
Conservative treatment of hepatic portal vein gas after transarterial chemoembolization treatment for liver metastasis of postoperative esophageal cancer: a case report
Highlight box
Key findings
• High risk factors leading to hepatic portal vein gas (HPVG) after transarterial chemoembolization (TACE) treatment for liver metastasis of postoperative esophageal cancer include gas accumulation in the small intestine before TACE treatment, long-term enteral nutrition (EN), and premature initiation of EN after TACE treatment
What is known and what is new?
• The causes of HPVG include intestinal necrosis, gastrointestinal dilatation, and iatrogenic factors.
• HPVG can develop after TACE treatment for liver metastases, which can be cured after active conservative treatment.
What is the implication, and what should change now?
• Parenteral nutrition support should be implemented instead of early EN support for elderly patients who need long-term EN support after TACE treatment. If such patients manifest HPVG, early conservative treatment can facilitate prompt recovery and avoid surgical treatment.
Introduction
Hepatic portal vein gas (HPVG) is a rare imaging manifestation of gas accumulation in the portal vein and its branches, which is often accompanied by serious intestinal diseases such as intestinal obstruction and necrotizing enteritis. HPVG often suggests a poor prognosis and is strongly associated with high mortality rates (1). The causes of HPVG include intestinal necrosis, gastrointestinal dilatation, iatrogenic factors, and so on (2). Timely treatment is the key to treating HPVG. The treatment methods include surgery and conservative treatment, but there is still no consensus on which method to use for treatment. Our study reports a rare case of HPVG after transarterial chemoembolization (TACE) treatment for liver metastasis after esophageal cancer surgery, which long term use of intestinal nutrition tubes for nutritional support. We have adopted conservative treatments such as early gastrointestinal decompression, fasting, and anti-infection treatment for the patient and achieved rapid cure with HPVG. We present the following article in accordance with the CARE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-213/rc).
Case presentation
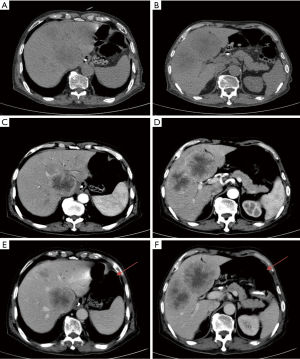
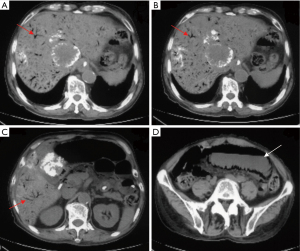
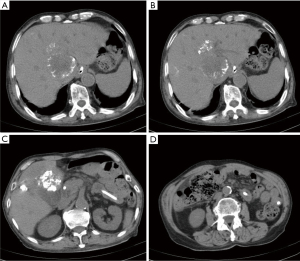
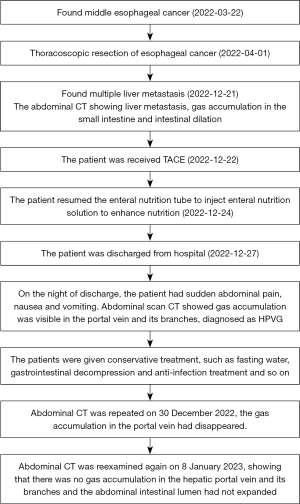
A 69-year-old man was diagnosed with middle esophageal cancer via gastroscopy on 22 March 2022, the pathology of which was poorly differentiated malignant tumor. Thoracoscopic resection of the esophageal cancer was performed in the Thoracic Surgery Department of Fourth Hospital of Hebei Medical University, Shijiazhuang, Hebei on 1 April 2022. The postoperative pathology indicated poorly differentiated adenocarcinoma with regional neuroendocrine differentiation. The patient developed anastomotic stoma-mediastinal fistula after surgery, and was administered symptomatic treatment including anti-infection, tracheal intubation, and enteral nutrition (EN) tube insertion. After active treatment, the anastomotic-mediastinal fistula healed, but due to prolonged postoperative endotracheal intubation, laryngoscopy revealed vocal cord paralysis on both sides. In order to reduce the risk of pneumonia aggravation caused by choking after eating, the thoracic surgeon decided not to remove the endotracheal tube and intestinal nutrition tube after consultation with the patient’s family, and the patient mainly used the intestinal nutrition tube for nutritional support. The patient underwent a repeat abdominal computed tomography (CT) scan on 21 December 2022, which revealed multiple round masses in the liver with borderline enhancement, and the patient was subsequently diagnosed with liver metastasis. The abdominal CT also revealed pneumatosis intestinalis and dilatation of the small intestine, although the imaging findings did not indicate intestinal obstruction (Figure 1). Examination revealed that only liver metastases were present; no other obvious metastases were found in other areas. After general discussion, the patient underwent TACE. Patient's daughter sign informed consent form. According to the pathological characteristics and imaging findings of the patient, we administered etoposide (0.1 g) and cisplatin (50 mg) perfusion chemotherapy during TACE, and used 15 mL lipiodol and 0.6 g embolic microsphere for tumor embolization. After the operation, the patient was provided with symptomatic treatment including hydration, liver protection, and nutritional support. The second day after