
A retrospective cohort study on the efficacy and safety for combination therapy of immunotherapy, targeted agent, and chemotherapy versus immunochemotherapy or chemotherapy alone in the first-line treatment of advanced biliary tract carcinoma
Highlight box
Key findings
• Anti-PD-1/PD-L1 immunotherapy in combination with anlotinib and gemcitabine showed promising efficacy and a acceptable safety profile.
What is known and what is new?
• Systemic chemotherapy based on gemcitabine or fluorouracil is still the main drug for first-line treatment of advanced BTC. In the era of new targeting and immunotherapy, targeted combined immunotherapy, targeted combined immunotherapy and chemotherapy, and immunotherapy combined chemotherapy are all superior to chemotherapy.
• We innovatively selected only gemcitabine as a chemotherapy drug and adjusted the usage of gemcitabine to once every 2 weeks. Under the condition of a better efficacy observed, adverse events did not increase significantly in this retrospective analysis.
What is the implication, and what should change now?
• Anti-PD-1/PD-L1 immunotherapy in combination with anlotinib and gemcitabine potentially provide promising efficacy with acce safety profile. It is suggested that a clinical randomized controlled study be conducted with a larger sample size to determine appropriate clinical use.
Introduction
Biliary tract carcinoma (BTC) is a spectrum of malignance primarily arising from the epithelial cells of the biliary duct and gallbladder. It can be divided into the cholangiocarcinoma (CCA), gallbladder carcinoma (GBC), and ampullary cancer (AMPAC) subtypes, each of which is a rare cancer with an incidence <6 per 100,000 (1). According to the anatomic location, CCA is classified into intrahepatic and extrahepatic CCA and accounts for approximately 2% of the annual cancer-related deaths worldwide (2). The incidence of intrahepatic CCA is on the rise, but the mortality rate of extrahepatic CCA and GBC is decreasing, and each subtype is distinct in respect to epidemiology, clinical behavior, and therapeutic characteristics (3). The pathogenesis of BTC is so unclear that the prognosis of BTC is quite poor due to the diagnosis occurring at a late stage of disease in the majority of patients, resulting in a 5-year survival rate of only 2%.
Currently, gemcitabine- or fluorouracil-based systemic chemotherapy remains the mainstay of the first-line treatment for advanced BTC. The regimen of gemcitabine and cisplatin (GEMCIS) confers a median overall survival (OS) of 11.7 months and a progression-free survival (PFS) of 8.0 months (4). On account of its better tolerability, oxaliplatin is extensively used in the treatment of BTC but has not been examined in a large-scale, prospective controlled study. Fiteni et al. analyzed the efficacy of GEMCIS and gemcitabine and oxaliplatin (GEMOX) by reviewing a total of 33 studies of 1,470 patients, which demonstrated that although GEMOX prolongs the median OS (9.7 vs. 9.5 months) and median PFS (6.3 vs. 4.9 months), it also increases the incidence of grade 3–4 toxicities (5). A randomized phase III trial comparing GEMOX and GEMCIS in GBC found that GEMOX yielded a longer median OS (9.0 vs. 8.3 months; P=0.057) than that of GEMCIS, with a relatively higher incidence of neuropathy and thrombocytopenia being associated with GEMOX and nephrotoxicity with GEMCIS (6). Investigators have attempted to intensify the magnitude of benefit via triplet-agent chemotherapy. In a phase II trial, a promising regimen of cisplatin and gemcitabine with nab-paclitaxel was demonstrated to improve the median OS and median PFS to 19.2 and 11.8 months, respectively, with a response rate of 45%, yet the sample was small while the incidence of grade >3 adverse events (AEs) reached 58% (7). A triplet regimen of cisplatin, gemcitabine, and S-1 has become a new treatment option in Japan on the basis of an improved median OS [13.5 vs. 12.6 months; hazard ratio (HR) 0.791; P=0.046]. However, a clinical benefit meeting statistical significance has yet to be attained. Another triplet regimen of folinic acid, fluorouracil, and oxaliplatin (FOLFOX) was reported to provide survival benefit in second line treatment of advanced BTC in the ABC-06 study (8), but has not been confirmed feasible in the first-line setting. The first-line therapy of BTC has changed little over recent years, with the largest obstacle being the toxicity of triplet chemotherapy. Thus, there is a pressing need to develop a new effective strategy to extend the benefit in first-line treatment of BTC, which is pivotal to improving the OS of these patients.
