
Efficacy and safety of toripalimab with fruquintinib in the third-line treatment of refractory advanced metastatic colorectal cancer: results of a single-arm, single-center, prospective, phase II clinical study
Highlight box
Key findings
• Fruquintinib, in combination with anti-PD-1 toripalimab, exerted a better antitumor activity and acceptable tolerance in refractory MSS and pMMR mCRC compared with the previous standard third-line therapy.
What is known and what is new?
• The use of fruquintinib combined with anti-PD-1 toripalimab therapy in patients with MSS mCRC is safe. Additionally, no significant toxicities of immunotherpy were observed. Responders to therapy demonstrated a survival benefit trend in this small and heterogeneous cohort.
• Cox regression analysis showed that primary lesion excision and peritoneal metastasis were predictive of benefit to the combination therapy for patients with mCRC and MSS or pMMR.
What is the implication, and what should change now?
• More in-depth research on this therapeutic combination is still required to evaluate its advantages in a larger group of patients and pinpoint independent the prognostic variables for MSS mCRC in more expansive populations.
Introduction
As the third most common visceral malignancy in the world, colorectal cancer (CRC) continues to be one of the main causes of cancer-related death (1). For patients with advanced or metastatic CRC diseases who are either poor surgical candidates or refuse surgery, the current guideline states oxaliplatin or irinotecan-containing regimens combined with an anti-epidermal growth factor receptor (anti-EGFR) antibody (only in the first-line treatment of patients with RAS wild-type left sided tumors) or an angiogenesis inhibitor as the standard therapy (2). However, patients’ prognosis with disease progression receiving second-line treatment is still poor. Regorafenib (3), fruquintinib, and trifluridine/tipiracil (TAS-102) are currently approved as the third-line treatments for metastatic CRC (mCRC) (2). The CORRECT study showed that patients who received regorafenib in addition to supportive care experienced longer progression-free survival (PFS: median of 2 vs. 1.7 months) and overall survival (OS: median of 6.4 vs. 5 months) than those who received placebo, despite an objective response rate which was only 1% (4). The FRESCO study indicated that fruquintinib extended the median OS (9.3 vs. 6.6 months) and PFS (3.7 vs. 1.8 months) when compared to placebo (5). The TERRA (6) study also indicated limited benefit for third-line monotherapy of advanced CRC. Median OS (7.8 vs. 7.1 months) and PFS (2 vs. 1.8 months) were also longer in the trifluridine/tipiracil arm versus the placebo arm. These studies suggested modest clinical efficacy of third-line treatment for colorectal cancer and new treatment strategies are needed. Indeed, the immune checkpoint inhibitors have shown efficacy only in a subset of patients with mCRC who are mismatch repair-deficient or have a high level of microsatellite instability high (MSI-H), whose objective response rate (ORR) may reach 65% (7). Importantly, patients with CRC and MSI-H status generally show good response to immunotherapy due to the presence of high-density infiltrating CD8+ T cells in MSI-H cancer tissues, leading to the abundance of neoantigens caused by hypermutation and corresponding high immunogenicity. On the other hand, due to poor immune cell infiltration (8), single-agent anti-programmed cell death protein 1 (anti-PD-1) or anti-programmed cell death ligand 1 (anti-PD-L1) blockade has not shown meaningful effect in the microsatellite-stable (MSS) or MMR-proficient (pMMR) mCRC subgroup (ORR =0%), a population that constitutes the majority of the patients with mCRC (9). Therefore, the optimum third-line treatment for mCRC remains controversial.
Immune checkpoint inhibitor (ICI) blocks tumor derived negative regulator signals that inhibit immune responses, thus amplifying host’s antitumor immunity. Nonetheless, a major and unsolved issue is the low response rate to immunotherapy, the selection of patients with advanced solid tumors who will benefit from ICI therapy represents a major challenge in clinical practice. Even though available predictive biomarkers such as PD-L1 expression, tumor mutation burden, mismatch-repair deficiency, and status of tumor-infiltrating lymphocytes are also useful factors for monitoring therapeutic effects and for prognostication in several malignancies, many questions remain unresolved about the frequent resistance to ICI monotherapy. However, accumulating evidence indicated that ICI resistance could be partially mitigated by combining anti-angiogenesis treatment with immunotherapy. Angiogenesis, mainly indicating the formation of new vasculature from preexisting vessels, take place in many adult physiological processes (such as wound healing) (10). At the same time, angiogenesis are often required for the growth and metastasis of solid tumor (11). Angiogenesis factors play immunosuppressive role through a variety of mechanisms, including directly suppressing the antigen-presenting cells as well as immune effector cells or enhancing the effect of regulatory T cells (Treg), myeloid-derived suppressor cells (MDSC), and tumor-associated macrophages (TAM) (12). Anti-angiogenesis therapy became an important treatment option for cancer treatment and received intensive attention earlier, yet, a considerable number of patients treated with anti-angiogenic drugs as single agents reap limited or no benefits at all.
Emerging evidence suggested that appropriate anti-angiogenesis administration could switch tumor immune environment from immunosuppressive to immunosupportive status (13). It is known that hypoxia in the tumor microenvironment forms a barrier to T cell infiltration and fosters resistance to antitumor immunotherapy. Antiangiogenic therapy worked by trimming tumor blood vessels and normalizing those remaining and it was a process whereby the abnormal, inefficient tumor blood vessels are restored to a more efficient, normalized state resulting in the reversal of hypoxia. Subsequently, alleviated hypoxia preferentially induces TAM polarization towards more antitumor M1-like phenotype (14). Besides, vessel normalization reduces immunosuppressive Treg and MDSC populations in tumor bearing body (10,15). Targeting VEGF agents block the inhibitory signal transduction during dendritic cell (DC) differentiation and reduce overall pool of MDSC (16).