TACE, because the patient had no obvious gastrointestinal symptoms such as nausea and vomiting, the EN tube was reinstated to enhance nutritional uptake via the injection of EN solution (about 1,000 mL of EN solution per day). The patient recovered smoothly after TACE and was discharged from hospital on 27 December 2022. On the night of discharge, the patient experienced sudden abdominal pain, nausea, and vomiting, and attended the emergency department of the local hospital. The physical examination showed that the abdomen was soft, peritoneal irritation was present, and bowel sounds were active. The abdominal plain scan CT in the local hospital showed that the abdominal intestinal lumen was significantly expanded, the liquid-gas plane shadow was visible, gas accumulation was visible in the portal vein and its branches, gas could also be seen in superior mesenteric vein,and lipiodol had deposited in the liver metastasis (Figure 2). The patient was diagnosed with HPVG and intestinal obstruction. The patient was transferred to the emergency department of Fourth Hospital of Hebei Medical University. Blood routine examination after admission revealed the following: white blood cell 9.19×109/L, neutrophil 88.87%, neutrophil 8.17×109/L, red blood cell 3.56×1012/L, and hemoglobin 111.4 g/L. Blood gas analysis showed a pH value of 7.491, carbon dioxide partial pressure of 34.2 mmHg, oxygen partial pressure of 32.3 mmHg, actual bicarbonate of 25.5 mmol/L, and standard bicarbonate of 26.4 mmol/L. The patient underwent water fasting and gastrointestinal decompression. To prevent serious infection caused by intestinal obstruction, anti-infection treatment was empirically administered, along with symptomatic treatment such as fluid replacement and correction of acid-base imbalance. About 4 hours after the attack of HPVG, the patient’s abdominal pain symptoms were significantly relieved. The physical examination showed that the abdomen was soft, the peritoneal irritation had disappeared, and the bowel sounds were still active. Since the patient’s abdominal pain symptoms were significantly reduced, the patient did not undergo further surgical treatment, and conservative treatment was continued. After the conservative treatment, abdominal CT was repeated on 30 December 2022, which revealed that the intestinal obstruction was relieved, and the gas accumulation in the portal vein had disappeared (Figure 3), which validated the effect of conservative treatment. Repeated blood routine revealed a white blood cell count of 4.88×109/L, neutrophil level of 75.79%, neutrophil count of 3.70×109/L, red blood cell count of 3.24×1012/L, and hemoglobin level of 100 g/L. Abdominal CT was reexamined again on 8 January 2023, showing that there was no gas accumulation in the hepatic portal vein and its branches, perihepatic effusion had disappeared, and the abdominal intestinal lumen had not expanded (Figure 4). The patient continued to receive treatment for bilateral lung inflammation after HPVG, and recovered well. The timeline of this case is shown in Figure 5.

All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient's daughter for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
The presence of HPVG was first reported in children in 1955, and the first adult case was described in 1960. HPVG is considered a sign of critical illness in both children and adults, with high mortality (3,4). With the continuous development of CT technology, high-sensitivity CT has become the preferred examination method for detecting HPVG, which gradually increases the detection rate of HPVG and enables HPVG to be treated in time (5-7). With the improvement of people’s awareness of HPVG, the treatment of HPVG is becoming more and more experienced, and its mortality is gradually decreasing (8,9). However, the pathogenesis of HPVG has not been fully clarified. At present, the mechanism of HPVG production is mainly considered to include the following factors (10): (I) increased intestinal pressure: the increased pressure in the intestinal cavity causes the gas plexus to transfer from the intestinal cavity to the portal vein system through the mesenteric vein. (II) Bacteria: the intestinal mucosa is damaged after bacterial infection, and the gas enters the mesenteric vein through the damaged intestinal mucosa, resulting in HPVG. (III) Other reasons: intestinal mucosal damage caused by diseases such as tumor, causing gas to enter the portal vein through the mesenteric vein. Seike et al. (2) summarized the common causes of portal vein pneumatosis, including gastrointestinal dilatation, inflammation, and iatrogenic factors.