With the advent of the immunotherapy era, the efficacy of immune-checkpoint inhibitors (ICIs) has been proven to have moderate efficacy in BTC. In the KEYNOTE-158 and KEYNOTE-028 trials, monotherapy with pembrolizumab, an inhibitor of programmed cell death protein 1 (PD-1), was reported to yield an objective response in 5.8% of all patients who underwent second-line treatment of BTC; however, this objective response only occurred in 13.0% of PD-L1-positive patients in the KEYNOTE-028 study (9). Furthermore, immunochemotherapy was demonstrated to be effective in another PD-1 inhibitor, camrelizumab, in combination with the FOLFOX4/GEMOX regimen, deriving an objective response rate (ORR) of 16.3% and a disease control rate (DCR) of 75.0% in first-line treatment in a phase II trial (10). In another a phase II trial, PD-1 inhibitors plus lenvatinib, an antiangiogenetic agent, reached an ORR of 42.1%, a DCR of 76.3%, and a 6-month OS rate of 87.1% in first-line treatment of advanced BTC (11). Similarly, therapy with camrelizumab and apatinib, a PD-1 inhibitor plus a selective vascular endothelial growth factor receptor-2 (VEGFR-2) inhibitor, was reported to achieve an ORR of 19% and a DCR of 71.4% in second-line treatment of advanced BTC (12). Inevitably, every benefit has its downside: the better clinical outcome conferred by quadruple treatment is associated with a higher toxicity profile. A phase II trial of GEMOX chemotherapy in combination with PD-1 antibody toripalimab and lenvatinib yielded an ORR and DCR of up to 80.0% and 93.3%, respectively, and a 12-month OS rate of 73.3% in patients with advanced intrahepatic CCA; however, the incidence of grade 3–4 AEs reached up to 50% in the enrolled patients (13). Thus, there is an urgent need for the effective treatment of BTC with better tolerance.
With the emergence of ICI therapy, novel inhibitors of PD-1/programmed cell death ligand-1 (PD-L1) have been investigated in relation to BTC by way of combination with targeted agents and/or chemotherapy. Camrelizumab, an anti-PD-1 antibody, in combination with GEMOX chemotherapy was reported to yield a median PFS and OS of 6.1 and 11.8 months, respectively, with the ORR being higher in the patients with advanced BTC and a PD-L1 tumor proportion score (TPS) ≥1% than in those with PD-L1 TPS <1% (80.0% vs. 53.8%) (14).
Targeted therapy has emerged as a prominent area of investigation within oncology research over the past several years. Previous trials targeting the epidermal growth factor receptor (EGFR) pathway demonstrating limited success, and inhibition of the vascular endothelial growth factor (VEGF) receptor pathway yielding suboptimal results, but these studies did not restrict drug administration to patient populations with corresponding target mutations (15). Recent evidence, including clinical trials employing the isocitrate dehydrogenase (IDH)-targeted agent ivosidenib in cholangiocarcinoma patients harboring IDH1 mutations and a cohort study utilizing a BRAF inhibitor in patients with BRAF V600E-mutated cholangiocarcinoma (16), has demonstrated efficacy, highlighting the importance of tailoring targeted therapies to specific patient populations with relevant mutations. However, the financial burden associated with genetic testing renders the selection of targeted therapeutic agents based on individual patient mutations impractical in a clinical setting. Anlotinib is a small molecular targeted agent with promising antitumor activity in intrahepatic CCA by way of inhibiting the phosphorylation of VEGFR-2 and inactivating PI3K/AKT signaling (17). Furthermore, a phase II trial found that after a median follow-up of 8.76 months, anlotinib in combination with sintilimab, an anti-PD-1 monoclonal antibody, could confer a median PFS of 6.50 months, an ORR of 40.0%, and a DCR of 86.67% with good tolerance in the second-line treatment of BTC (18).
The application of targeted immunotherapy combinations in the field of hepatocellular carcinoma has become well-established, with a wealth of evidence supporting the safety and efficacy of such regimens (19,20). GEMOX chemotherapy is the standard treatment for biliary tract carcinoma. By incorporating targeted and immunotherapy agents to the chemotherapy foundation, we aim to achieve improved treatment efficacy. The combination of targeted immunotherapy combination with chemotherapy was explored with the use of tislelizumab combined with lenvatinib and the GEMOX regimen for first-line treatment of advanced biliary tract carcinoma. The results of this trial demonstrated an ORR of 80%, with median OS, PFS, and duration of response of 22.5, 10.2, and 11.0 months, respectively. A total of 56.7% of patients experienced grade ≥3 adverse events, primarily neutropenia and leukopenia, with no new safety signals observed, further indicating the feasibility of such combination strategy (21). In the present study, we retrospectively compared the efficacy and safety of immunotherapy plus targeted therapy and gemcitabine-based chemotherapy. We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-218/rc).