With reference to the above basic research findings, a number of animal studies have been carried out successively. As early as 2013, study of immunotherapy with antiangiogenic agents by Yasuda et al. observed the synergistic effect in mice bearing colon adenocarcinoma (17). The combination of fruquintinib plus PD-1 inhibitors was also shown, in mouse experiments, to increase antitumor activity and the ability to reprogram the immunosuppressive TME (18). Apart from findings in colorectal cancer, Wu et al. also identified that ICI plus anti-angiogenesis could significantly prolong OS of mice bearing kidney and mammary tumors (19). As mentioned above, it can be seen that the interaction between immunity and angiogenesis leads to tumor immune escape and treatment resistance. Owing to the encouraging early-phase pre-clinical results with this combination therapy, many clinical studies of ICI combined with anti-angiogenesis therapies have been conducted or are ongoing to investigate the synergistic effect in cancer patients (20,21). Motzer et al. reported the results of phase III clinical study which aimed to investigate the efficacy of avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma patients (22). The results showed that median PFS was 13.8 months with avelumab plus axitinib, as compared with 7.2 months with sunitinib (HR, 0.61; P=0.0001). Among the patients with PD-L1-positive tumors, the objective response rate was 55.2% with avelumab plus axitinib and 25.5% with sunitinib. Finn et al. has announced that the combination of atezolizumab and bevacizumab demonstrated superior OS (19.2 vs. 13.4 months, P=0.0009) and PFS (6.9 vs. 4.3 months, P<0.001) compared to sorafenib in the first-line treatment of advanced hepatocellular carcinoma (HCC) (23). Data from preclinical and clinical studies have suggested that ICIs in combination with antiangiogenic/vasculature-targeting agents mutually enhanced effect of antitumor. On the one hand, anti-angiogenesis enhances the anti-tumour activity of ICI by blocking tumour-induced immune-suppressive cells and increasing T-cell infiltration into tumours. On the other hand, ICI therapy could modulate the tumor immunosuppressive microenvironment and enhance the immune system’s ability to block tumor angiogenesis. In 2022 Gou et al. (24) reported the efficacy of fruquintinib in combination with PD-1 inhibitors in patients with refractory non-MSI-H/pMMR metastatic colorectal cancer, the ORR was much higher than that of single-agent fruquintinib (11.1% vs. 4.9%), disease control rate (DCR) was 62.2% (28/45), median PFS equal 3.8 months, and median OS was 14.9 months.
In China, toripalimab (Tuoyi) a selective, recombinant, humanized immunoglobulin G4 (IgG4) monoclonal antibody against PD-1, was approved by the National Medical Product Administration (NMPA) in 2018 as a curative drug for unresectable or metastatic melanoma that has not responded to prior systemic therapy (25). Toripalimab has exhibited remarkable antitumoral activity in metastatic melanoma (26) and more recently in non-small cell lung cancer (27), digestive tract tumors (28-30), hepatobiliary (31), pancreatic tumors (32), neuroendocrine neoplasms (33), nasopharyngeal carcinoma (34), and urothelial carcinoma (35). Due to satisfactory antitumor effect and long-term survival benefits in China, the US Food and Drug Administration (FDA) designated toripalimab as an orphan drug for the treatment of refractory advanced solitary malignant tumors.
Toripalimab has also demonstrated a similar response rate to pembrolizumab or nivolumab as a monotherapy in many preclinical studies and phase Ib/II clinical trials for several cancer types (36-39). Recent studies have identified that simultaneous inhibition of PD-1 and vascular endothelial growth factor receptors (VEGFRs) could have a synergistic antitumor effect that leads to highly durable clinical responses with acceptable toxicity profiles (40-42).
Fruquintinib is a new-generation small molecule VEGFR inhibitor with strong potency and high kinase selectivity targeting of VEGFR1/2/3. It can suppress tumor proliferation, metastasis, and angiogenesis because it strongly inhibits VEGFR family members while weakly inhibiting FGFR-1, RET, and c-kit kinases (43,44). More interestingly, selective VEGFR inhibition might enhance the efficacy of immunotherapy with immune checkpoint inhibitors. Mechanistically, administration of anti-angiogenesis molecules in combination with PD-1 inhibitors has been shown to reduce angiogenesis; alter the vascular structure; enhance T-cell priming and activation by promoting DC maturation; increase the infiltration of CD8+ T cells (P<0.05), CD8+TNFα+ T cells (P<0.05), and CD8+IFNγ+ T cells (P<0.05); and decrease the ratios of myeloid-derived suppressor cells and macrophages in mouse models (21,45,46).
Based on the above-mentioned results, we hypothesized that combining fruquintinib with anti-PD-1/anti-PD-L1 antibodies may yield a significant clinical benefit for patients with mCRC and MSS who have failed prior standard chemotherapy regimens. This paper reports the clinical outcomes of a phase II trial evaluating the combination of fruquintinib and toripalimab in third-line treatment and beyond for refractory advanced CRC. We present the following article in accordance with the TREND reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-108/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of the First Hospital of Lanzhou University (No. LDYYLL2019-248) and informed consent was taken from all the patients.
