
Association between the systemic immune inflammation index and recurrence or metastasis after interventional therapy in patients with primary liver cancer- a retrospective cohort study
Highlight box
Key findings
• Elevated systemic immune inflammation index is associated with recurrence and metastasis after interventional therapy in patients with primary liver cancer (PLC).
What is known and what is new?
• The systemic immune inflammation index can reflect the inflammatory state and immune function status in patients with malignant tumors.
• Elevated systemic immune inflammation index is associated with recurrence and metastasis after interventional therapy in patients with PLC and can be used as a predictor of prognosis.
What is the implication, and what should change now?
• Elevated systemic immune inflammation index is associated with recurrence and metastasis after interventional therapy in patients with PLC, and treatment should be intensified in patients with elevated systemic immune inflammation index, which may help reduce the risk of recurrence and metastasis.
Introduction
Primary liver cancer (PLC) is a common fatal disease with a high degree of malignancy, accounting for about 3–6% of cancer deaths per year (1). Transcatheter arterial chemoembolization (TACE) is the preferred non-surgical treatment of PLC and can improve the long-term survival rate of patients (2-4). For patients with small liver cancer, TACE or radiofrequency ablation can be used as a radical treatment. However, due to the high degree of malignancy of liver cancer, the recurrence and metastasis rates after TACE or radiofrequency ablation can be as high as 20% (5), and it is of great significance to accurately identify the recurrence and metastasis risks after TACE or radiofrequency ablation therapy in patients with liver cancer. Vascular tumor thrombus and hepatitis B are risk factors for recurrence of liver cancer patients after operation. Moreover, Alpha-fetoprotein (AFP) is the most commonly used biological indicator in patients with PLC, which has a high value in distinguishing PLC from metastatic liver cancer (6-8) and also has some value in identifying recurrence or metastasis after treatment of PLC. However, the timeliness of AFP is poor: after treatment, patients with markedly elevated AFP often already have recurrence or metastasis and therefore AFP cannot be used as an early predictor of the risk of recurrence or metastasis after PLC TACE or radiofrequency ablation therapy (9,10). The systemic immune inflammation index can reflect the inflammatory state and immune function status of patients with malignant tumors and has been used to evaluate the prognosis of patients with a variety of malignant tumors (11-15), but there is a lack of relevant studies in evaluating the predicting value of systemic immune inflammation index in patients with PLC after TACE or radiofrequency ablation therapy. This study aimed to investigate the predictive value of systemic immune inflammation index for recurrence or metastasis after TACE or radiofrequency ablation therapy in patients with PLC. We present the following article in accordance with the STARD reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-104/rc).
Methods
General information
The 5-year recurrence rate of liver cancer after surgery was about 50% (16). Based on the principle of 10 events per variable, the sample size for multivariate regression analysis was determined. Before the study started, we speculated that 5-10 variables would be analyzed, so the minimum sample size should be greater than 200 cases. A total of 272 patients with PLC who were admitted to 941st Hospital of PLA Joint Logistics Support Force from January 2016 to December 2017 were continuously retrospectively collected. All patients received interventional treatment, there were no residual lesions after interventional treatment, and the patients were followed up for 5 years to observe whether the patients had recurrence or metastasis after interventional treatment. Then, the patients were divided into a recurrence or metastasis group (n=112) and a control group (n=160). The inclusion criteria were as follows: (I) small PLC (liver cancer in which the maximum diameter of a single cancer nodule does not exceed 3 cm or the sum of the diameters of 2 cancer nodules does not exceed 3 cm); (II) age ≥18 years old; (III) receiving TACE treatment or radiofrequency ablation therapy; (IV) no residual lesions after treatment. The exclusion criteria were as follows: (I) metastatic liver cancer; (II) PLC with distant metastasis; (III) combined with other malignant tumors; (IV) major organs dysfunction; (V) previous abnormal immune system function, such as ulcerative colitis, systemic lupus erythematosus, and other diseases; (VI) lost to follow-up. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the 941st Hospital of PLA Joint Logistics Support Force (No. 202200231) and individual consent for this retrospective analysis was waived.