Methods
Patients
We conducted a retrospective study in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics board of The First Affiliated Hospital of Guangxi Medical University (No. ChiECRCT20210082). Because of the retrospective nature of the study, patient consent for inclusion was waived. Data from consecutive patients who were treated between February 2018 and August 2021 at the First Affiliated Hospital of Guangxi Medical University were retrieved, and patients were enrolled according to the following eligibility criteria: 18 years or older with physiologically identified advanced BTC; an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0–1; Child-Pugh class A–B liver function; received gemcitabine-based chemotherapy as the first-line treatment, which could include regimens of chemotherapy only, in combination with targeted agent, or with immunotherapy of camrelizumab or other PD-1/PD-L1 inhibitor; and with at least 1 measurable intrahepatic lesion according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. The exclusive criteria included patients with other malignant tumors; those with severe respiratory, cardiovascular, or kidney disease; those pregnant or lactating; those with incomplete medical information; and those loss to follow-up.
Treatments
Patients were divided into 3 arms according to the regimens. Arm A included patients who were treated with ICIs, a targeted agent with anlotinib (8–12 mg, day 1–14, orally, q3w), and chemotherapy with gemcitabine (1 g/m2 d1, q2w); arm B included patients treated with ICIs and GEMOX chemotherapy; and arm C included patients treated with GEMOX regimen chemotherapy only. In arm A and arm B, ICIs included camrelizumab (200 mg q2w, IV) or other PD-1/PD-L1 inhibitors. The GEMOX regimen consisted of 1 g/m2 of gemcitabine on day 1 and day 8 with 85 mg/m2 of oxaliplatin on day 1 (q3w). Patients were treated until disease progression or intolerable toxicities. Patients were followed up weekly, based on the drug administration cycle.
Data collection and study objectives
The medical records were retrospectively reviewed to collect the clinical data and imaging data. The baseline clinical factors were assessed in accordance with routine clinical treatment procedures. Data for analysis included: sex, age, ECOG PS score, primary location of tumor, and disease stage according to the American Joint Commission on Cancer (AJCC) staging system. All imaging data had to be independently evaluated by two radiologists. If two radiologists differed in their classifications, the final decision was made by another more senior radiologist. PFS was defined as the time from the commencement treatment of corresponding regimens to progressive disease (PD) on the basis of the RECIST v. 1.1 or death for any cause, whichever occurred first. OS was defined as the time from the commencement of treatment to death from any cause. And ORR was calculated as the proportion of patients with complete response (CR) or partial response (PR) according RECIST version 1.1. The DCR was defined as the proportion of patients with objective response plus stable disease (SD). AEs were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events v. 4.03.
Statistical analysis
The demographic data, outcomes, and other clinical characteristics were summarized. Frequency was used to present categorical variables, and age variable was expressed as median with interquartile range (IQR). The median PFS and OS were estimated from the Kaplan-Meier curves with their corresponding 95% CIs reported. For ORR and DCR, point estimates and exact Clopper-Pearson confidence intervals were calculated. The Fisher exact test was used to compare the ORR and DCR among the arms. All the statistical data were analyzed using IBM SPSS version 26.0 (IBM Corp., Armonk, NY, USA). All P values were 2-sided, with P values <0.05 being considered significant.
Results
Patient characteristics
From February 2018 to August 2021, a total of 35 patients with pathologically identified advanced BTC who were treated with gemcitabine-based chemotherapy or in combination with targeted therapy of anlotinib, and/or camrelizumab or other PD-1/PD-L1 inhibitors as first-line were enrolled. According to the treatment regimen, 11 patients who were treated with immunotherapy of camrelizumab or other PD-1/PD-L1 inhibitors plus anlotinib and gemcitabine were included in arm A. Meanwhile, 12 patients treated with the GEMOX regimen combined with camrelizumab or other PD-1/PD-L1 inhibitors were included in arm B, and 12 patients treated with GEMOX chemotherapy were included in arm C. The baseline characteristics are summarized in Table 1. Most patients were women (51.4%) and had an ECOG performance status of 0 (77.1%), Child-Pugh class A (94.3%), intrahepatic CCA (88.6%), metastatic BTC (94.3%), baseline CA199 >37 U/mL (54.3%), cholinesterase ≥5,000 U/L (80.0%), and prealbumin <250 mg/L (85.7%).