Study design and participants
This study was a single-arm, single-center, prospective, phase II trial which patients were recruited from the First Hospital of Lanzhou University. The trial was registered at the Chinese Clinical Trial Registry (http://www.chictr.org/cn/, identifier ChiCTR2000028965). Patient demographics, extent of disease at diagnosis, prior chemotherapy, prior radiotherapy, subsequent surgical therapy, and Eastern Cooperative Oncology Group (ECOG) performance status were collected at the time of enrollment. From December 2019 to August 2022, we assessed the outcomes of MSS patients with refractory advanced CRC who received fruquintinib in combination with toripalimab at the First Hospital of Lanzhou University. These patients had previously undergone at least second-line treatment. The regimens were based on oxaliplatin and irinotecan and/or combined with bevacizumab or cetuximab. The inclusion criteria for the research were as follows: (I) cases had pathologically confirmed CRC; (II) patients ranged in age from 18 to 75 years; (III) patients had recurrent/metastatic CRC and had previously undergone at least 2 lines of standard therapy that included oxaliplatin, irinotecan, and fluoropyrimidine or raltitrexed, with prior target treatment, such as bevacizumab or cetuximab, also being permitted; (IV) the physical status was 0 or 1 according to the ECOG; (V) all enrolled patients with liver metastases underwent a multi-disciplinary team (MDT) discussion, and liver metastases were considered unresectable; and (VI) informed consent has been signed. The exclusion criteria included the following: (I) a medication history of fruquintinib; (II) severe heart, brain, lung, liver, or kidney insufficiency or other serious underlying diseases; (III) a history of immunodeficiency or an active or documented history of chronic or recurrent autoimmune diseases; (IV) no measurable lesions at baseline according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1; and (V) no history of receiving previous immunotherapy before. Sample size estimation was based on ORR. According to the previous studies (5,47), the ORR of fruquintinib in the treatment of relapsed or metastatic advanced colorectal cancer is 5%, while the ORR of PD-1 monoclonal antibody combined with TKI in mCRC is 33%. Therefore, we assumed that the ORR is 25%, 24 cases were needed in this study with a two-side α of 0.05 and the power of 0.9. Considering the loss to follow-up rate of 20%, the total number was 30 cases.
Treatment
Patients received intravenous toripalimab 240 mg every 3 weeks in addition to oral fruquintinib 5 mg once daily on a 21-day on–7-day off schedule until disease progression or intolerance to adverse events (AEs).
Assessment
Until disease progression or before subsequent treatment, the patients underwent computed tomography scans every 2 treatment cycles. Antitumor efficacy was evaluated with RECIST v1.1. The ORR was defined as the percentage of patients with confirmed complete response (CR) or confirmed partial response (PR) as the best overall response during combination therapy. DCR was calculated as the proportion of patients with CR, PR, and stable disease (SD). The time between the start of treatment and the earliest date at which the disease progressed or death occurred due to any cause was considered to be the PFS. Various treatment responses were performed by independent evaluators at our center. AEs were evaluated according to the Common Terminology Criteria for Adverse Events 5.0 standard (CTCAE 5.0). All enrolled patients were followed up every 6 weeks from the end of treatment. During the follow up, patients’ disease and survival status were examined and recorded.
Statistical analysis
Baseline characteristics of the enrolled patients, efficacy results, and AE data in categorical format are presented as numbers and percentages, and the 95% confidence interval (CI) was calculated as appropriate. The Kaplan-Meier method was used to evaluate the end point of event arrival time (including PFS, OS, and DOR). Univariate Cox regression was applied to estimate the significance of clinical factors on prognosis. Confounding factors were adjusted in multivariate Cox regression models by choosing the baseline covariates from univariate analysis covariates with a P value <0.1. Cox multiple regression analysis was used to perform multifactor analysis on the features that influenced OS and PFS. A 2-sided P value <0.05 was considered statistically significant. The statistical analyses were carried out using GraphPad Prism 8.0 (GraphPad Software) and R version 4.2.0 (The R Foundation for Statistical Computing).
Results
Patient characteristics
In total, 19 patients with refractory advanced CRC (12 male and 7 female) with MSS or pMMR were enrolled, with a median age was 52 years. The final outcome analysis was as follows: 14 patients with colorectal cancers were left sided and 5 patients were right sided; the majority of patients (78.9%) had the primary lesions resected; of the stage IV patients, 13/19 (68.4%) had lung metastases, 13/19 (68.4%) had liver metastases, 2/19 (10.5%) had lymph node metastases. All patients previously received irinotecan-, oxaliplatin- and fluorouracil-based chemotherapy before enrollment. Fifteen patients (78.9%) had been treated with bevacizumab, 6 (31.6%) with cetuximab and 3 (15.7%) with raltitrexed. Sixteen patients had gene mutation testing and 8 patients have mutations in RAS oncogene. The patient characteristics are detailed in Table 1.
Table 1
| Characteristic (n=19) | Value |
|---|---|
| Age, years | |
| Median [range] | 52 [30–71] |
| <60 years | 16 (84.21) |
| Men | 12 (63.16) |
| ECOG PS =0 | 8 (42.11) |
| Primary tumor location of left | 14 (73.68) |
| Metastases | |
| Liver | 13 (68.42) |
| Lung | 13 (68.42) |
| Lymph node | 2 (10.53) |
| Peritoneum | 1 (5.26) |
| Primary lesion resected | 15 (78.95) |
| RAS mutant (n=16) | 8 (50.00) |
| BRAFV600Emutant (n=19) | 0 (0.00) |
| Prior medication | |
| Fluorouracil | 19 (100.00) |
| Oxaliplatin | 19 (100.00) |
| Irinotecan | 19 (100.00) |
| Raltitrexed | 3 (15.79) |
| Bevacizumab | 15 (78.95) |
| Cetuximab | 6 (31.58) |
Data are reported as number (percentage) unless otherwise indicated. ECOG PS, Eastern Cooperative Oncology Group performance status.