Treatment
All patients were treated with TACE or radiofrequency ablation. The methods of TACE were as follows: percutaneous right femoral artery puncture, selective insertion of a catheter into the hepatic intrinsic artery (the target artery of tumor blood supply). Then, we injected a contrast agent to observe the mass. After the location, diameter, and blood supply of the mass had been clarified, chemotherapeutic drugs such as pirarubicin, oxaliplatin, 5-fluorouracil, and iodide oil mixture were injected at an appropriate speed, and then microspheres were injected for embolization. Radiofrequency ablation was conducted as follows: under ultrasound guidance, the radiofrequency ablation electrode was pierced into the tumor site, and the radiofrequency ablation instrument sent out radiofrequency pulses under computer control to increase the local temperature of tumor tissue to 80 ℃ and to kill tumor cells.
Observation indicators
Age, gender, body mass index, combined with Hepatitis B, tumor size, number of lesions, vascular cancer thrombus, vascular invasion, Child-Pugh Grade, tumor capsule, AFP, albumin, neutrophil ratio, lymphocyte ratio, platelet count, systemic immune inflammation index, and recurrence rate or metastasis rate 5 years after treatment.
Definitions
(I) Recurrence rate or metastasis: at least once a year after surgery, liver magnetic resonance imaging, abdominal computed tomography, head computed tomography, and chest computed tomography examinations should be performed. If imaging suggests recurrence or metastasis, lesion biopsy should be performed to confirm the presence of recurrence or metastasis. (II) Systemic immune inflammation index: Platelet count × Neutrophil count/Lymphocyte count (15).
Statistical analysis
The software SPSS 26.0 (IBM Corp., Armonk, NY, USA) was used to complete the data analysis of this study, and P<0.05 indicated that the difference was statistically significant (2-tailed). The measurement data of the 2 groups were expressed by mean ± standard deviation, and the differences between the 2 groups were analyzed by independent sample t-test. The patient count data of the 2 groups were expressed by n (%), and the chi-square test was used to analyze the difference between the 2 groups. The receiver operating characteristic (ROC) curve was used to analyze the predictive value of systemic immune inflammation index on recurrence or metastasis after interventional treatment in patients with PLC. Multivariate regression analysis was used to explore the risk factors of recurrence or metastasis.
Results
Comparison of clinical features of the two groups
The patient inclusion process diagram was shown in Figure 1. Compared with the control group, the proportion of patients with ≥2 lesions in the recurrence or metastasis group was significantly increased (19.64% vs. 8.12%, P=0.005); the proportion of patients with vascular invasion was significantly increased in the recurrence or metastasis group (10.71% vs. 4.38%, P=0.044); albumin decreased significantly in the recurrence or metastasis group (39.69±6.17 vs. 41.69±6.82 g/L, P=0.014); neutrophils (%) were significantly increased in the recurrence or metastasis group (0.70±0.08 vs. 0.64±0.08, P<0.001); lymphocytes (%) were significantly reduced in the recurrence or metastasis group (0.25±0.06 vs. 0.30±0.06, P<0.001); and platelet count was significantly increased in the recurrence or metastasis group (179.22±39.52 vs. 160.81±34.13 109/L, P<0.001). The systemic immune inflammation index was significantly increased in the recurrence or metastasis group (535.23±174.05 vs. 357.84±120.21, P<0.001) (see Table 1).