Table 1
| Variables | All patients (N=35) | Cohort A (N=11) | Cohort B (N=12) | Cohort C (N=12) |
|---|---|---|---|---|
| Age (years), median [range] | 53.9 [28–77] | 56.5 [33–69] | 50.0 [28–76] | 55.3 [28–77] |
| Sex, n (%) | ||||
| Male | 17 (48.6) | 6 (54.5) | 5 (41.7) | 6 (50.0) |
| Female | 18 (51.4) | 5 (45.5) | 7 (58.3) | 6 (50.0) |
| ECOG performance status, n (%) | ||||
| 0 | 27 (77.1) | 9 (81.8) | 10 (83.3) | 8 (66.7) |
| 1 | 8 (22.9) | 2 (18.2) | 2 (16.7) | 4 (33.3) |
| Child-Pugh class, n (%) | ||||
| A | 33 (94.3) | 10 (90.9) | 12 (100.0) | 11 (91.7) |
| B | 2 (5.7) | 1 (9.1) | 0 | 1 (8.3) |
| C | 0 | 0 | 0 | 0 |
| Primary site, n (%) | ||||
| Intrahepatic CCA | 31 (88.6) | 10 (90.9) | 11 (91.7) | 10 (83.3) |
| GBC | 4 (11.4) | 1 (9.1) | 1 (8.3) | 2 (16.7) |
| Disease stage, n (%) | ||||
| Locally advanced | 2 (5.7) | 0 | 0 | 2 (16.7) |
| Metastatic | 33 (94.3) | 11 (100.0) | 12 (100.0) | 10 (83.3) |
| CA199 >37 U/mL, n (%) | 19 (54.3) | 5 (45.5) | 8 (66.7) | 6 (50.0) |
| Cholinesterase ≥5,000 U/L, n (%) | 28 (80.0) | 8 (72.7) | 10 (83.3) | 10 (83.3) |
| Prealbumin ≥250 mg/L, n (%) | 5 (14.3) | 2 (18.2) | 2 (16.7) | 1 (8.3) |
ECOG, Eastern Cooperative Oncology Group performance status; CCA, cholangiocarcinoma; GBC, gallbladder cancer; CA, carbohydrate antigen.
Efficacy
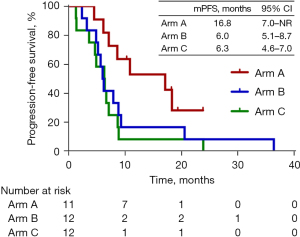
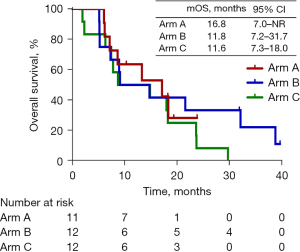
All 35 patients had received at least 3 cycles within 1-week on or off schedule and were included into the efficacy analysis. As of January 9, 2022, with a median follow-up of 31.9 months (range, 23.8–39.7 months), the distribution of response among all patients was 1 (2.9%) patient with CR, 13 (37.1%) with PR, 19 (54.3%) with SD, and 2 (5.7%) with PD. The overall ORR and DCR were 40.0% (95% CI: 23.9–57.9%) and 94.3% (95% CI: 80.8–99.3%), respectively. In arm A, the ORR and DCR were 63.6% (95% CI: 30.8–89.1%) and 100% (95% CI: 71.5–100%), respectively, with 7 patients with PR and 4 with SD. In arm B, the ORR and DCR were 33.3% (95% CI: 9.9–65.1%) and 100% (95% CI: 61.6–99.8%), respectively, with 1 patient with CR, 3 with PR, and 8 with SD. In arm C, the ORR and DCR were and 25.0% (95% CI: 5.5–57.2%) and 83.3% (95% CI: 61.6–99.8%), respectively, with 3 patients with PR and 7 with SD (Table 2). In arm A, the median PFS was 16.9 months [95% CI: 7.0–not reached (NR)]; 3 patients were assessed as PR and discontinued treatment, while 4 patients did not reach PD at the cutoff date. In arm B, the median PFS was 6.0 months (95% CI: 5.1–8.7 months), with all patients assessed as PD. In arm C, the median PFS was 6.3 months (95% CI: 4.6–7.0 months) with all patients assessed as PD. In arm A, the median OS was 16.9 months (95% CI: 7.0–NR) with 4 patients still alive at the cutoff date. In arm B, the median OS was 11.8 months (95% CI: 7.2–31.7 months) with 2 patients alive and on the second-line treatment at the cutoff date. In arm C, the median OS was 11.6 months (95% CI: 7.3–18.0 months). However, the median OS across the 3 arms showed no significant difference. When compared with arm C, arm A and B showed numerically longer PFS and OS. Figure 1 and Figure 2 show the Kaplan-Meier curves for the PFS and OS, respectively, of arm A, B, and C.
