
- Hua C, Boussemart L, Mateus C, et al. Association of Vitiligo With Tumor Response in Patients With Metastatic Melanoma Treated With Pembrolizumab. JAMA Dermatol 2016;152:45-51. [Crossref] [PubMed]
- Serna-Higuita LM, Amaral T, Forschner A, et al. Association between Immune-Related Adverse Events and Survival in 319 Stage IV Melanoma Patients Treated with PD-1-Based Immunotherapy: An Approach Based on Clinical Chemistry. Cancers (Basel) 2021;13:6141. [Crossref] [PubMed]
- Geisler AN, Phillips GS, Barrios DM, et al. Immune checkpoint inhibitor-related dermatologic adverse events. J Am Acad Dermatol 2020;83:1255-68. [Crossref] [PubMed]
- Suh KJ, Kim SH, Kim YJ, et al. Post-treatment neutrophil-to-lymphocyte ratio at week 6 is prognostic in patients with advanced non-small cell lung cancers treated with anti-PD-1 antibody. Cancer Immunol Immunother 2018;67:459-70. [Crossref] [PubMed]
- Verzoni E, Cartenì G, Cortesi E, et al. Real-world efficacy and safety of nivolumab in previously-treated metastatic renal cell carcinoma, and association between immune-related adverse events and survival: the Italian expanded access program. J Immunother Cancer 2019;7:99. [Crossref] [PubMed]
- Wang F, Qin S, Sun X, et al. Reactive cutaneous capillary endothelial proliferation in advanced hepatocellular carcinoma patients treated with camrelizumab: data derived from a multicenter phase 2 trial. J Hematol Oncol 2020;13:47. [Crossref] [PubMed]
- Nishino M, Chambers ES, Chong CR, et al. Anti-PD-1 Inhibitor-Related Pneumonitis in Non-Small Cell Lung Cancer. Cancer Immunol Res 2016;4:289-93. [Crossref] [PubMed]
- Zhang Q, Tang L, Zhou Y, et al. Immune Checkpoint Inhibitor-Associated Pneumonitis in Non-Small Cell Lung Cancer: Current Understanding in Characteristics, Diagnosis, and Management. Front Immunol 2021;12:663986. [Crossref] [PubMed]
- Sears CR, Peikert T, Possick JD, et al. Knowledge Gaps and Research Priorities in Immune Checkpoint Inhibitor-related Pneumonitis. An Official American Thoracic Society Research Statement. Am J Respir Crit Care Med 2019;200:e31-43. [Crossref] [PubMed]
- Zhao Z, Wang X, Qu J, et al. Immune-Related Adverse Events Associated With Outcomes in Patients With NSCLC Treated With Anti-PD-1 Inhibitors: A Systematic Review and Meta-Analysis. Front Oncol 2021;11:708195. [Crossref] [PubMed]
- Thompson JA, Schneider BJ, Brahmer J, et al. Management of Immunotherapy-Related Toxicities, Version 1.2019. J Natl Compr Canc Netw 2019;17:255-89. [Crossref] [PubMed]
- Allouchery M, Lombard T, Martin M, et al. Safety of immune checkpoint inhibitor rechallenge after discontinuation for grade ≥2 immune-related adverse events in patients with cancer. J Immunother Cancer 2020;8:e001622. [Crossref] [PubMed]
- Dolladille C, Ederhy S, Sassier M, et al. Immune Checkpoint Inhibitor Rechallenge After Immune-Related Adverse Events in Patients With Cancer. JAMA Oncol 2020;6:865-71. [Crossref] [PubMed]
(English Language Editor: J. Gray)