Table 1
| Variables | Recurrence or metastasis group (n=112) | Control group (n=160) | t/χ2 value | P value |
|---|---|---|---|---|
| Age (years) (mean ± standard deviation) | 54.96±10.22 | 56.31±10.37 | 1.064 | 0.289 |
| Gender [n (%)] | 0.146 | 0.702 | ||
| Male | 67 (59.82%) | 92 (57.50%) | ||
| Female | 45 (40.18%) | 68 (42.50%) | ||
| Body mass index (kg/m2) (mean ± standard deviation) | 24.64±2.03 | 24.48±2.03 | 0.644 | 0.520 |
| Hepatitis B [n (%)] | 0.522 | 0.470 | ||
| Yes | 90 (80.36%) | 134 (83.75%) | ||
| No | 22 (19.64%) | 26 (16.25%) | ||
| Tumor sizes (cm) (mean ± standard deviation) | 1.98±0.59 | 2.02±0.57 | 0.656 | 0.513 |
| Number of lesions [n (%)] | 7.795 | 0.005 | ||
| 1 | 90 (80.36%) | 147 (91.88%) | ||
| ≥2 | 22 (19.64%) | 13 (8.12%) | ||
| Vascular cancer thrombus [n (%)] | 10 (8.93%) | 7 (4.38%) | 2.331 | 0.127 |
| Vascular invasion [n (%)] | 12 (10.71%) | 7 (4.38%) | 4.075 | 0.044 |
| Child-Pugh grade [n (%)] | 0.000 | 1.000 | ||
| A grade | 98 (87.50%) | 140 (87.50%) | ||
| B grade | 14 (12.50%) | 20 (12.50%) | ||
| Tumor capsule [n (%)] | 0.384 | 0.535 | ||
| Yes | 102 (91.07%) | 142 (88.75%) | ||
| No | 10 (8.93%) | 18 (11.25%) | ||
| AFP (µg/L) (mean ± standard deviation) | 231.45±98.03 | 211.41±94.03 | 1.699 | 0.090 |
| Albumin (g/L) (mean ± standard deviation) | 39.69±6.17 | 41.69±6.82 | 2.474 | 0.014 |
| Neutrophil ratio (%) (mean ± standard deviation) | 0.70±0.08 | 0.64±0.08 | 5.685 | <0.001 |
| Lymphocyte ratio (%) (mean ± standard deviation) | 0.25±0.06 | 0.30±0.06 | 6.914 | <0.001 |
| Platelet count (109/L) (mean ± standard deviation) | 179.22±39.52 | 160.81±34.13 | 4.102 | <0.001 |
| Systemic immune inflammation index (mean ± standard deviation) | 535.23±174.05 | 357.84±120.21 | 9.945 | <0.001 |
AFP, alpha fetoprotein.
Predictive values of different biological indexes on recurrence or metastasis after interventional treatment in patients with PLC
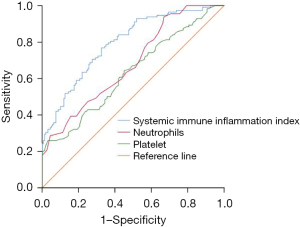
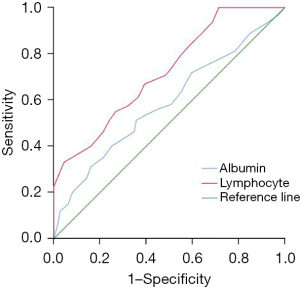
Systemic immune inflammation index, neutrophils, platelets, lymphocytes, and albumin were all valuable in predicting recurrence or metastasis after interventional treatment in patients with PLC, among which systemic immune inflammation index had the highest predictive value, and the area under the curve (AUC) was 0.795 (95% CI: 0.742–0.848, P<0.001) (see Tables 2,3 and Figures 2,3).
Table 2
| Variables | AUC (95% CI) | Standard error | P value | Best diagnostic threshold | Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| Systemic immune inflammation index | 0.795 (0.742–0.848) | 0.027 | 0.000 | 405.08 | 0.768 | 0.675 |
| Neutrophils | 0.682 (0.619–0.745) | 0.032 | 0.000 | 0.69 | 0.518 | 0.681 |
| Platelet | 0.632 (0.565–0.700) | 0.034 | 0.000 | 161.50 | 0.643 | 0.550 |
AUC, area under the curve.
Table 3
| Variables | AUC (95% CI) | Standard error | P value | Best diagnostic threshold | Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| Albumin | 0.587 (0.519–0.654) | 0.034 | 0.015 | 42.5 | 0.513 | 0.643 |
| Lymphocyte | 0.722 (0.662–0.782) | 0.030 | 0.000 | 0.27 | 0.669 | 0.607 |
AUC, area under the curve.
Risk factors of recurrence or metastasis after interventional treatment in patients with PLC
Systemic immune inflammation index >405.08 was an independent risk factor of recurrence or metastasis after interventional treatment in patients with PLC [relative risk (95% CI: 1.878–5.329), P=0.000]. See Table 4.