Table 2
| Variables | All patients (N=35) | Arm A (N=11) | Arm B (N=12) | Arm C (N=12) |
|---|---|---|---|---|
| Complete response, n (%) | 1 (2.9) | 0 | 1 (8.3) | 0 |
| Partial response, n (%) | 13 (37.1) | 7 (63.7) | 3 (25.0) | 3 (25.0) |
| Stable disease, n (%) | 19 (54.3) | 4 (36.4) | 8 (66.6) | 7 (58.3) |
| Progressive disease, n (%) | 2 (5.7) | 0 | 0 | 2 (16.7) |
| ORR, n (%) | 14 (40.0) | 7 (63.6) | 4 (33.3) | 3 (25.0) |
| DCR, n (%) | 33 (94.3) | 11 (100.0) | 12 (100.0) | 10 (83.3) |
| PFS, median (95% CI), months | – | 16.9 (7.0–NR) | 6.0 (5.1–8.7) | 6.3 (4.6–7.0) |
| OS, median (95% CI), months | – | 16.9 (7.0–NR) | 11.8 (7.2–31.7) | 11.6 (7.3–18.0) |
RECIST, Response Evaluation Criteria in Solid Tumors; ORR, objective response rate; DCR, disease control rate; PFS, progression-free survival; OS, overall survival; NR, no reached.
Safety
In this retrospective study, any-grade AEs occurred in 94.3% of the overall population, with 2 patients in arm A having no AEs. The AE statistics are shown in Table 3. Of the 35 patients included in the safety analysis, the most common AEs of any grade (incidence >15%) were transaminase elevation (54.3%), neutrophil count decrease (34.3%), blood bilirubin increase (17.1%), and fatigue (17.1%). Meanwhile, grade 3–4 AEs occurred in 13 of 35 (37.1%) patients, 1 of 11 (9.1%) in arm A (fatigue), 7 of 12 (58.3%) in arm B, and 5 of 12 (41.7%) in arm C. Grade 3–4 AEs in all patients included neutrophil count decrease (14.3%), aspartate aminotransferase increase (8.6%), alanine aminotransferase increase (8.6%), fatigue (5.7%), and blood bilirubin increase (5.7%). The GEMOX chemotherapy seemed to be correlated with a higher frequency of neutrophil count decrease. Palmar-plantar erythrodysesthesia syndrome only occurred in patients treated with anlotinib, with an incidence of 45.5%. Three patients (8.6% of all patients) in arm A and B developed grade 1–2 hypothyroidism, which could be attributable to immunotherapy. No grade 5 AEs occurred.
Table 3
| Adverse events | Any grade, n (%) | Grade 3/4, n (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| All patients (N=35) | Arm A (N=11) | Arm B (N=12) | Arm C (N=12) | All patients (N=35) | Arm A (N=11) | Arm B (N=12) | Arm C (N=12) | ||
| Hypertension | 4 (11.4) | 3 (27.3) | 0 | 1 (8.3) | 0 | 0 | 0 | 0 | |
| Palmar-plantar erythrodysesthesia syndrome | 5 (14.3) | 5 (45.5) | 0 | 0 | 0 | 0 | 0 | 0 | |
| Fatigue | 6 (17.1) | 3 (27.3) | 1 (8.3) | 2 (16.7) | 2 (5.7) | 1 (9.1) | 1 (8.3) | 0 | |
| Vomiting | 5 (14.3) | 1 (9.1) | 2 (16.7) | 2 (16.7) | 0 | 0 | 0 | 0 | |
| Neutrophil count decrease | 12 (34.3) | 2 (18.2) | 5 (41.7) | 5 (41.7) | 5 (14.3) | 0 | 2 (17.7) | 3 (25.0) | |
| Blood bilirubin increase | 6 (17.1) | 2 (18.2) | 2 (16.7) | 2 (16.7) | 2 (5.7) | 0 | 1 (8.3) | 1 (8.3) | |
| Aspartate aminotransferase increase | 19 (54.3) | 6 (54.5) | 5 (41.7) | 8 (66.7) | 3 (8.6) | 0 | 2 (16.7) | 1 (8.3) | |
| Alanine aminotransferase increase | 19 (54.3) | 6 (54.5) | 6 (50.0) | 7 (58.3) | 3 (8.6) | 0 | 2 (16.7) | 1 (8.3) | |
| Hypothyroidism | 3 (8.6) | 1 (9.1) | 2 (16.7) | 0 | 0 | 0 | 0 | 0 | |
Discussion
In this retrospective real-world study, immunotherapy plus chemotherapy with or without targeted therapy showed promising antitumor activity with a manageable safety profile in patients with advanced BTC. This study was the first investigation of the combined treatment strategy with ICIs plus targeted agents and chemotherapy in first-line treatment of advanced BTC. The triplet treatment strategy with ICIs, targeted agents, and gemcitabine in arm A conferred a much higher ORR of 63.6% than the 33.3% ORR yielded with ICIs plus GEMOX in arm B and the 25% yielded with GEMOX chemotherapy in arm C.