Iatrogenic jejunal feeding tube implantation and hepatic artery embolization are the precursors of HPVG (2). Reviewing this case, we found that the patient had relied upon EN tube for nutritional support for a long time before TACE, and EN support through EN tube was initiated the second day after TACE. Amenu et al. (11) reported the case of a postoperative pancreatic cancer patient who chose percutaneous jejunostomy for EN support due to an eating disorder and underwent systemic chemotherapy, in whom HPVG occurred suddenly 2 months after chemotherapy. He considered that HPVG was caused by feeding-related intestinal ischemia or non-occlusive bowel ischemia (NOBI) related to EN. The similarities between their case and ours include that before HPVG, the patients had a long-term feeding history related to EN and chemotherapy, but the difference is that HPVG occurred 5 days after TACE in our case. Sun et al. (12) described the case of a radical total gastrectomy patient who received EN support through an EN tube on the second day after surgery, and HPVG occurred after the infusion of EN solution, which is similar to our case. Therefore, we infer that this case involved long-term EN tube implantation combined with small intestinal pneumatosis and dilation before TACE treatment, then the intestinal function did not fully recover after TACE, and early EN support damaged the intestinal mucosa, resulting in intestinal injury, which caused intestinal obstruction. Intestinal obstruction increased the intestinal pressure and made the gas in the intestine move into the portal vein through the superior mesenteric vein. This is the main reason for HPVG generation in this case, and the age of the patient may also be one of the important factors for HPVG generation (13,14). Although Xu et al. (15) reported that in a case of primary liver cancer treated with multiple TACE, liver abscess caused by necrosis of liver cancer led to HPVG, the abdominal CT reexamination in our case showed that there was no massive necrosis of liver metastases and no formation of liver abscess. Therefore, we concluded that the TACE treatment of this case was not the direct cause of HPVG.
Timely treatment of the etiology is the key to the treatment of HPVG, with treatment methods including surgical and conservative approaches (16). HPVG is not a direct risk factor for death (1), which means that the choice of treatment should be determined by the cause rather than HPVG. Nelson et al. (1) outlined the “ABC” grading treatment strategy for clinical treatment of HPVG: (I) if the patient has a sudden onset and critical clinical manifestation, the mortality rate could reach 75% when the CT scan indicates intestinal ischemia and intestinal necrosis are present. Surgical treatment should be carried out as soon as possible. (II) If the patient has a relatively slow onset and mild clinical manifestation, the mortality rate is between 20% and 30%. At the initial stage, the patient can be closely observed and treated conservatively, and surgery can be performed if necessary. (III) If HPVG occurs with no clinical manifestations, conservative treatment such as fasting and gastrointestinal decompression can be implemented. Gonda et al. (17) also suggested that strategic non-surgical treatment should be adopted for HPVG patients with 0–1 high mortality factors (ascites, peritoneal irritation, and shock), and challenging surgery can be considered for patients with 2–3 of the above factors, regardless of their background. Although these treatment strategies have become common methods to treat HPVG, there is no specific treatment standard to treat HPVG, and most cases still treat the cause or potential cause of HPVG (18,19). The clinical manifestations, imaging, and laboratory evidence of the patient determine the treatment plan for HPV (13). Koami et al. (20) reported that HPVG could not be used as a predictor of emergency surgery and high mortality in the absence of significant clinical evidence of intestinal ischemia or necrosis. For this case, although the patient exhibited sudden onset, peritoneal irritation sign, and slightly elevated neutrophil and blood pH, his vital signs were stable and there was no ascites, because his physique was weak, we chose conservative treatment such as gastrointestinal decompression to reduce the pressure of intestinal obstruction and antibiotic treatment to avoid aggravation of infection. After 4 hours of conservative treatment, the patient’s abdominal pain symptoms improved significantly, and the peritoneal irritation sign disappeared. Abdominal CT reexamination on the third day after treatment showed that intestinal obstruction and HPVG had disappeared. Therefore, we believe that the key to the conservative treatment of HPVG caused by long-term nutritional support and early EN after TACE treatment is to relieve intestinal obstruction as soon as possible, cease EN, and carry out anti-infection treatment.