Table 4
| Variables | B value | Standard error | Wald value | P value | Relative risk (95% CI) |
|---|---|---|---|---|---|
| Number of lesions ≥2 | 0.829 | 0.393 | 4.448 | 0.035 | 2.292 (1.060–4.953) |
| Vascular invasion | 0.935 | 0.519 | 3.241 | 0.072 | 2.547 (0.920–7.049) |
| Albumin <35 g/L | 0.365 | 0.299 | 1.486 | 0.223 | 1.440 (0.801–2.588) |
| Systemic immune inflammation index >405.08 | 1.152 | 0.266 | 18.751 | <0.001 | 3.164 (1.878–5.329) |
| Constant | −5.310 | 1.403 | 14.333 | <0.001 | 0.005 |
Discussion
PLC has high incidence and mortality rates, and recurrence or metastasis after treatment is the main factor leading to death of PLC patients. This study was conducted to explore the predictive value of systemic immune inflammation index for recurrence or metastasis after interventional therapy in patients with PLC. We found that systemic immune inflammation index, neutrophils, platelets, lymphocytes, and albumin were all valuable in predicting recurrence or metastasis after interventional treatment in patients with PLC, among which systemic immune inflammation index had the highest predictive value, with an AUC of 0.795 (95% CI: 0.742–0.848, P=0.000).
The systemic immune inflammation index has the advantage of convenient detection and dynamic monitoring, and is calculated by neutrophils, platelets, and neutrophils (17,18). Neutrophils are inflammatory cells, and an increase in neutrophils levels indicates an increase in systemic immune inflammation index in patients with PLC, which is conducive to the generation of local blood vessels in tumor tissues, promoting the tumor cell proliferation and metastasis (19-21). Platelets are small pieces of cytoplasm shed from the cytoplasm of mature megakaryocytes in the bone marrow, and are active participants in all steps of tumorigenesis, including tumor growth, tumor cell extravasation, and tumor metastasis. At the same time, platelets play an important role in protecting cancer cells from chemotherapy-induced apoptosis and maintaining the integrity of tumor vasculature (6,22). Lymphocytes include T cells, B cells, and natural killer (NK) cells, which are the main immune cells of the body to kill tumors, and reduction of lymphocytes indicates that patients with PLC have a reduced ability to kill tumor cells (23-25). Systemic immune inflammation index = (neutrophils * platelets)/lymphocytes, so when the systemic immune inflammation index is elevated, it indicates an increase in neutrophils and platelets, and a decrease in lymphocyte levels, which indicates that tumor cells are more likely to proliferate and metastasize, resulting in recurrence or metastasis in patients with PLC after treatment. A study in patients with intrahepatic cholangiocarcinoma has shown that elevated systemic immune inflammation index is a risk factor for poor prognosis in liver transplant patients (26). A study in patients with hepatocellular carcinoma has also shown that elevated systemic immune inflammation index is a risk factor for poor prognosis in liver transplant patients, and systemic immune inflammation index was valuable in predicting the survival, the area under the ROC curve was 0.632 (27). The previous studies supported our study, but our study predominantly involved PLC patients after interventional treatment, which is different from previous studies.
Limitations
This study was a retrospectively clinical study and we failed to explore the molecular mechanism of systemic immune inflammation index leading to recurrence or metastasis after interventional therapy in patients with PLC.
Conclusions
The study of biological indicators related to the prognosis of different diseases is a research hotspot (28-33). The elevated systemic immune inflammation index is associated with recurrence and metastasis after interventional therapy in patients with PLC, and treatment should be intensified in patients with elevated systemic immune inflammation index, which may help reduce the risk of recurrence or metastasis.