As one of the most malignant but rare tumors, BTC has challenged investigators all over the word. Since the ABC-02 study reported a median OS of 11.7 months and a median PFS of 8.0 months from gemcitabine and cisplatin, gemcitabine-based chemotherapy has been identified as fundamental in treatment for BTC (4). With the emergence of targeted therapy and immunotherapy, antiangiogenesis via targeted agents and immunotherapy have been proven to be effective in the treatment of BTC. Nonetheless, chemotherapy, targeted therapy, and immunotherapy possess individual strengths and shortcomings; for example, immunotherapy has unsatisfactory efficacy (9), and GEMOX plus ICIs and targeted agents has increased toxicity (13) and is limited by a low incidence of mutation (22). With multiple factors being considered, chemotherapy-based treatment combined with immunotherapy and targeted therapy might be the most effective regimen. Indeed, a retrospective analysis indicated gemcitabine to be the only chemotherapy agent to reduce the incidence of AEs (23). In our retrospective study, immunotherapy with PD-1/PD-L1 inhibitor plus targeted agents with anlotinib and gemcitabine conferred the highest ORR and best survival benefit among the 3 arms by prolonging the median PFS and OS. This was consistent with our assumption that a triple-combination of immunotherapy plus targeted therapy and gemcitabine would confer the greatest benefit. Even though gemcitabine was used as monochemotherapy in arm A, gemcitabine in combination with camrelizumab or other PD-1/PD-L1 inhibitors and anlotinib in arm A yielded a better median PFS than that of arm C. The regimen in arm A exhibited nonsignificant superiority in prolonging OS, partly due to the small sample size in the 3 arms. Moreover, 3 patients in arm A who discontinued treatment after PR died of disease progression, which decreased the median OS of arm A.
As a new treatment strategy, immunotherapy in combination of targeted therapy and chemotherapy has not been studied in-depth. In this study, compared with the toripalimab plus lenvatinib and GEMOX examined in a previous phase Ⅱ trial, we replaced the GEMOX chemotherapy with gemcitabine monotherapy to reduce the incidence of AEs. The treatment strategy brought a substantial increase in ORR, DCR, median PFS, and median OS in our study compared with the previous one (13).
In the context of the great improvement in clinical benefit derived from immunotherapy plus targeted therapy in several carcinoma types, the importance of chemotherapy should also be noted. In the treatment of BTC, immunotherapy plus targeted therapy is always used in second-line treatment of advanced cases. In previous reports, PD-1/PD-L1 inhibitors plus targeted agents, such as camrelizumab plus apatinib, sintilimab plus anlotinib, and pembrolizumab plus lenvatinib, conferred an ORR of 10–31.58%, a DCR of 68–82.35%, a median PFS of 4.4–6.50 months, and a median OS of 8.6–13.1 months in patients with previously treated advanced BTC (12,18,24). In the few investigations of immunotherapy plus targeted therapy in the first-line treatment of BTC, lenvatinib plus PD-1 inhibitors yielded an ORR of 42.1%, a DCR of 76.3%, and a 6-month OS rate of 87.1%. In arm A of this study, gemcitabine was added to the immunotherapy plus targeted therapy regimen, which proved that the combination with chemotherapy may be a better option when compared with the “chemo-free” strategy of immunotherapy and targeted therapy in the first- and second-line treatment of BTC.