Efficacy
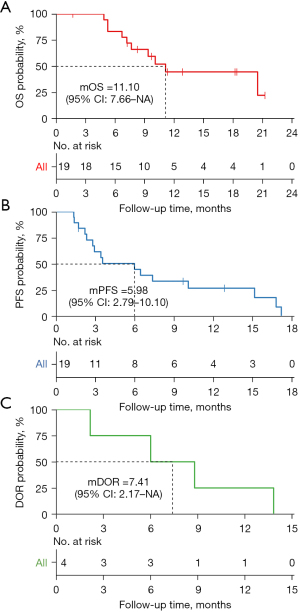
The evaluations of therapeutic response was performed according to the RECIST v. 1.1. It was regarded as effective if the patients achieved CR, PR, or SD. Patients who were evaluated as progressive disease (PD) indicated an ineffective. Accordingly, 4 patients (21.05%) received PR, 10 patients (52.63%) experienced SD, and 4 patients (21.05%) experienced PD (Figure 1). The global ORR was 21.05%, and the DCR was 73.68% (Table 2). Median PFS (mPFS) was 5.98 months (95% CI: 2.79–10.10), the 1-year PFS rate was 26.95% (95% CI: 5.83–48.10%), and the median OS (mOS) was 11.10 months (95% CI: 7.66–NA). For the 4 patients who achieved objective response, the median duration of response (DOR) was 7.41 months (95% CI: 2.17–NA). Notablely, in our study, there were 6 patients without liver metastasis, among which 3 patients had lung metastasis, 2 patients had pelvic metastasis, and 1 patient had retroperitoneal metastasis concurrently. After receiving combination therapy, 2 patients had PR, 1 patient had SD, and 3 patients had PD, the ORR of them was 33% and DCR was 50%. Additionally, 13 patients died, and 6 patients survived at the end of follow-up (Figure 2). Interestingly, patient no. 6 experienced PD on day 29, but she later received monotherapy with fruquintinib again and maintained SD until day 191.
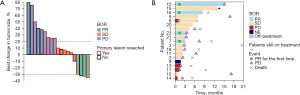
Table 2
| Best overall response | No. (%) |
|---|---|
| CR | 0 (0) |
| PR | 4 (21.05) |
| SD | 10 (52.63) |
| PD | 4 (21.05) |
| NE | 1 (5.26) |
| ORR | 4 (21.05, 95% CI: 6.05–45.57) |
| DCR | 14 (73.68, 95% CI: 48.80–90.85) |
CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; NE, not evaluable; DCR, disease control rate; ORR, objective response rate.
Univariate analysis
In univariate analysis, patients who had previously received excision of the primary lesion received more benefit in PFS (P=0.029), while those who had peritoneal metastasis had a poorer PFS (P=0.043). There were no significant differences in effectiveness in age, ECOG status, tumor location, liver metastasis, lung metastasis, lymph node metastasis, previous cetuximab or bevacizumab medication, number of previous chemotherapy lines, or RAS gene status (P>0.05).
Multivariate analysis
The HR and P value were adjusted by multivariate Cox regression. The inclusion threshold was set as a P value <0.1 (in the univariate Cox regression result). The results showed that there was no clinical characteristics indicated statistical significance.
Safety
Overall, 19 patients were enrolled for safety analysis. The most frequent treatment-related adverse events (TRAEs) were fatigue (57.89%), hepatic dysfunction (42.11%), hypertension (36.84%), abdominal pain (26.32%), hand-foot syndrome (21.05%), diarrhea (15.79%), anorexia (10.53%), fever (10.53%), hoarseness (5.26%), and hypothyroidism (5.26%). Moreover, 7 patients (36.84%) experienced grade 3–4 TRAEs (including hypertension, hepatic dysfunction, and hand-foot syndrome). No treatment-related deaths occurred. TRAEs leading to either fruquintinib or toripalimab discontinuation occurred in 3 (15.7%) patients for each drug. Most patients with mild adverse reactions could continue to receive combination therapy after symptomatic treatment. The results are shown in Table 3.
Table 3
| Adverse event | All grades, n (%) | Grade 3–4, n (%) |
|---|---|---|
| Fatigue | 11 (57.89) | 0 (0) |
| Hepatic dysfunction | 8 (42.11) | 3 (15.79) |
| Hypertension | 7 (36.84) | 3 (15.79) |
| Abdominal pain | 5 (26.32) | 0 (0) |
| Hand-Foot syndrome | 4 (21.05) | 1 (5.26) |
| Diarrhea | 3 (15.79) | 0 (0) |
| Anorexia | 2 (10.53) | 0 (0) |
| Fever | 2 (10.53) | 0 (0) |
| Hoarseness | 1 (5.26) | 0 (0) |
| Hypothyroidism | 1 (5.26) | 0 (0) |
Discussion
Since being used in clinical practice, the strong antitumor effects of immune checkpoint inhibitors are likely to be broadly applicable to all the solid tumors, such as melanoma, renal clear cell carcinoma, and liver cancer (48). However, a subset of patients with mCRC low MSI (MSI-L)/MSS or pMMR have primary resistance to anti-PD-1 antibodies (49). To help MSS CRC transform from an immune-excluded to an immune-responsive malignancy, numerous combination immunotherapies have been thoroughly investigated. However, the inconsistencies in the results of these related studies have led to great challenges in the application of immune checkpoint inhibitors in “immune-excluded or immune-desert” tumors. In the clinical IMblaze 370 trial, the combined effect of atezolizumab (an anti-PD-L1 antibody) and cobimetinib (an MEK inhibitor) was found in only 3% of patients, and the primary end point of improving OS was not met (50). Dual checkpoint blockades of nivolumab plus ipilimumab led to a poor response of 0–10%. The efficacy of toripalimab and fruquintinib in our study was superior to that reported for the combination of regorafenib plus avelumab in the REGOMUNE study (ORR =0%; mOS =10.8 months) (51). The results in our study were well supported by numerous clinical studies using other PD-1–PD-L1 axis blockade and VEGF-targeted therapy, reporting ORRs ranging from 5% to 11% (24,52,53). These studies included atezolizumab plus capecitabine and bevacizumab or pembrolizumab plus capecitabine and bevacizumab, as well as a retrospective study of combined PD-1 blockade and VEGF–tyrosine kinase inhibitor (TKI) therapy (sintilimab plus fruquintinib). According to the REGOTORI study from China, patients with resistant MSS CRC who received the combination of regorafenib and toripalimab had an OS of 15.5 months, indicating that the antitumor activity of this regimen was long-lasting, and the ORR was up to 30% in the participants without hepatic metastases (41). These results suggest that although a similar rationale applies to PD-L1 inhibitors, which work by targeting and blocking the PD-1-PD-L1 signaling pathway, their binding sites and antitumor mechanisms are different.