Conclusions
In this case, HPVG was developed after TACE treatment for liver metastases, and literature regarding this circumstance was rare. The patient had several high-risk factors for HPVG, including age, gas accumulation in the small intestine before TACE treatment, TACE treatment, long-term EN, and premature EN after TACE treatment. These high-risk factors lead to intestinal obstruction after TACE: the high intestinal pressure caused the gas accumulated in the small intestine to transfer to the portal vein, leading to HPVG. Therefore, parenteral nutrition support should be implemented instead of early EN support for elderly patients who need long-term EN support after TACE treatment. If such patients manifest HPVG, early conservative treatment could help expediate resolution and avoid surgical treatment.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-213/rc
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-213/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-213/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient's daughter for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nelson AL, Millington TM, Sahani D, et al. Hepatic portal venous gas: the ABCs of management. Arch Surg 2009;144:575-81; discussion 581. [Crossref] [PubMed]
- Seike T, Suda T, Oishi N. Conservative treatment of hepatic portal venous gas resulting from non-occlusive mesenteric ischemia: a case report. Clin J Gastroenterol 2021;14:1404-10. [Crossref] [PubMed]
- Wolfe JN, Evans WA. Gas in the portal veins of the liver in infants; a roentgenographic demonstration with postmortem anatomical correlation. Am J Roentgenol Radium Ther Nucl Med 1955;74:486-8. [PubMed]
- Susman N, Senturia HR. Gas embolization of the portal venous system. Am J Roentgenol Radium Ther Nucl Med 1960;83:847-50. [PubMed]
- Moussa M, Marzouk I, Abdelmoula K, et al. Role of Computed tomography in predicting prognosis of Hepatic portal venous gas. Int J Surg Case Rep 2017;30:177-82. [Crossref] [PubMed]
- Alqahtani S, Coffin CS, Burak K, et al. Hepatic portal venous gas: a report of two cases and a review of the epidemiology, pathogenesis, diagnosis and approach to management. Can J Gastroenterol 2007;21:309-13. [Crossref] [PubMed]
- Faberman RS, Mayo-Smith WW. Outcome of 17 patients with portal venous gas detected by CT. AJR Am J Roentgenol 1997;169:1535-8. [Crossref] [PubMed]
- Zhou C, Kilpatrick MD, Williams JB, et al. Hepatic Portal Venous Gas: A Potentially Lethal Sign Demanding Urgent Management. Am J Case Rep 2022;23:e937197. [Crossref] [PubMed]
- Liu C, Wu CH, Zheng XD, et al. Hepatic portal venous gas: A case report and analysis of 131 patients using PUBMED and MEDLINE database. Am J Emerg Med 2021;45:506-9. [Crossref] [PubMed]
- Hu SF, Liu HB, Hao YY. Portal vein gas combined with pneumatosis intestinalis and emphysematous cystitis: A case report and literature review. World J Clin Cases 2022;10:8945-53. [Crossref] [PubMed]
- Amenu E, Karim S, Da Silva RC. Tube Feeding-Related Bowel Ischemia Presenting As Extensive Intestinal Pneumatosis Complicated With Hepatic Portal Venous Gas. Cureus 2022;14:e24313. [Crossref] [PubMed]
- Sun S, Zheng X, Zhang H, et al. Hepatic portal venous gas associated with rapid infusion of postoperative early enteral nutrition after radical total gastrectomy. Nutrition 2022;101:111685. [Crossref] [PubMed]
- Zhang Y, Liu HL, Tang M, et al. Clinical features and management of 20 patients with hepatic portal venous gas. Exp Ther Med 2022;24:525. [Crossref] [PubMed]
- Koizumi C, Michihata N, Matsui H, et al. In-Hospital Mortality for Hepatic Portal Venous Gas: Analysis of 1590 Patients Using a Japanese National Inpatient Database. World J Surg 2018;42:816-22. [Crossref] [PubMed]
- Xu L, Wang Y, Li W. A case report of fatal hepatic portal venous gas after transcatheter arterial chemoembolization in a patient with hepatocellular carcinoma. Transl Cancer Res 2021;10:5437-42. [Crossref] [PubMed]
- Li Z, Su Y, Wang X, et al. Hepatic portal venous gas associated with colon cancer: A case report and literature review. Medicine (Baltimore) 2017;96:e9352. [Crossref] [PubMed]
- Gonda M, Osuga T, Ikura Y, et al. Optimal treatment strategies for hepatic portal venous gas: A retrospective assessment. World J Gastroenterol 2020;26:1628-37. [Crossref] [PubMed]
- Wayne E, Ough M, Wu A, et al. Management algorithm for pneumatosis intestinalis and portal venous gas: treatment and outcome of 88 consecutive cases. J Gastrointest Surg 2010;14:437-48. [Crossref] [PubMed]
- Abboud B, El Hachem J, Yazbeck T, et al. Hepatic portal venous gas: physiopathology, etiology, prognosis and treatment. World J Gastroenterol 2009;15:3585-90. [Crossref] [PubMed]
- Koami H, Isa T, Ishimine T, et al. Risk factors for bowel necrosis in patients with hepatic portal venous gas. Surg Today 2015;45:156-61. [Crossref] [PubMed]
(English Language Editor: J. Jones)