Immunochemotherapy is becoming an important treatment strategy for advanced BTC. In reported clinical trials, immunochemotherapy yielded an ORR of 16.3–21.7%, a DCR of 75.0–80.4%, a median PFS of 4.9–5.8 months, and a median OS of 9.5–12.4 months in patients with advanced BTC (10,25). Immunotherapy has shown superiority to standard chemotherapy in treatment of advanced BTC (5). However, when compared with the standard doublet chemotherapy in arm C in our study, immunochemotherapy produced a similar median PFS and OS in arm B.
In terms of safety, the three regimens showed manageable toxicities. In this retrospective study, the incidence of AEs of any grade was observed in 94.3% of the overall population. The incidence of grade 3–4 AEs was much lower in arm A (1/11, 9.1%) than in arm B (7/12, 58.3%) and arm C (5/12, 41.7%), which could be attributed to gemcitabine monochemotherapy. It was further found that the combination of PD-1/PD-L1 inhibitor, anlotinib, and gemcitabine was well tolerated, and its safety was in line with the safety profile of sintilimab plus anlotinib; moreover, the combination had a lower incidence of grade 3 AEs than the 34.2% found with lenvatinib plus PD-1 treatment (11). Thus, anlotinib may be associated with fewer AEs and better safety. Compared with the incidence of grade 3 AEs in ≥50% patients with immunotherapy, targeted therapy, and GEMOX, that of chemotherapy with gemcitabine only was reduced, indicating more favorable tolerability. To prevent AEs, we assessed patients for high-risk factors of immune-related adverse events prior to initiating the treatment regimen, routinely monitored relevant laboratory parameters to identify trends indicative of adverse events or early stages of AEs, and promptly intervened as necessary, such as administering leukocyte elevation therapy or hepatoprotective treatments. In addition, depending on the patient’s condition, adjusting the medication dosage or temporarily discontinuing treatment in a timely manner was considered. Furthermore, gemcitabine was used once in a 2-week cycle, rather than on D1 and D8 in a 3-week cycle. The regimen in arm A was thus more compatible with efficacy and safety.
There were some intrinsic limitations in this study. First, the population sample was small, which means there could be some inevitable bias. And the influence of individual participants on the results was substantial, making statistical tests less meaningful. Consequently, we did not perform tests to compare baseline data across groups or conduct adjusted analyses on the results. Second, because of the retrospective nature of the study design, some patients were not managed properly, so that many patients were excluded for not receiving the scheduled treatment on time, which made the situation worse. As such, the findings in our study are descriptive and should be interpreted with caution, serving as a reference only.
Conclusions
In conclusion, immunotherapy plus targeted therapy and chemotherapy with gemcitabine showed promising antitumor activity with acceptable safety in advanced BTC population included in this study. A subsequent large-sample study to investigate the strategy of immunotherapy, targeted therapy, and chemotherapy in patients with advanced BTC is warranted. To further improve the treatment strategy, anlotinib dosing could be optimized based on patient weight or body surface area to reduce risk for AEs. Moreover, corporating local therapies such as interventional procedures and radiotherapy, into the combination regimen could be considered to enhance efficacy. While the specific improvements would need to be carefully evaluated in the context of potential toxicities. The optimal approach requires further exploration in future trials.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-218/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-218/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-218/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-218/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics board of The First Affiliated Hospital of Guangxi Medical University (No. ChiECRCT20210082). Because of the retrospective nature of the study, patient consent for inclusion was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhuravleva E, O'Rourke CJ, Andersen JB. Mutational signatures and processes in hepatobiliary cancers. Nat Rev Gastroenterol Hepatol 2022;19:367-82. [Crossref] [PubMed]