Notably, the ORR in our study outperformed that of patients who received fruquintinib alone (ORR =4.7%) (5) in the third-line setting for refractory mCRC, highlighting a possible synergic effect between antitumor angiogenesis therapy and immunotherapy. The therapeutic effects of combination methods were better than those of PD-1 blockade alone because the MSS/pMMR mCRC patients are highly unlikely to respond to anti-PD-1 antibodies (49) despite the fact that there are few related research data on the use of toripalimab in mCRC. In the present study, 4 patients (21.05%) achieved partial response, 10 patients (52.63%) experienced stable disease, and 4 patients (21.05%) experienced progressive disease. The ORR was 21.05%, and the DCR was 73.68%. In contrast, nivolumab plus regorafenib provided a robust clinical benefit in MSS patients with CRC in the REGONIVO study (47), with high ORRs in 8 out of 24 (33%; 8 patients had PR). The response rate in our study was not better than those of the REGONIVO trial. In contrast to the North American REGONIVO study (54), which has a response rate of only 7.1%, with 5 patients achieving PR and 22 experience SD, our study suggested that a combination regimen can also be effective in third-line therapy. The REGONIVO study was a phase Ib dose-escalation and dose-expansion trial that sought to determine the safety and recommended doses, and the enrolled participants were carefully chosen, which may account for the differences between the results of the different studies. Only 50% of patients (n=12) had target lesions in the liver, which is a proportion significantly lower than that observed in routine practice and in our study. It is well known that the liver has an immune microenvironment that is permissive to tumor growth. Combination strategies with currently used targeting anti-angiogenesis and anti-PD-1/PD-L1 antibodies could convert the tumor immune-suppressive microenvironment into an immune-permissive one, which will in turn strengthen the antitumor effect of immune checkpoint inhibitors (55). Second, the effectiveness of the combination therapy might vary because of the different anti-PD-1 antibodies, their combined use with various angiogenesis agents, and the patient selection. Thus, future clinical studies with larger cohorts may be able to determine the actual response rates in a combination regimen.
Patient no. 6 presented with disease progression on day 29. However, the patient was treated with fruquintinib monotherapy at the recommendation of a local doctor after a short discontinuation of treatment. To our surprise, the patient sustained SD until day 191 from then on. We have no additional evidence to exclude the possibility that the initial PD was pseudoprogression. However, a more interesting hypothesis is that fruquintinib has efficacy in patients who fail in the first challenge. Nonetheless, further trials are required to validate this hypothesis.
In terms of PFS, it was reported that the median PFS in the REGONIVO (47), the FRESCO (5), and the TAS-102 (56) studies was 6.3, 3.7, and 2.0 months, respectively. The mPFS in patients in our study was 5.98 months (95% CI: 2.79–10.10), which was comparable to that of the REGONIVO study and better than that of other studies of third or subsequent line treatment in mCRC. Fruquintinib combined with toripalimab yielded an obvious antitumor effect, and patients had a longer PFS in this study; however, it should be noted that a number of factors that could have affected response to investigational drugs, whereas randomized controlled clinical trials were able to exclude potentially confounding conditions. Furthermore, despite the fact that both regorafenib and fruquintinib are potent orally administered inhibitors of angiogenesis, regorafenib is a multitargeted TKI, mainly targeting VEGFR2, PDGFR, and FGFR tyrosine kinase (3), while fruquintinib is a potent, highly selective and active inhibitor of VEGFR1/2/3 tyrosine kinases (43), implying that both TKIs’ regulatory mechanisms for these active binding sites are distinct. In addition, our analyses highlighted the molecular properties of PD-1–targeted antibodies as another factor influencing the effects. These differences in PD-1 binding sites between nivolumab and pembrolizumab may account for the difference in efficacy observed in treatments for solid tumors (57). The median OS time in our study was 11.10 months, which is significantly longer than the 9.3 months of the FRESCO study, which was the longest OS ever reported in the third-line standard treatment. This result might be attributable to differences in baseline demographics compared to the FRESCO study. Moreover, our results may not be directly comparable to those of the FRESCO or REGONIVO studies because further validation of the variations in OS or PFS in a larger sample size is warranted.
Accordingly, we further assessed whether clinical characteristics were correlated with clinical outcomes. Univariate regression analysis showed that primary lesion excision and peritoneal metastasis were independent prognostic factors of PFS (P=0.029 and P=0.043). Patients with unresected primary lesions experience poor efficacy with combination therapy. There were no statistically significant differences in OS or PFS for sex, age, ECOG, liver metastasis, lung metastasis, lymph node metastasis, cetuximab medication, or other factors (P>0.05) by multivariate Cox regression. The REGONIVO study (47) found that all patients who responded were males with lung metastases and had a PS score of 0, which was incongruent with the results of our study. Thus, the data were insufficiently consistent to draw a firm inference concerning the clinical factors that influence outcomes. To elucidate the impact of these factors on combination therapy outcomes, additional analyses with larger sample sizes are necessary.
Overall, the results of this study showed that the safety profile of fruquintinib and toripalimab seemed to be manageable and acceptable. The combination’s incidence and type of AEs and TRAEs seemed to be generally consistent with the safety profiles of the individual drugs. No other toxicities were identified compared with either treatment alone (58). Only 3 patients with hepatic dysfunction and hand-foot syndrome experienced the majority of grade 3 and 4 AEs, and they were treated for their symptoms and with systemic corticosteroids as necessary. Moreover, no grade-5 TRAEs or treatment-related deaths occurred. In summary, the combination of toripalimab and fruquintinib at 5 mg appeared to have a similar safety profile and was well-tolerated (46,59).