Acknowledgments
Funding: This study was funded by Yunnan Province High-level Health and Family planning Personnel Training Project (Yunwei Science and Education Development [2017] No. 14); Basic Research Joint Special General Project of Yunnan Provincial Local Universities (part) (Nos. 2018FH001-076, 2018FH001-080).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-104/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-104/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-104/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-104/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the 941st Hospital of PLA Joint Logistics Support Force (No. 202200231) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]
- Cun J, Xu Y, Li W, et al. Analysis of factors affecting the prognosis of transcatheter arterial chemoembolization for hepatitis B-related hepatocellular carcinoma. J Interv Med 2021;4:66-70. [Crossref] [PubMed]
- Feng GY, Cheng Y, Xiong X, et al. Conversion therapy of hepatic artery ligation combined with transcatheter arterial chemoembolization for treating liver cancer: A case report. World J Clin Cases 2021;9:9151-8. [Crossref] [PubMed]
- Zhu C, Chen H, Fang Q, et al. Improvement in the condition of patients with primary liver cancer with transcatheter arterial chemoembolization before and after microwave ablation interventional therapy. Am J Transl Res 2021;13:11908-16. [PubMed]
- Yu Y, Fu J, Xia P, et al. A systematic review and meta-analysis on the efficacy and safety of transcatheter arterial chemoembolization combined with radiofrequency ablation in the treatment of primary liver cancer. Transl Cancer Res 2022;11:1297-308. [Crossref] [PubMed]
- Guo C, Liang H, Yuan W, et al. Analysis on the value of soluble intercellular adhesion molecule-1 (sICAM-1), alpha fetoprotein (AFP), and aspartate aminotransferase/platelet ratio index (APRI) in predicting the prognostic survival of patients with primary liver cancer after radiofrequency ablation. Ann Palliat Med 2021;10:4760-7. [Crossref] [PubMed]
- Pho-Iam T, Punnakitikashem P, Somboonyosdech C, et al. PLGA nanoparticles containing alpha-fetoprotein siRNA induce apoptosis and enhance the cytotoxic effects of doxorubicin in human liver cancer cell line. Biochem Biophys Res Commun 2021;553:191-7. [Crossref] [PubMed]
- Yu K, Tang J, Wu JL, et al. Risk factors for intraocular metastasis of primary liver cancer in diabetic patients: Alpha-fetoprotein and cancer antigen 125. World J Diabetes 2021;12:158-69. [Crossref] [PubMed]
- Zhang G, Yun Y, Lin C, et al. Predictive Value of MRI with Serum Lectin-Reactive Alpha-Fetoprotein for Liver Cancer Recurrence after Percutaneous Radiofrequency Ablation. Evid Based Complement Alternat Med 2022;2022:5132135. [Crossref] [PubMed]
- Sun LY, He Y, Liu Q, et al. Effect of delta alpha-fetoprotein on the detection of liver cancer recurrence. Transl Cancer Res 2020;9:6263-74. [Crossref] [PubMed]
- Wang S, Yang X, Yu Z, et al. The Values of Systemic Immune-Inflammation Index and Neutrophil-Lymphocyte Ratio in Predicting Biochemical Recurrence in Patients With Localized Prostate Cancer After Radical Prostatectomy. Front Oncol 2022;12:907625. [Crossref] [PubMed]
- Yekedüz E, Dogan İ, Kaya DM, et al. Systemic Immune-Inflammation Index as a Prognostic Marker of Late Recurrence in Operable Gastric Cancer: a Dual-Center Study. J Gastrointest Cancer 2022;53:870-9. [Crossref] [PubMed]
- Chien TM, Li CC, Lu YM, et al. The Predictive Value of Systemic Immune-Inflammation Index on Bladder Recurrence on Upper Tract Urothelial Carcinoma Outcomes after Radical Nephroureterectomy. J Clin Med 2021;10:5273. [Crossref] [PubMed]
- Zeng Q, Li J, Sun N, et al. Preoperative systemic immune-inflammation index predicts survival and recurrence in patients with resected primary pulmonary sarcomatoid carcinoma. Transl Lung Cancer Res 2021;10:18-31. [Crossref] [PubMed]
- Yang J, Bao Y, Chen W, et al. Nomogram Based on Systemic Immune Inflammation Index and Prognostic Nutrition Index Predicts Recurrence of Hepatocellular Carcinoma After Surgery. Front Oncol 2020;10:551668. [Crossref] [PubMed]