- Banales JM, Marin JJG, Lamarca A, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol 2020;17:557-88. [Crossref] [PubMed]
- Valle JW, Kelley RK, Nervi B, et al. Biliary tract cancer. Lancet 2021;397:428-44. [Crossref] [PubMed]
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81. [Crossref] [PubMed]
- Fiteni F, Nguyen T, Vernerey D, et al. Cisplatin/gemcitabine or oxaliplatin/gemcitabine in the treatment of advanced biliary tract cancer: a systematic review. Cancer Med 2014;3:1502-11. [Crossref] [PubMed]
- Sharma A, Kalyan Mohanti B, Pal Chaudhary S, et al. Modified gemcitabine and oxaliplatin or gemcitabine + cisplatin in unresectable gallbladder cancer: Results of a phase III randomised controlled trial. Eur J Cancer 2019;123:162-70. [Crossref] [PubMed]
- Shroff RT, Javle MM, Xiao L, et al. Gemcitabine, Cisplatin, and nab-Paclitaxel for the Treatment of Advanced Biliary Tract Cancers: A Phase 2 Clinical Trial. JAMA Oncol 2019;5:824-30. [Crossref] [PubMed]
- Lamarca A, Palmer DH, Wasan HS, et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): a phase 3, open-label, randomised, controlled trial. Lancet Oncol 2021;22:690-701. [Crossref] [PubMed]
- Piha-Paul SA, Oh DY, Ueno M, et al. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: Results from the KEYNOTE-158 and KEYNOTE-028 studies. Int J Cancer 2020;147:2190-8. [Crossref] [PubMed]
- Chen X, Qin S, Gu S, et al. Camrelizumab plus oxaliplatin-based chemotherapy as first-line therapy for advanced biliary tract cancer: A multicenter, phase 2 trial. Int J Cancer 2021;149:1944-54. [Crossref] [PubMed]
- Zhang Q, Liu X, Wei S, et al. Lenvatinib Plus PD-1 Inhibitors as First-Line Treatment in Patients With Unresectable Biliary Tract Cancer: A Single-Arm, Open-Label, Phase II Study. Front Oncol 2021;11:751391. [Crossref] [PubMed]
- Wang D, Yang X, Long J, et al. The Efficacy and Safety of Apatinib Plus Camrelizumab in Patients With Previously Treated Advanced Biliary Tract Cancer: A Prospective Clinical Study. Front Oncol 2021;11:646979. [Crossref] [PubMed]
- Jian Z, Fan J, Shi GM, et al. Gemox chemotherapy in combination with anti-PD1 antibody toripalimab and lenvatinib as first-line treatment for advanced intrahepatic cholangiocarcinoma: A phase 2 clinical trial. J Clin Oncol 2021;39:abstr 4094.
- Chen X, Wu X, Wu H, et al. Camrelizumab plus gemcitabine and oxaliplatin (GEMOX) in patients with advanced biliary tract cancer: a single-arm, open-label, phase II trial. J Immunother Cancer 2020;8:e001240. [Crossref] [PubMed]
- Martinez FJ, Shroff RT. Biliary tract cancers: systemic therapy for advanced disease. Chin Clin Oncol 2020;9:5. [Crossref] [PubMed]
- Wainberg ZA, Lassen UN, Elez E, et al. Efficacy and safety of dabrafenib (D) and trametinib (T) in patients (pts) with BRAF V600E-mutated biliary tract cancer (BTC): A cohort of the ROAR basket trial. J Clin Oncol 2019;37:abstr 187.
- Song F, Hu B, Cheng JW, et al. Anlotinib suppresses tumor progression via blocking the VEGFR2/PI3K/AKT cascade in intrahepatic cholangiocarcinoma. Cell Death Dis 2020;11:573. [Crossref] [PubMed]
- Zong H, Zhong Q, Zhao R, et al. Phase II study of anlotinib plus sintilimab as second-line treatment for patients with advanced biliary tract cancers. J Clin Oncol 2021;39:abstr 307.
- Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med 2020;382:1894-905. [Crossref] [PubMed]
- Ren Z, Xu J, Bai Y, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2-3 study. Lancet Oncol 2021;22:977-90. [Crossref] [PubMed]
- Shi GM, Huang XY, Wu D, et al. Toripalimab combined with lenvatinib and GEMOX is a promising regimen as first-line treatment for advanced intrahepatic cholangiocarcinoma: a single-center, single-arm, phase 2 study. Signal Transduct Target Ther 2023;8:106. [Crossref] [PubMed]
- Mahipal A, Tella SH, Kommalapati A, et al. FGFR2 genomic aberrations: Achilles heel in the management of advanced cholangiocarcinoma. Cancer Treat Rev 2019;78:1-7. [Crossref] [PubMed]
- Eckel F, Schmid RM. Chemotherapy and targeted therapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Chemotherapy 2014;60:13-23. [Crossref] [PubMed]
- Villanueva L, Lwin Z, Chung HCC, et al. Lenvatinib plus pembrolizumab for patients with previously treated biliary tract cancers in the multicohort phase 2 LEAP-005 study. J Clin Oncol 2021;39:abstr 4080.
- Gou M, Zhang Y, Liu T, et al. PD-1 Inhibitors Could Improve the Efficacy of Chemotherapy as First-Line Treatment in Biliary Tract Cancers: A Propensity Score Matching Based Analysis. Front Oncol 2021;11:648068. [Crossref] [PubMed]