There are some limitations to our study. First, the sample size was small, and all the MSS patients were recruited from a single center. Second, although the enrolled patients were thoroughly screened, the subset of patients with lung- or liver-specific metastases was relatively small. Third, several studies suggested that tumor mutation burden (TMB), PD-L1 expression, circulating tumor DNA (ctDNA) levels, tumor-infiltrating lymphocytes (TILs), gene expression profiling (GEP) of an inflammatory microenvironment, and neoantigen prediction have become independent predictors of immunotherapy in multiple solid tumors (60-62); however, these were not evaluated in our study, and whether these biomarkers can be used as an independent predictor of response to combination therapy is unclear and requires additional study. Further research is needed to explore effective biomarkers on treatment outcomes with this combined therapy.
Conclusions
Our study demonstrated that fruquintinib, in combination with the anti-PD-1, toripalimab, exerted antitumor activity and acceptable tolerance in patients with refractory and MSS or pMMR mCRC compared with the previous standard third-line therapy. Nevertheless, numerous unevaluated clinical features could have affected the efficacy of antiangiogenic and anti–PD-L1 combination therapy. More extensive research on the combination is still required to evaluate its advantages and pinpoint independent prognostic variables for MSS patients with mCRC.
Acknowledgments
This study was presented at the American Society of Clinical Oncology 2022 Annual Meeting (Chicago, USA; June 3 to June 7, 2022). The authors would like to thank Chao Zhao and Zheng Wang from HUTCHMED Limited contributed to the data analysis and review of the manuscript. The authors would also like to thank the patients who participated in this study.
Funding: This study was sponsored by HUTCHMED Limited and Shanghai Junshi Biosciences.
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-108/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-108/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-108/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-108/coif). All authors report that this study was sponsored by HUTCHMED Limited and Shanghai Junshi Biosciences. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of the First Hospital of Lanzhou University (No. LDYYLL2019-248) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Deo SVS, Sharma J, Kumar S. GLOBOCAN 2020 Report on Global Cancer Burden: Challenges and Opportunities for Surgical Oncologists. Annals of Surgical Oncology 2022; [Crossref] [PubMed]
- Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 2016;27:1386-422. [Crossref] [PubMed]
- Loupakis F, Antonuzzo L, Bachet JB, et al. Practical considerations in the use of regorafenib in metastatic colorectal cancer. Ther Adv Med Oncol 2020;12:1758835920956862. [Crossref] [PubMed]
- Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303-12. [Crossref] [PubMed]
- Li J, Qin S, Xu RH, et al. Effect of Fruquintinib vs Placebo on Overall Survival in Patients With Previously Treated Metastatic Colorectal Cancer: The FRESCO Randomized Clinical Trial. Jama 2018;319:2486-96. [Crossref] [PubMed]
- Xu J, Kim TW, Shen L, et al. Results of a Randomized, Double-Blind, Placebo-Controlled, Phase III Trial of Trifluridine/Tipiracil (TAS-102) Monotherapy in Asian Patients With Previously Treated Metastatic Colorectal Cancer: The TERRA Study. J Clin Oncol 2018;36:350-8. [Crossref] [PubMed]
- André T, Lonardi S, Wong KYM, et al. Nivolumab + low-dose ipilimumab in previously treated patients with microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: 4-year follow-up from CheckMate 142. Ann Oncol 2022;33:1052-60. [Crossref] [PubMed]
- Giannakis M, Mu XJ, Shukla SA, et al. Genomic Correlates of Immune-Cell Infiltrates in Colorectal Carcinoma. Cell Rep 2016;15:857-65. [Crossref] [PubMed]
- Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015;372:2509-20. [Crossref] [PubMed]
- Ramjiawan RR, Griffioen AW, Duda DG. Anti-angiogenesis for cancer revisited: Is there a role for combinations with immunotherapy? Angiogenesis 2017;20:185-204. [Crossref] [PubMed]
- Kerbel RS. Tumor angiogenesis. N Engl J Med 2008;358:2039-49. [Crossref] [PubMed]
- Rahma OE, Hodi FS. The Intersection between Tumor Angiogenesis and Immune Suppression. Clin Cancer Res 2019;25:5449-57. [Crossref] [PubMed]
- Fukumura D, Kloepper J, Amoozgar Z, et al. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol 2018;15:325-40. [Crossref] [PubMed]
- Huang Y, Yuan J, Righi E, et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci U S A 2012;109:17561-6. [Crossref] [PubMed]
- Du Four S, Maenhout SK, Niclou SP, et al. Combined VEGFR and CTLA-4 blockade increases the antigen-presenting function of intratumoral DCs and reduces the suppressive capacity of intratumoral MDSCs. Am J Cancer Res 2016;6:2514-31. [PubMed]
- Horikawa N, Abiko K, Matsumura N, et al. Expression of Vascular Endothelial Growth Factor in Ovarian Cancer Inhibits Tumor Immunity through the Accumulation of Myeloid-Derived Suppressor Cells. Clin Cancer Res 2017;23:587-99. [Crossref] [PubMed]