- Yin Z, Jin H, Ma T, et al. A meta-analysis of long-term survival outcomes between surgical resection and radiofrequency ablation in patients with single hepatocellular carcinoma </= 2 cm (BCLC very early stage). Int J Surg 2018;56:61-7. [Crossref] [PubMed]
- Jung SH, Hao J, Shivakumar M, et al. Development and validation of a novel strong prognostic index for colon cancer through a robust combination of laboratory features for systemic inflammation: a prognostic immune nutritional index. Br J Cancer 2022;126:1539-47. [Crossref] [PubMed]
- Zhu M, Chen L, Kong X, et al. The Systemic Immune-Inflammation Index is an Independent Predictor of Survival in Breast Cancer Patients. Cancer Manag Res 2022;14:775-820. [Crossref] [PubMed]
- Siwicki M, Gort-Freitas NA, Messemaker M, et al. Resident Kupffer cells and neutrophils drive liver toxicity in cancer immunotherapy. Sci Immunol 2021;6:eabi7083. [Crossref] [PubMed]
- Wang X, Hu LP, Qin WT, et al. Identification of a subset of immunosuppressive P2RX1-negative neutrophils in pancreatic cancer liver metastasis. Nat Commun 2021;12:174. [Crossref] [PubMed]
- Palmieri V, Lazaris A, Mayer TZ, et al. Neutrophils expressing lysyl oxidase-like 4 protein are present in colorectal cancer liver metastases resistant to anti-angiogenic therapy. J Pathol 2020;251:213-23. [Crossref] [PubMed]
- Wang YH, Qiu H, He XL, et al. Prevention of venous thromboembolism after resection of primary liver cancer with low molecular weight heparin and its association with P-selectin, lysosomal granule glycoprotein, platelet activating factor and plasma D-dimer. Eur Rev Med Pharmacol Sci 2018;22:4657-62. [PubMed]
- Li X, Zhang Y, Ma W, et al. An Elevated Neutrophil-to-Lymphocyte Ratio Predicts Poor Prognosis in Patients with Liver Cancer after Interventional Treatments. Biomed Res Int 2022;2022:6141317. [Crossref] [PubMed]
- Lin N, Li J, Yao X, et al. Prognostic value of neutrophil-to-lymphocyte ratio in colorectal cancer liver metastasis: A meta-analysis of results from multivariate analysis. Int J Surg 2022;107:106959. [Crossref] [PubMed]
- Fang C, Huang Y, Chen C, et al. The Prognostic Value of Serum Apolipoprotein A-I Level and Neutrophil-to-Lymphocyte Ratio in Colorectal Cancer Liver Metastasis. J Oncol 2022;2022:9149788. [Crossref] [PubMed]
- Ren A, Li Z, Cheng P, et al. Systemic Immune-Inflammation Index Is a Prognostic Predictor in Patients with Intrahepatic Cholangiocarcinoma Undergoing Liver Transplantation. Mediators Inflamm 2021;2021:6656996. [Crossref] [PubMed]
- Fu H, Zheng J, Cai J, et al. Systemic Immune-Inflammation Index (SII) is Useful to Predict Survival Outcomes in Patients After Liver Transplantation for Hepatocellular Carcinoma within Hangzhou Criteria. Cell Physiol Biochem 2018;47:293-301. [Crossref] [PubMed]
- Teng D, Xia S, Hu S, et al. miR-887-3p Inhibits the Progression of Colorectal Cancer via Downregulating DNMT1 Expression and Regulating P53 Expression. Comput Intell Neurosci 2022;2022:7179733. [Crossref] [PubMed]
- Chen Y, Wang J, Zhang X, et al. Correlation between apparent diffusion coefficient and pathological characteristics of patients with invasive breast cancer. Ann Transl Med 2021;9:143. [Crossref] [PubMed]
- Qi A, Li Y, Yan S, et al. Effect of postoperative chemotherapy on blood glucose and lipid metabolism in patients with invasive breast cancer. Gland Surg 2021;10:1470-7. [Crossref] [PubMed]
- He XC, Chen HY, Qiu Y, et al. Associations of iron status with breast cancer risk factors in adult women: Findings from National Health and Nutrition Examination Survey 2017-2018. J Trace Elem Med Biol 2021;68:126867. [Crossref] [PubMed]
- Han R, Tian Z, Jiang Y, et al. Prognostic significance of systemic immune-inflammation index and platelet-albumin-bilirubin grade in patients with pancreatic cancer undergoing radical surgery. Gland Surg 2022;11:576-587. [Crossref] [PubMed]
- Wang J, Yin S, Chen K. Predictive value of the systemic immune-inflammation index for the efficacy of neoadjuvant chemotherapy and prognosis in patients with stage III ovarian cancer—a retrospective cohort study. Gland Surg 2022;11:1639-46. [Crossref] [PubMed]
(English Language Editor: J. Jones)