- Yasuda S, Sho M, Yamato I, et al. Simultaneous blockade of programmed death 1 and vascular endothelial growth factor receptor 2 (VEGFR2) induces synergistic anti-tumour effect in vivo. Clin Exp Immunol 2013;172:500-6. [Crossref] [PubMed]
- Wang Y, Wei B, Gao J, et al. Combination of Fruquintinib and Anti-PD-1 for the Treatment of Colorectal Cancer. J Immunol 2020;205:2905-15. [Crossref] [PubMed]
- Wu FTH, Xu P, Chow A, et al. Pre- and post-operative anti-PD-L1 plus anti-angiogenic therapies in mouse breast or renal cancer models of micro- or macro-metastatic disease. Br J Cancer 2019;120:196-206. [Crossref] [PubMed]
- Li RR, Yin XL, Zeng DY, et al. Efficacy and safety of anti-PD-1 antibody plus regorafenib in refractory microsatellite stable metastatic colorectal cancer: a retrospective single-arm cohort study. Ann Transl Med 2022;10:880. [Crossref] [PubMed]
- Yi M, Jiao D, Qin S, et al. Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment. Mol Cancer 2019;18:60. [Crossref] [PubMed]
- Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med 2019;380:1103-15. [Crossref] [PubMed]
- Finn R, Qin S, Ikeda M, et al. IMbrave150: Updated overall survival (OS) data from a global, randomized, open-label phase III study of atezolizumab (atezo) plus bevacizumab (bev) versus sorafenib (sor) in patients (pts) with unresectable hepatocellular carcinoma (HCC). J Clin Oncol 2021;39:267. [Crossref]
- Gou M, Qian N, Zhang Y, et al. Fruquintinib in Combination With PD-1 Inhibitors in Patients With Refractory Non-MSI-H/pMMR Metastatic Colorectal Cancer: A Real-World Study in China. Front Oncol 2022;12:851756. [Crossref] [PubMed]
- Keam SJ. Toripalimab: First Global Approval. Drugs 2019;79:573-8. [Crossref] [PubMed]
- Tang B, Chi Z, Chen Y, et al. Safety, Efficacy, and Biomarker Analysis of Toripalimab in Previously Treated Advanced Melanoma: Results of the POLARIS-01 Multicenter Phase II Trial. Clin Cancer Res 2020;26:4250-9. [Crossref] [PubMed]
- Yang J, Dong L, Yang S, et al. Safety and clinical efficacy of toripalimab, a PD-1 mAb, in patients with advanced or recurrent malignancies in a phase I study. Eur J Cancer 2020;130:182-92. [Crossref] [PubMed]
- Wang ZX, Cui C, Yao J, et al. Toripalimab plus chemotherapy in treatment-naïve, advanced esophageal squamous cell carcinoma (JUPITER-06): A multi-center phase 3 trial. Cancer Cell 2022;40:277-88.e3. [Crossref] [PubMed]
- Wang F, Wei XL, Wang FH, et al. Safety, efficacy and tumor mutational burden as a biomarker of overall survival benefit in chemo-refractory gastric cancer treated with toripalimab, a PD-1 antibody in phase Ib/II clinical trial NCT02915432. Ann Oncol 2019;30:1479-86. [Crossref] [PubMed]
- Hu H, Kang L, Zhang J, et al. Neoadjuvant PD-1 blockade with toripalimab, with or without celecoxib, in mismatch repair-deficient or microsatellite instability-high, locally advanced, colorectal cancer (PICC): a single-centre, parallel-group, non-comparative, randomised, phase 2 trial. Lancet Gastroenterol Hepatol 2022;7:38-48. [Crossref] [PubMed]
- He M, Ming S, Lai Z, et al. A phase II trial of lenvatinib plus toripalimab and hepatic arterial infusion chemotherapy as a first-line treatment for advanced hepatocellular carcinoma (LTHAIC study). J Clin Oncol 2021;39:4083. [Crossref]
- Cheng K, Lv WR, Li X, et al. Toripalimab with nab-paclitaxel/gemcitabine as first-line treatment for advanced pancreatic adenocarcinoma: Updated results of a single-arm, open-label, phase Ib/II clinical study. J Clin Oncol 2021;39:e16213. [Crossref]
- Shen L, Yu X, Lu M, et al. Surufatinib in combination with toripalimab in patients with advanced neuroendocrine carcinoma: Results from a multicenter, open-label, single-arm, phase II trial. J Clin Oncol 2021;39:e16199. [Crossref]
- Wang FH, Wei XL, Feng J, et al. Efficacy, Safety, and Correlative Biomarkers of Toripalimab in Previously Treated Recurrent or Metastatic Nasopharyngeal Carcinoma: A Phase II Clinical Trial (POLARIS-02). J Clin Oncol 2021;39:704-12. [Crossref] [PubMed]
- Sheng X, Chen H, Hu B, et al. Safety, Efficacy, and Biomarker Analysis of Toripalimab in Patients with Previously Treated Advanced Urothelial Carcinoma: Results from a Multicenter Phase II Trial POLARIS-03. Clin Cancer Res 2022;28:489-97. [Crossref] [PubMed]
- Zhang L, Hao B, Geng Z, et al. Toripalimab: the First Domestic Anti-Tumor PD-1 Antibody in China. Front Immunol 2021;12:730666. [Crossref] [PubMed]
- Fu J, Wang F, Dong LH, et al. Preclinical evaluation of the efficacy, pharmacokinetics and immunogenicity of JS-001, a programmed cell death protein-1 (PD-1) monoclonal antibody. Acta Pharmacol Sin 2017;38:710-8. [Crossref] [PubMed]
- Liu H, Guo L, Zhang J, et al. Glycosylation-independent binding of monoclonal antibody toripalimab to FG loop of PD-1 for tumor immune checkpoint therapy. MAbs 2019;11:681-90. [PubMed]
- Wei XL, Ren C, Wang FH, et al. A phase I study of toripalimab, an anti-PD-1 antibody, in patients with refractory malignant solid tumors. Cancer Commun (Lond) 2020;40:345-54. [Crossref] [PubMed]
- Sheng X, Yan X, Chi Z, et al. Axitinib in Combination With Toripalimab, a Humanized Immunoglobulin G(4) Monoclonal Antibody Against Programmed Cell Death-1, in Patients With Metastatic Mucosal Melanoma: An Open-Label Phase IB Trial. J Clin Oncol 2019;37:2987-99. [Crossref] [PubMed]
- Wang F, He MM, Yao YC, et al. Regorafenib plus toripalimab in patients with metastatic colorectal cancer: a phase Ib/II clinical trial and gut microbiome analysis. Cell Rep Med 2021;2:100383. [Crossref] [PubMed]
- Lin H, Ma J, Zhuo M, et al. Updated results of the phase II ALTER-H003 trial: Anlotinib plus toripalimab as a first-line treatment for patients with unresectable hepatocellular carcinoma. J Clin Oncol 2022;40:446. [Crossref]
- Sun Q, Zhou J, Zhang Z, et al. Discovery of fruquintinib, a potent and highly selective small molecule inhibitor of VEGFR 1, 2, 3 tyrosine kinases for cancer therapy. Cancer Biol Ther 2014;15:1635-45. [Crossref] [PubMed]
- Chen Z, Jiang L. The clinical application of fruquintinib on colorectal cancer. Expert Rev Clin Pharmacol 2019;12:713-21. [Crossref] [PubMed]
- Doleschel D, Hoff S, Koletnik S, et al. Regorafenib enhances anti-PD1 immunotherapy efficacy in murine colorectal cancers and their combination prevents tumor regrowth. J Exp Clin Cancer Res 2021;40:288. [Crossref] [PubMed]
- Li Q, Cheng X, Zhou C, et al. Fruquintinib Enhances the Antitumor Immune Responses of Anti-Programmed Death Receptor-1 in Colorectal Cancer. Front Oncol 2022;12:841977. [Crossref] [PubMed]
- Fukuoka S, Hara H, Takahashi N, et al. Regorafenib Plus Nivolumab in Patients With Advanced Gastric or Colorectal Cancer: An Open-Label, Dose-Escalation, and Dose-Expansion Phase Ib Trial (REGONIVO, EPOC1603). J Clin Oncol 2020;38:2053-61. [Crossref] [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Five-Year Survival and Correlates Among Patients With Advanced Melanoma, Renal Cell Carcinoma, or Non-Small Cell Lung Cancer Treated With Nivolumab. JAMA Oncol 2019;5:1411-20. [Crossref] [PubMed]
- Toh JWT, de Souza P, Lim SH, et al. The Potential Value of Immunotherapy in Colorectal Cancers: Review of the Evidence for Programmed Death-1 Inhibitor Therapy. Clin Colorectal Cancer 2016;15:285-91. [Crossref] [PubMed]
- Eng C, Kim TW, Bendell J, et al. Atezolizumab with or without cobimetinib versus regorafenib in previously treated metastatic colorectal cancer (IMblaze370): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol 2019;20:849-61. [Crossref] [PubMed]
- Cousin S, Cantarel C, Guegan JP, et al. Regorafenib-Avelumab Combination in Patients with Microsatellite Stable Colorectal Cancer (REGOMUNE): A Single-arm, Open-label, Phase II Trial. Clin Cancer Res 2021;27:2139-47. [Crossref] [PubMed]
- Bocobo AG, Wang R, Behr S, et al. Phase II study of pembrolizumab plus capecitabine and bevacizumab in microsatellite stable (MSS) metastatic colorectal cancer (mCRC). J Clin Oncol 2022;40:3565. [Crossref]
- Mettu NB, Ou FS, Zemla TJ, et al. Assessment of Capecitabine and Bevacizumab With or Without Atezolizumab for the Treatment of Refractory Metastatic Colorectal Cancer: A Randomized Clinical Trial. JAMA Netw Open 2022;5:e2149040. [Crossref] [PubMed]
- Fakih M, Raghav KPS, Chang DZ, et al. Single-arm, phase 2 study of regorafenib plus nivolumab in patients with mismatch repair-proficient (pMMR)/microsatellite stable (MSS) colorectal cancer (CRC). J Clin Oncol 2021;39:3560. [Crossref]
- Song Y, Fu Y, Xie Q, et al. Anti-angiogenic Agents in Combination With Immune Checkpoint Inhibitors: A Promising Strategy for Cancer Treatment. Front Immunol 2020;11:1956. [Crossref] [PubMed]
- Mayer RJ, Ohtsu A, Yoshino T, et al. TAS-102 versus placebo plus best supportive care in patients with metastatic colorectal cancer refractory to standard therapies: Final survival results of the phase III RECOURSE trial. J Clin Oncol 2016;34:634. [Crossref]
- Fessas P, Lee H, Ikemizu S, et al. A molecular and preclinical comparison of the PD-1-targeted T-cell checkpoint inhibitors nivolumab and pembrolizumab. Semin Oncol 2017;44:136-40. [Crossref] [PubMed]
- Li J, Guo W, Bai Y, et al. Safety Profile and Adverse Events of Special Interest for Fruquintinib in Chinese Patients with Previously Treated Metastatic Colorectal Cancer: Analysis of the Phase 3 FRESCO Trial. Adv Ther 2020;37:4585-98. [Crossref] [PubMed]
- Sun L, Huang S, Li D, et al. Efficacy and Safety of Fruquintinib Plus PD-1 Inhibitors Versus Regorafenib Plus PD-1 Inhibitors in Refractory Microsatellite Stable Metastatic Colorectal Cancer. Front Oncol 2021;11:754881. [Crossref] [PubMed]
- Gjoerup O, Brown CA, Ross JS, et al. Identification and Utilization of Biomarkers to Predict Response to Immune Checkpoint Inhibitors. AAPS J 2020;22:132. [Crossref] [PubMed]
- Sivapiragasam A, Ashok Kumar P, Sokol ES, et al. Predictive Biomarkers for Immune Checkpoint Inhibitors in Metastatic Breast Cancer. Cancer Med 2021;10:53-61. [Crossref] [PubMed]
- Duffy MJ, Crown J. Biomarkers for Predicting Response to Immunotherapy with Immune Checkpoint Inhibitors in Cancer Patients. Clin Chem 2019;65:1228-38. [Crossref] [PubMed]
(English Language Editor: J. Gray)


